
Isolation and Characterization of Chromium Reducing
Bacteria
Y Gao
1
, Q Cheng
1
, T T Hu
1
, H J Ji
1
, Z Y Zhu
1
, Q Xu
2
, A M Li
2
and Y Yang
1,3,*
1
School of Minerals Processing and Bioengineering, Central South University,
Changsha, Hunan, China, 410083
2
Hunan Coal Science Research Institute, Changsha, Hunan, China, 410004
Central South University, 932 South Lushan Road, Changsha, 410083, China.
3
Key Laboratory of Biometallurgy of Ministry of Education, Changsha, Hunan,
China, 410083
Corresponding author and E-mail: Y Yang , csuyangyu@csu.edu.cn.
Abstract. Removal of highly toxic Cr (VI) using bioremediation can start a new way for
effective treatment of chromium contamination. Therefore, the chromate-reducing strain G-13
under alkaline conditions was isolated from chromium factory. G-13 was identified as
Micrococcus sp..The strain of G-13 was selected to study the resistance of Cr (VI) and its
ability to reduce Cr (VI) under different culture conditions. The results showed that the
optimum temperature and pH for the strain were found to be 30℃ and 7.0 or 8.0, respectively.
The percent reduction of Cr(VI) for 50,100, 200, 400 and 500 mg/L of initial concentration at
96 h of incubation were 82.1%, 63.6%, 34.5%, 21.2% and 9.1%, respectively. The
Micrococcus sp.G-13 strain was remarkable under the condition of glycerol and sodium
lactate as the electron donor, which could reduce 50 mg/L Cr (VI) to 0 within 36 h, and the
reduction rate was 100%. Consequently, the isolation of bacteria can be exploited for the
bioremediation of Cr (VI) pollution. It is hoped that this study can provide theoretical basis
for the treatment of environmental chromium pollution.
1. Introduction
Chromium is one of the most widely and heavily used heavy metals in various industrial processes,
such as metallurgy, wood preservation, nuclear power plants and
so on[1]. The widespread use of
chromium compounds can result in large quantities of chromium being released into the environment
[2]. Therefore, chromium contamination has been often reported in many industrial sites due to
improper disposals, poor storage and accidental leakages measures. In natural systems, chromium
generally exists in two stable oxidation states, hexavalent chromium (Cr (VI)) and trivalent
chromium (Cr (III))[3].Soluble hexavalent chromium [Cr (VI)], such as [CrO
4
2-
, HCrO
4
-
] is highly
toxic, and shows mutagenic and carcinogenic effects on biological systems due to its strong oxidizing
nature [4].While trivalent chromium (Cr (III)) like [Cr (OH)
3
] is an essential micronutrient for
organisms (50-200 ug/day). It is 100-fold less toxic than Cr (VI) due to its lower cell permeability
and insolubility [5].
Since Cr (VI) poses a great threat to humans, cleaning up this contaminant from soil and water is
Gao, Y., Cheng, Q., Hu, T., Ji, H., Zhu, Z., Xu, Q., Li, A. and Yang, Y.
Isolation and Characterization of Chromium Reducing Bacteria.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 605-613
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
605

essential and reduction to Cr (III) may be considered a satisfactory solution in eliminating the
toxicity of Cr (VI)[6]. Over the past few decades, several technologies based on this reason have
been developed to remove chromium from the environment. Far-ranging conventional methodologies
have been used for the removal of Cr (VI) from industrial wastewaters including ion-exchange resins
[7], filtration [8], chemical precipitation [9], chemical oxidation or reduction and so on [10].
However, these methods tend to generate large amount of secondary waste products, resulting in
secondary pollution of the environment. Thus, to overcome these disadvantages, it is important to
develop an innovative, cost-effective and eco-friendly methods for extraction of hazardous materials
present in environment.
Microbial remediation, is a potential alternative for the removal of chromium [Cr (VI)] in
environmental studies because of the advantages of its environmental friendliness, cost effectiveness
compared with conventional methods [11]. Soil microorganisms play a dominant role in reducing the
toxic charge of heavy metals through conversion of toxic Cr(VI) to relatively nontoxic Cr(III)[12].In
some cases, the microorganisms remediation occurs spontaneously that reduces the toxic Cr(VI) to
Cr(III) because the essential materials required for bacterial growth are naturally present at the
contaminated sites[13,14].Hence, the present study is aimed at the isolation of potential hexavalent
chromium reducing bacteria from a contaminated soil and to study their hexavalent chromium
reduction characteristics.
