
Geochemical Study of the Geothermal Field of Νigrita,
Greece
G Diamantopoulos
1,*
, D Poutoukis
2
, B Raco
3
, A Arvanitis
4
, P
Karalis
1
and E
Dotsika
1
1
Stable Isotopes Unit, N.C.S.R. “Demokritos”, Institute of Nanoscience and
Nanotechnology, 15310, Ag. Paraskevi Attikis, Greece
2
General Secretariat for Research and Technology, Mesogion 14-18, 11510, Athens,
Greece
3
Institute of Geosciences and Earth Resources, Via G. Moruzzi 1, 56124 Pisa, Italy
4
Institute of Geology and Mineral Exploration, (I.G.M.E.), S. Loui 1, 3rd entrance of
Olympic Village, 13677, Athens, Greece
*Corresponding author. Tel.: +30 210 6503305; Fax: +30 210 6519430
e-mail address, g.diamantopoulos@inn.demokritos.gr
Abstract. In order to investigate the mineralisation process, we conducted geochemical and
isotopic analyses (major ions,
18
O,
2
H) of the thermal waters of springs and boreholes of
Nigrita. This study shows that the thermal waters are of meteoric origin. Appropriate
geothermometers were applied on selected samples of thermal waters for the determination of
the deep aquifer temperature.
1. Introduction
The thermal springs of Nigrita are located in North Greece. The geothermal anomaly manifests itself
mainly by the intersection of the fault systems of the area. The main thermal reservoir is located at
the basalt conglomerate containing water at a highest temperature of 600°C. Appropriate
geothermometers were applied on selected samples of thermal waters for the determination of the
deep aquifer temperature.
2. Geology
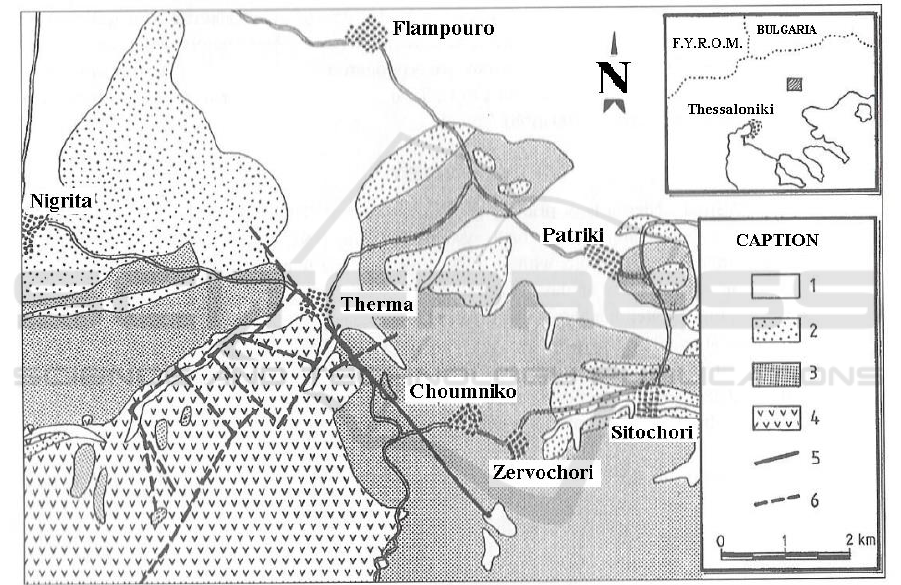
The geological background (Figure 1) of the area consists of metamorphic rocks of the
Serbomacedonian mass and thick sedimentary deposits of Neogene age, which are rich in clay and
marl components, and present poor hydraulic characteristics.
The aquifer body consists of a basal conglomerate formation which develops at the depth of 70 to
500 m. The reservoir presents pressurized heads, and measured temperatures range from 40 to 64°C.
3. Sampling and analytical methods
The water samples were collected from the area of Nigrita-Therma for isotopic analysis. For the
chemical analyses, water was sampled in plastic bottles of 700ml. Two bottles of waters were taken
for the chemical analyses, one acidized (HNO
3
1:1), for cation analysis and one not-acidized for
Diamantopoulos, G., Poutoukis, D., Raco, B., Arvanitis, A., Karalis, P. and Dotsika, E.
Geochemical Study of the Geothermal Field of Nigrita, Greece.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 221-227
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
221
anion analysis. Chemical analyses were conducted at the Institute of Geosciences and Earth Resource,
C.N.R., Pisa. One glass bottle of water (50ml) was taken for the isotopic analyses (
18
O and
2
H).