2. Materials and methods
2.1. Sample collection
Soil sample was collected from the surface horizon (0-10 cm) of the chromium pollution sites of a
chromate factory in Changsha, China. Soil samples collected in sterilized plastic bags were brought
to the laboratory and stored at 4°C refrigerated condition until use.
2.2. Preparation of media
The medium selected was Luria Broth (LB) medium to isolate and the reduction characteristics of
chromium-reducing bacteria. The medium specific ingredients [15] was tryptone 10 g/L, yeast extract
5 g/L, NaCl 5 g/L,MgSO
4
.
7H
2
O 0.2 g/L,K
2
HPO
4
0.05 g/L, distilled water 1 L, Agar 2%(w/v).The pH
value of the medium was adjusted to 8.0 by adding aliquots of either 1 mol/L H
2
HSO
4
or 1 mol/L
NaOH. The medium were autoclaved at 121°C for 20 min.
2.3. Isolation of Cr (VI) - tolerant bacterial strains
Isolation of the bacterial culture was done by an enrichment culture technique [16]. Luria Broth was
amended with filter sterilized 200 mg/L K
2
Cr
2
O
7
as Cr(VI) and 10 g soil which was incubated at
30°C ,170 r/min for 12 h on a rotary shaker. After 12 h enriched bacterial strains were isolated by
plating on Luria agar plate amended with 200 mg/L of K
2
Cr
2
O
7
.This process was serially diluted with
sterile water of soil sample and plated onto Luria Broth (LB) agar and incubated at 30°C for 24–36 h
to isolate Cr (VI)-resistant bacteria. Bacterial colonies of different morphologies were obtained
through many round streaking and purification on the same agar medium. From this preliminary
screening strains showing resistance to chromium were selected for further studies. The ability of the
isolates to reduce Cr (VI) was determined by Cr (VI) reduction experiments.
2.4. Screening of Cr (VI)-reducing strain
The selected Cr (VI)-resistant strain were inoculated into 100 mL of liquid LB medium containing 50
mg/L of Cr(VI) as K
2
Cr
2
O
7
and incubated in an orbital shaker(170 r/min) at 30°C .1 mL liquid of
culture were taken out in sterilized tube at regular time interval and were centrifuged at 10,000 r/min
for 5 min at room temperature. The supernatant was used to measure Cr (VI) concentration at 540 nm
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
606

using UV 754 N model spectrophotometer.
2.5. Identification of the isolated strain
The isolated strain was identified based on standard biochemical tests [17]. In addition, molecular
identification was done by 16S rRNA analysis using universal bacterial 16S rRNA gene primers
27F (5’-AGAGTTTGATCCTGGCTGGCTCAG-3’) and 1492R
(5’-GGTTACCTTGTTACGACTT-3’) for polymerase chain reaction (PCR) amplification of the
16S rRNA gene [18]. Purified PCR products were conducted by Platinum Sequencing Company,
Shanghai, China. The resulting 16S rRNA gene sequences were initially analyzed with known 16S
rRNA sequences in the GenBank database to identify the most similar sequence alignment and to
download the corresponding sequence. The phylogenetic tree was then constructed by
neighbor-joining method using the MEGA 7.0 package version.
2.6. Determination of optimum growth temperature and pH of the isolated strain
For optimum growth of the bacterial isolates, two parameters, i.e., temperature and pH were
considered [19]. For the determination of optimum temperature, the logarithmic strain seed solution
were inoculated into sterilized 100 mL LB medium at 5% inoculum concentration (250 mL culture
flasks, pH 9.0) and incubated at 25°C , 30°C , 35°C , 40°C and 45°C , respectively. In addition, to
determine the optimum pH of the strain, a set of liquid LB medium of the same specifications was
prepared and the pH values were set to 3.0, 5.0, 7.0, 8.0, 9.0, 10.0 and 11.0, respectively. Then
inoculated with 5% inoculum concentration into the sterilized LB medium, placed in 30°C , 170 r/min
incubated on a rotary shaker, observed at regular time intervals the OD
600
value of the culture
medium, each experiment was carried out in triplicate. Data are the mean of three replications.
2.7. Determination of chromium reduction experiments
2.7.1. Effects of initial chromium concentration on chromium reduction. To determine the effect of
initial chromium concentration on Cr (VI) removal of strain, the logarithmic strain seed solution of
G-13 was inoculated into sterilized 100 mL LB liquid medium at a 5% inoculum concentration (250
mL culture flasks, pH 9.0) amended with variable concentration of Cr (VI) as K
2
CrO
4
[20],
respectively 50, 100, 200, 400 and 500 mg/L and incubated on a rotary shaker at 30°C , 170 r/min.
The chromium removal was measured at regular time intervals by measuring the residual Cr (VI) in
the cell-free supernatant. Each experiment was carried out in triplicate. Data are the mean of three
replications.
2.7.2. Effects of various electron donors on chromium reduction. To determine the effect of carbon
source on Cr (VI) removal of strain, the logarithmic strain seed solution of G-13 was inoculated with
5% inoculum concentration into 100 mL LB liquid medium (250 mL culture flasks, 50 mg/L Cr (VI),
pH 9.0) amended with a certain concentration of carbon source as electron donors [21], respectively
glucose, sucrose, sodium lactate, glycerol and incubated on a rotary shaker at 30°C , 170 r/min. The
chromium removal was measured at regular time intervals by measuring the residual Cr (VI) in the
cell-free supernatant following centrifugation. Each experiment was carried out in triplicate. Data are
the mean of three replications.
2.8. Analytical methods
Cr (VI) concentration in the supernatant was determined colorimetrically with a spectrophotometer
using diphenylcarbazide reagent in acid solution method. The reagent was prepared by adding 0.2 g
of diphenylcarbazide to 50 mL of acetone and then made up to a final volume of 100 mL with
distilled water. To the cooling solution were added 12.5 mL H
2
SO
4
© and H
2
PO
4
©, respectively. The