In situ, conductivity, temperature, pH and bicarbonates were measured. For this purpose, several
instruments were used, including digital pH-meter, control-solutions pH 4 and 7 for the calibration of
the pH-meter, automatic digital conductometer for conductivity and temperature measurements,
control-solutions for the calibration of the conductometer, dense nitric acid, ΗΝΟ
3
65%, 1,40 Kg/l
density, portable fridge for the storage of the samples.
The isotopic composition of the waters was conducted according to the isotopic method for the
18
O [1] and
2
H analysis [2]. The results of the stable isotope are expressed in delta (δ) ‰ vs SMOW
(Standard Mean Ocean Water). The error for δ
18
O is ± 0.2 ‰ and for δ
2
H ± 2‰. Isotopic analyses
were conducted at the Unit of Stable Isotopes, Institute of Nanoscience and Nanotechnology,
N.C.S.R. “Demokritos” and at the Institute of Geosciences and Earth Resource, C.N.R., Pisa.
Figure 1. Simplified geological map of Therma-Nigrita area, on the basis of the geological map of
I.G.M.E. [1. Sandy clays, sands, gravels, alluvial fans (Holocene), 2. Sands, gravels, clays, loams
(Pleistocene), 3. Fine-layered silts, clays, sandy clays, lacustrine limestones, marls, sandy marls,
marly limestones, silts, intecalations of gravel layers, sands, basal conglomerate (Neogene), 4.
Ophiolites, 5. Faults, 6. Propable faults].
4. Hydrochemistry
9 samples of water were collected for this study: 3 cold waters from boreholes (samples N-4, N-6, N-
8), 2 semi thermal water from borehole (N-5, N-9: 22 and 27°C respectively) and 4 from thermal
springs (samples TH-1, TH-2, TH-3, TH-5). Bibliographic data were also considered [3]. The
sampling was carried out between June 2013 and July 2015. The temperatures of borehole, waters
varied between 17 and 27 °C, although the ambient temperature for the last 30 years averaged
17.5 °C. All the boreholes samples are of Ca-HCO
3
type (Figure 2).
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
222
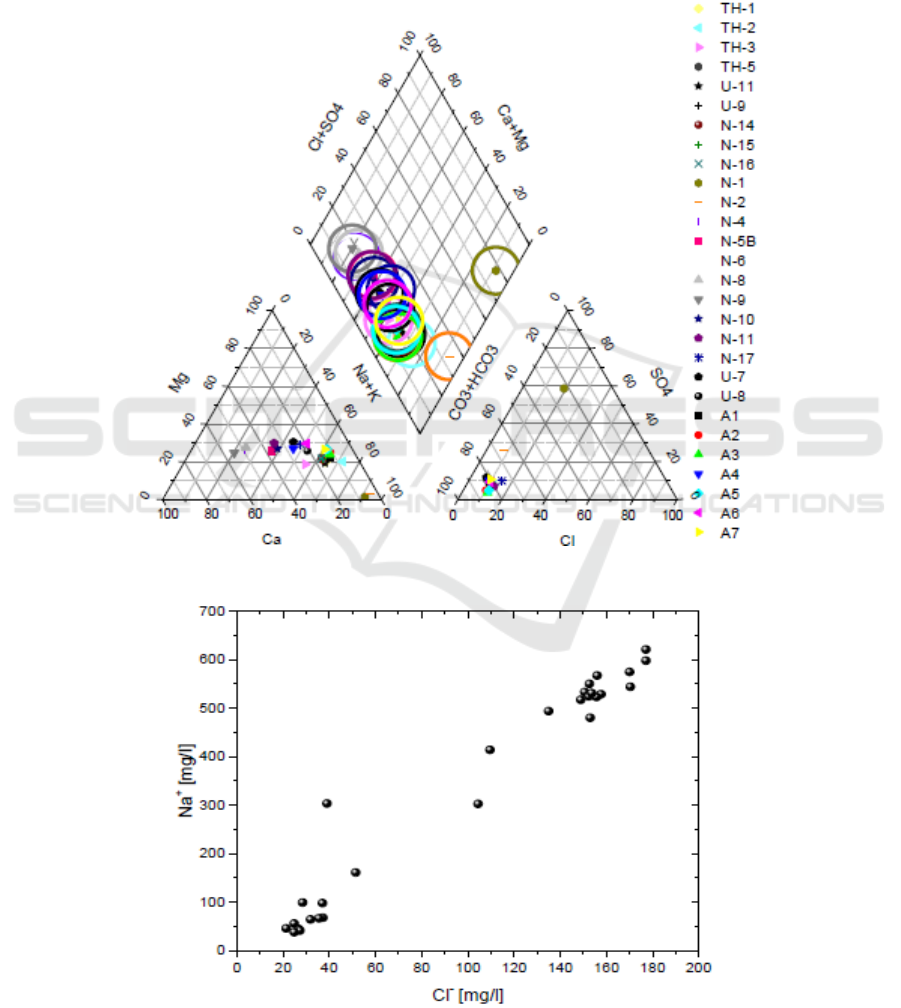
In the Na versus Cl diagram (Figure 2) the samples follow a relation between the low TDS fresh
cold borehole water with the thermal springs. This indicates the occurrences of mixing between a low
Na-Cl component and a high Na-Cl component. The first is probably diluted ground water while the
later maybe water of geothermal reservoir. The relatively low concentration of Cl
−
in these waters
excludes contamination of shallow aquifers with seawater. On the other hand, the possibility that
some of the Na
+
and Cl
−
ions contained in well waters come from marine spray cannot be excluded
(Figure 3).
Figure 2. Chemical types of the waters.
Figure 3. Na versus Cl contents.
Geochemical Study of the Geothermal Field of Nigrita, Greece
223
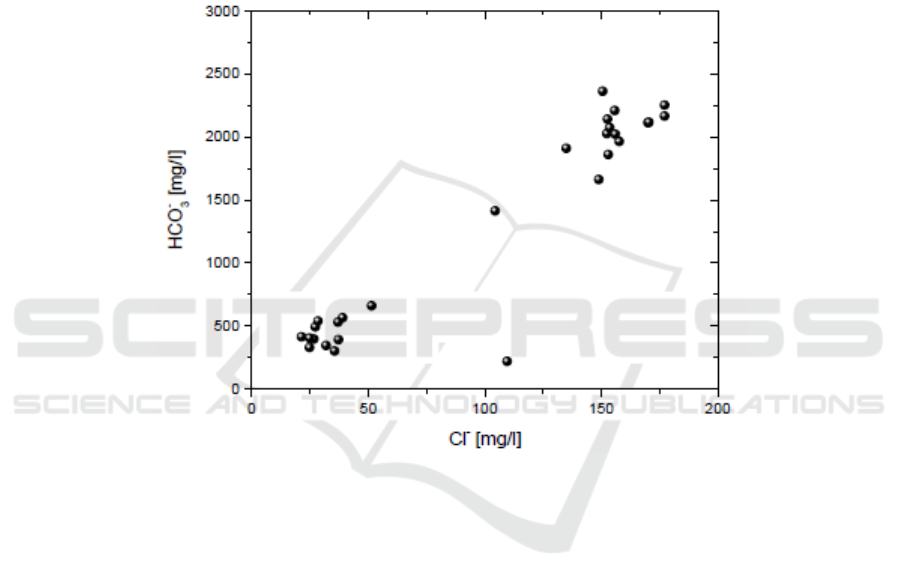
These waters in general are superficial waters in their first stages of interaction with the rocks. In
fact the TDS in the Ca-HCO
3
type waters from borehole is in the interval of 0.5 to 1.5g/L. The
sampled thermal waters of this area are Na-HCO
3
type with high B contents (3 mg/L) showing that
the supply of boron by rock leaching is significant. The relatively high HCO
3
−
, which is observed in
the hot springs, relates with absorption of CO
2
-bearing gases or with condensation of CO
2
geothermal
steam. The waters, in which the condensation of the geothermal steam took place, have a high
composition in bicarbonates ([i.e. 1600-2300 mg/L) (Figure 4) and are found in the marginal zone of
geothermal liquid-domined systems. The differentiation of hot waters, rich in B and HCO
3
content
from the others and the gradual increase of Cl
−
content confirm that these waters are mixed with a
deeper geothermal fluid.
Figure 4. HCO
3
versus Cl contents.