Isolation and Characterization of Chromium Reducing Bacteria
607
reagent was stored in a brown bottle at 4°C until used. The residual Cr (VI) concentration in the
culture was measured at 540 nm by UV 754N model spectrophotometer. The growth of cells was
routinely monitored by measuring optical density (OD) at 600 nm.
3. Results and discussion
3.1. Identification of the isolated Cr (VI)-reducing strain
The colony morphology, cell morphology and biochemical test results of the isolated strain are
presented in Table 1 and the partial amplification sequences was sequenced by 16S rRNA gene. The
result was compared using the BLSAT function provided by NCBI database to identify the most
similar sequence alignment. The results revealed that the strain was 99 % homologous to
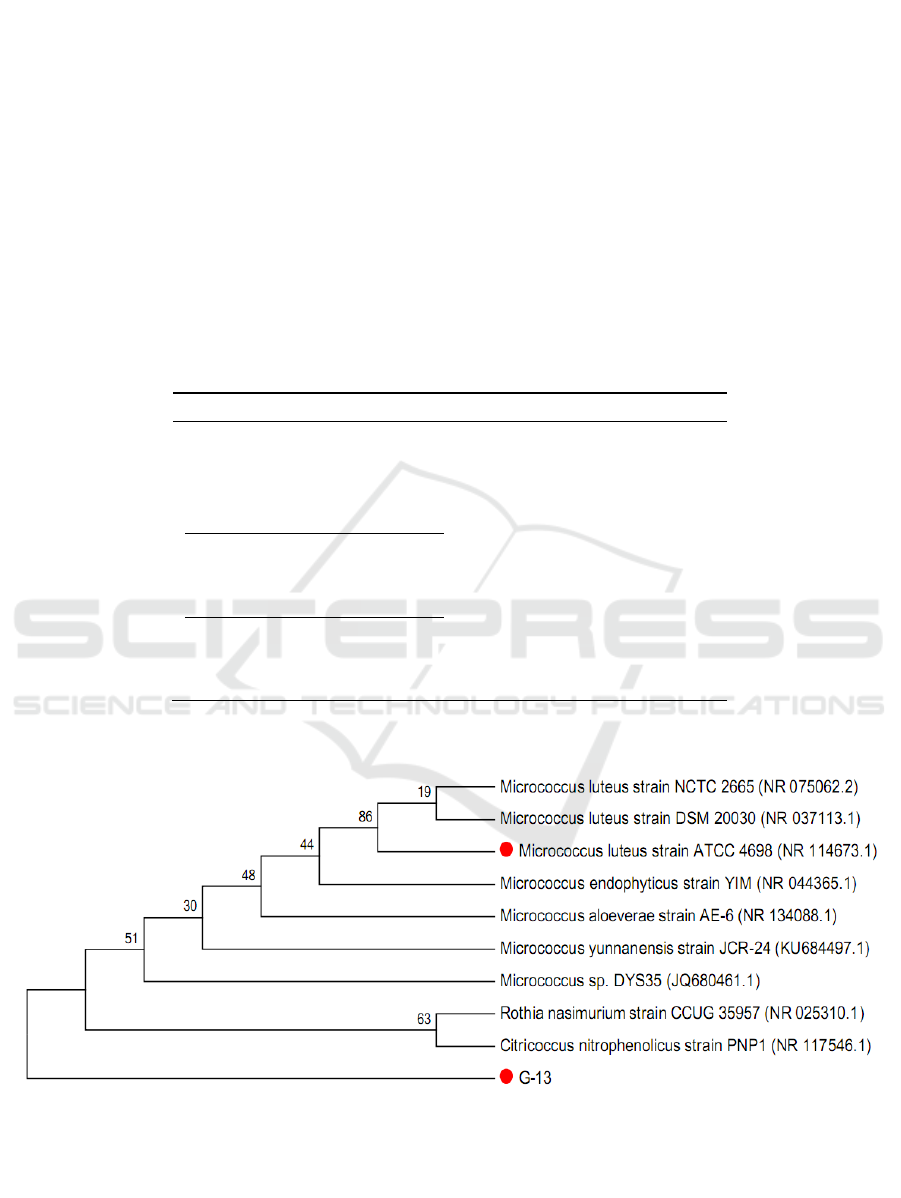
Micrococcus luteus strain ATCC 4698 (NR 114673.1) (Figure 1).Hence, the isolates strain was
identified from this result as Micrococcus sp.
Table 1. Cell morphology and biochemical test results for the isolate strain.
Biochemical characteristics
Results
Cell / Colony morphology
Round
Colony Color
Yellow
Colony edge
Smooth
Motility
+
Gram staining
+
V-P test
-
Citrate test
+
Indole test
+
Voges-proskauer
-
Lactose fermentation test
+
Note: positive : +; negative : -
Figure 1. Phylogenetic tree based on 16S rRNA gene sequence for Micrococcus sp.G-13.
3.2. Determination of optimum growth temperature and pH of the isolated strain
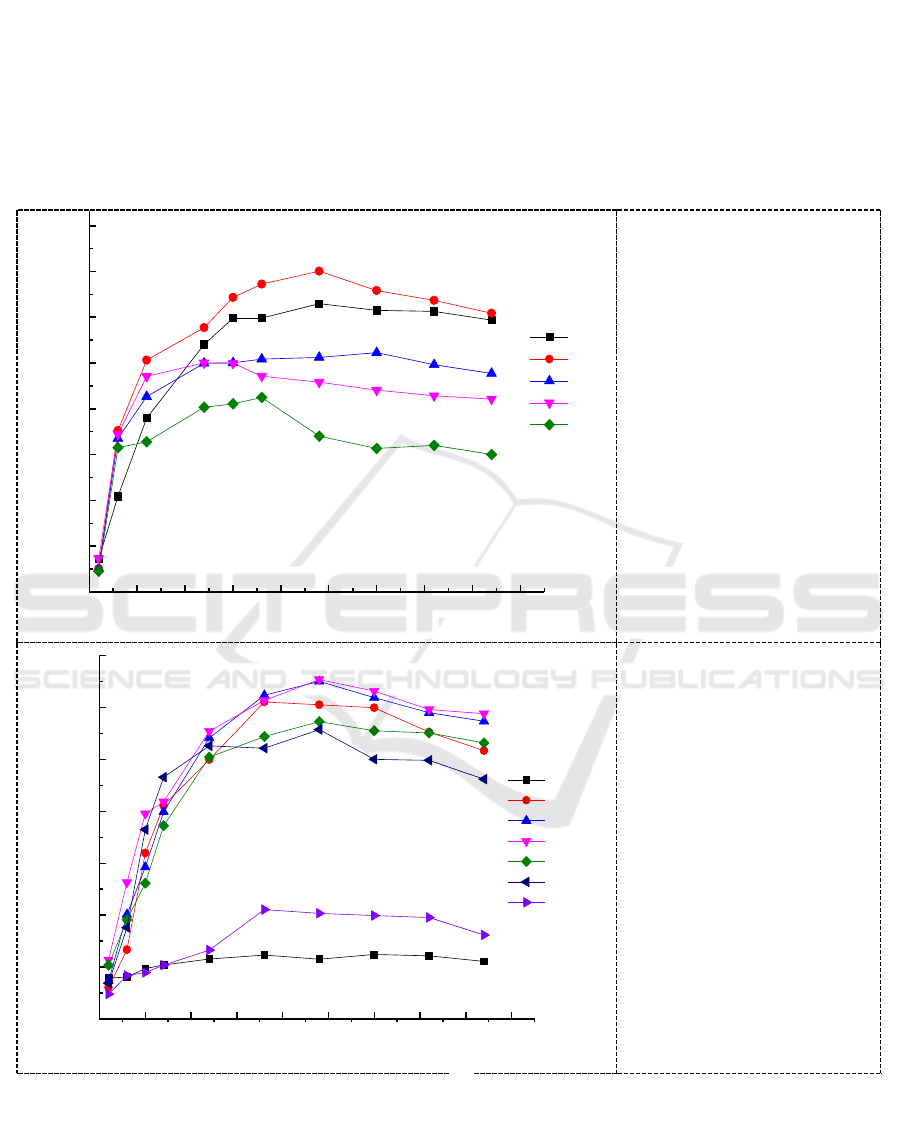
Temperature and pH play a crucial role in the growth rate and chromium reduction of the
Micrococcus sp.G-13 strain. The variation in temperature and pH of the LB medium affects the
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
608
viability of the cells, the activity of chromium reductases and causes changes in the ionic form of
active sites [22].Therefore, the optimum growth temperature and pH of the isolated bacteria