5. Stable isotopes of water
Analyses of δ
2
H and δ
18
O have been performed on all samples. The stable isotope contents of δ
18
O
ranging from −8.8 to -8.5‰ and δ
2
H from −56 to –55.9‰. The isotopic values of the region’s waters
are plotted in the diagram of Figure 4 along with the Global Meteoric Water Line and the Eastern
Mediterranean Water Line. The cold and thermal waters are plotted between these two lines (Global:
δ
2
H = 8δ
18
Ο+10, [4] and E. Mediterranean: δ
2
H = 8δ
18
Ο+22, [5, 6]). In Figure 5 it is shown that the
composition of the Nigrita waters is mainly meteoric, ruling out the isotopic exchange with the
geological environment under temperature, evaporation or mixing with isotopically different waters.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
224
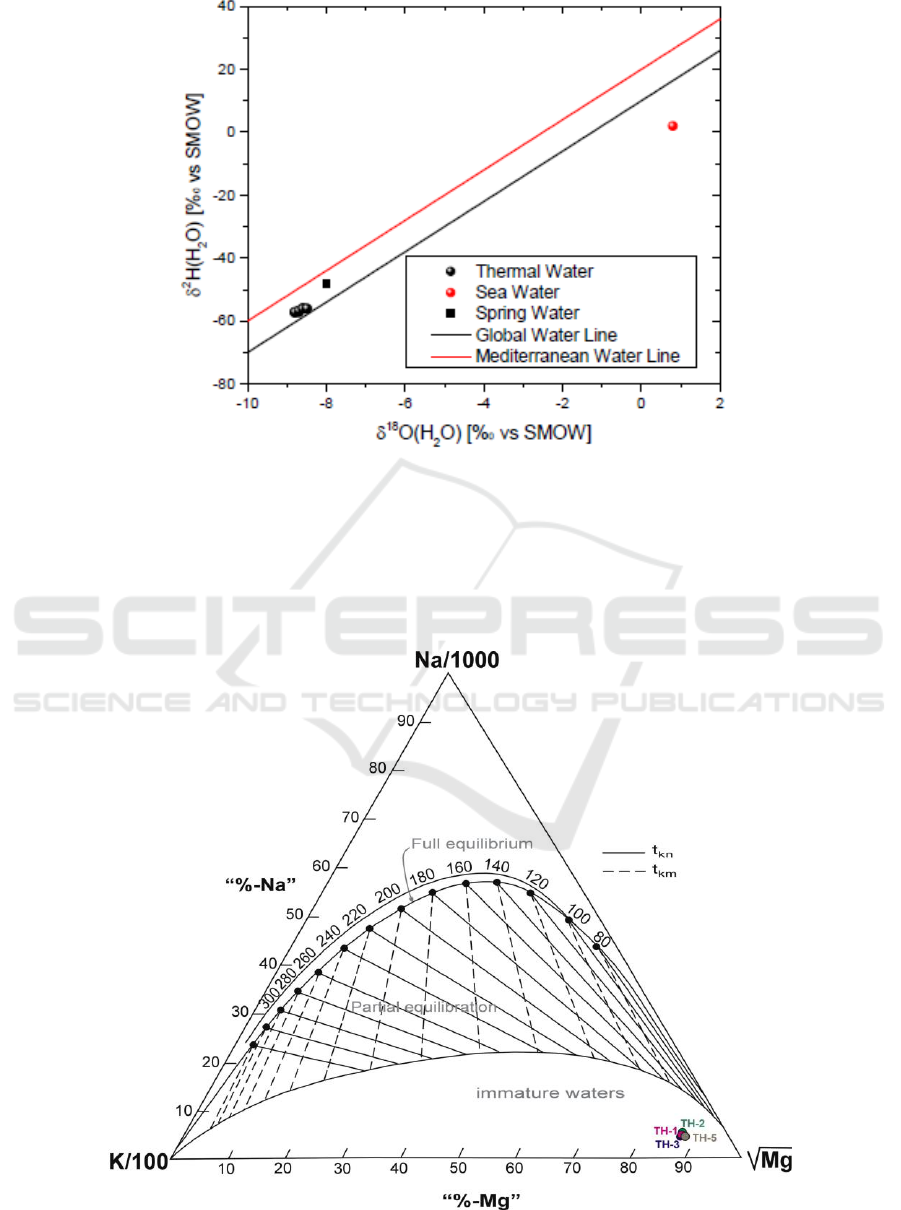
Figure 5. Diagram δ
18
Ο vs δ
2
Η.