Micrococcus sp.G-13 were studied and the results are shown in Figure 2.In the temperature range of
25 ~ 45°C , the Micrococcus sp. G-13 has the best growth trend at 30°C . However, the Micrococcus
sp. G-13 strain can also grow in other temperature ranges but its growth is significantly inhibited at
45°C , which may be due to the decrease of membrane fluidity hindered cell growth. In addition, it
was found that the optimum pH of Micrococcus sp. G-13 was pH 7.0 or pH 8.0, but it cannot tolerate
extreme acid and extreme alkali environments (Figure 3).
Figure 2. Growth curve of
the Micrococcus sp. G -13
strain at different
temperatures.
Figure 3. Growth curve of
the Micrococcus sp. G -13
strain at different pH .
3.3. Effects of initial chromium concentration on chromium reduction
Research reported that the concentration of Cr (VI) in the process of chromium reduction can affect
the growth and chromium reduction of chromium reduction strain [23].With the increase of
chromium concentration, the reduction rate of chromium gradually decreased and the total reduction
0 10 20 30 40 50 60 70 80 90
0.0
0.3
0.6
0.9
1.2
1.5
1.8
2.1
2.4
25℃
30℃
35℃
40℃
45℃
OD
600
Time(h)
0 10 20 30 40 50 60 70 80 90
0.0
0.3
0.6
0.9
1.2
1.5
1.8
2.1
pH 3
pH 5
pH 7
pH 8
pH 9
pH 10
pH 11
OD
600
Time(h)
Isolation and Characterization of Chromium Reducing Bacteria
609
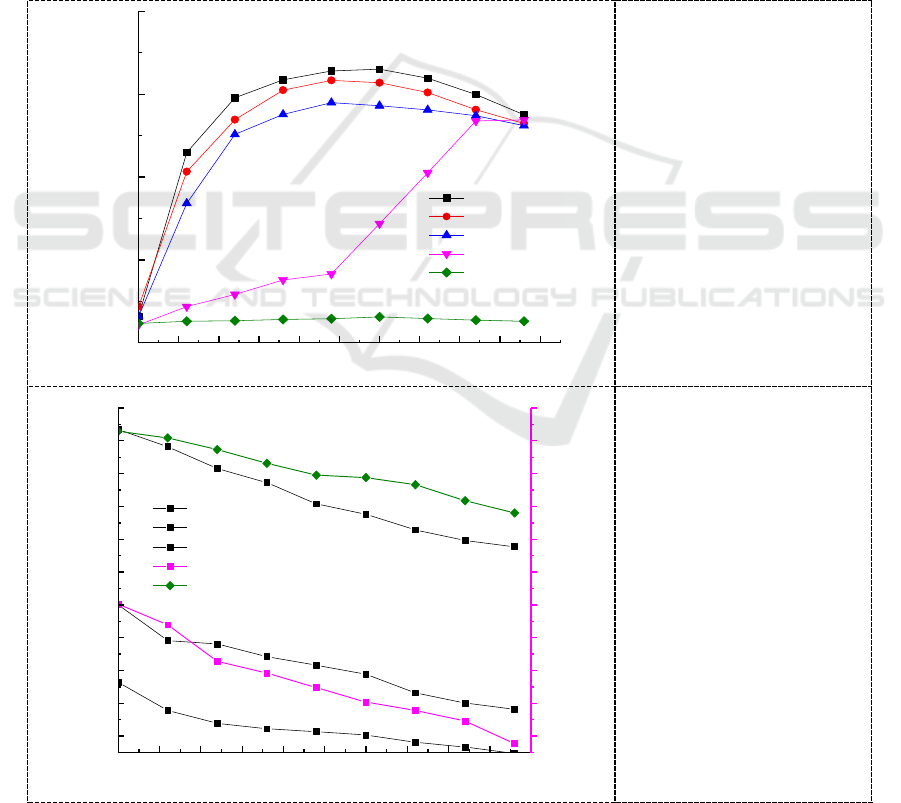
time of chromium increased. The effect of initial concentration on biomass growth and Cr (VI)
reduction by Micrococcus sp.G-13 was studied over an initial concentration range of 50 mg/L~500
mg/L and the results are shown in Figure 4. Higher amount of biomass concentration was observed at
an initial concentration of 50~200 mg/L and increase in initial concentration of Cr (VI) decreased the
biomass concentration. The growth of the Micrococcus sp.G-13 strain was significantly delayed at