6. Geothermometry of the waters
For the determination of the temperature of the deep geothermal fluids, the chemical
geothermometers applied are SiO
2
[7], Na/K [8], Na-K-Ca [9], Νa-Li [10], K-Mg [11] and Li-Mg
[12] on the geothermal waters of Nigrita basin. The results of the application of these chemical
geothermometers are reported in Table 1 and Figure 6.
Figure 6. Giggenbach diagram.
Geochemical Study of the Geothermal Field of Nigrita, Greece
225

The resulting temperatures are different for each geothermometer with moderate to large variation
with the exception of the temperatures resulting from the Li-Mg geothermometer, which are almost
identical to the measured temperatures of the emerging thermal waters. Probably the high
temperatures of the other geothermometers, are due to the state of non-equilibrium of the water in the
thermal reservoir as calculated by the saturation indexes (PHREEQC). Contrary, the waters are
sursaturated in quartz [13] allowing the use of SiO
2
geothermometer. The temperature proposed is
about 110 ° C that we accept as the lowest for this geothermic system. These temperatures are close
to that estimated (130-150° C) from [3] with the use of the isotopic geothermometer (sulfur isotopic
geothermometer is based on the equilibration of δ
18
O between SO
4
2-
-H
2
O).
Table 1. Estimation of the temperature (
ο
C) of the deep reservoir by the use of geothermometers for
the geothermal waters of the Nigrita region.
T (°C)
T (°C)
T (°C)
T (°C)
T (°C)
T (°C)
SiO
2
Na/K
Na-K-Ca
Na-Li
K/Mg
Li-Mg
TH-1
118.9
244.7
210.5
138.3
91.5
76.3
TH-2
118.9
241.3
217.2
92.0
TH-3
102.2
266.2
214.2
94.4
94.8
60.0
TH-5
107.0
257.4
215.9
97.0
92.0
60.2
7. Conclusions
The chemical data of the thermal water samples indicate the mixing between deep geothermal water
and cold water. Furthermore, the high B contents measured in these thermal waters show that the
supply of boron by rock leaching is significant. The use of chemical geothermometer attributes a
temperature greater than 110°C to the deep geothermal field.
References
[1] Epstein S and T M 1953 Variation of 18O content of water from natural sources Geochim.
Cosmochim Acta 4: p 213–224
[2] Coleman M L and et al 1982 Reduction of water with zinc for hydrogen isotope analysis
Analytical chemistry 54(6): p 993-995
[3] Dotsika E 1991 Utilisation du geothermometre isotopique sulfate-eau en milieux de haute
temperature sous influence marine potentielle: Les systemes geothermaux de Grece Thesis
Paris 11
[4] Craig H 1961 Isotopic variations in meteoric waters Science 133(3465): p 1702-1703
[5] Bowen G J and Wilkinson B 2002 Spatial distribution of δ18O in meteoric precipitation
Geology 30(4): p 315-318
[6] Aouad A and et al 2004 Etude isotopique de la pluie et de la neige sur le Mont Liban:
premiers résultats/Isotope study of snow and rain on Mount Lebanon: preliminary results
Hydrological Sciences Journal 49(3)
[7] Fournier R 1981 Application of water geochemistry to geothermal exploration and reservoir
engineering, Principles and Case Histories Geothermal systems p 109-143
[8] Arnorsson S, Gunnlaugsson E and Svavarsson H 1983 The chemistry of geothermal waters in
Iceland. III. Chemical geothermometry in geothermal investigations Geochimica et
Cosmochimica Acta 47(3): p 567-577
[9] Fournier R and Truesdell A 1973 An empirical Na K Ca geothermometer for natural
waters Geochimica et Cosmochimica Acta 37(5): p 1255-1275
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
226

[10] Fouillac C and Michard G 1981 Sodium/lithium ratio in water applied to geothermometry of
geothermal reservoirs Geothermics 10(1): p 55-70
[11] Giggenbach W and et al 1983 Isotopic and chemical composition of Parbati valley geothermal
discharges, north-west Himalaya, India Geothermics 12(2-3): p 199-222
[12] Kharaka Y K and Mariner R H 1989 Chemical geothermometers and their application to
formation waters from sedimentary basins, in Thermal history of sedimentary basins
Springer p 99-117
[13] Parkhurst D L and C Appelo 1999 User's guide to PHREEQC (Version 2): A computer
program for speciation, batch-reaction, one-dimensional transport, and inverse
geochemical calculations
Geochemical Study of the Geothermal Field of Nigrita, Greece
227