500 mg/L Cr (VI) concentration compared to 400 mg/L, it may be that when the initial concentration
of chromium in the culture medium exceeded a certain limited concentration, the toxic effects on the
cells of the strain and Cr (VI) reducing strain is irreversibly inactivated.
Bacterial Cr (VI) reduction is enzyme mediated. Rate of this enzyme-catalyzed reaction increases
with the increase in the number of active collisions, as Cr (VI) occupies more enzyme active sites
[20]. Consequently, Cr (VI) reduction increases with reflecting increased enzyme activity until Cr
(VI) concentration saturates the enzyme, but the rate of reduction was decreased with increase in
incubation time and Cr (VI) concentration. The trend observed in the present study was the maximum
Cr (VI) reduction obtained for 50,100,200,400 and 500 mg/L of initial concentration at 96 h of
incubation were 82.1%, 63.6%, 34.5%, 21.2% and 9.1%, respectively (Figure 5).
Figure 4. Growth curve
of the Micrococcus sp. G
-13 strain at different
initial Cr (VI)
concentrations.
0 10 20 30 40 50 60 70 80 90 100
20
40
60
80
100
120
140
160
180
200
220
Cr(Ⅵ) (mg/L)
50mg/L
100mg/L
200mg/L
400mg/L
500mg/L
Time(h)
Cr(Ⅵ) (mg/L)
320
340
360
380
400
420
440
460
480
500
520
Figure 5. Chromium
reducing ability the
Micrococcus sp. G -13
strain at different initial
Cr (VI) concentrations.
0 10 20 30 40 50 60 70 80 90 100
0.0
0.5
1.0
1.5
2.0
50mg/L
100mg/L
200mg/L
400mg/L
500mg/L
OD
600
Time(h)
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
610
3.4. Effects of various electron donors/carbon sources on chromium reduction
The types of carbon sources include four types of carbohydrates, organic acids and lipids which can
be used as electron donors in the process of oxidative reduction to participate in the transmission of
electrons in the process of microbial growth. However, Cr (VI) reducing strains often preferentially
utilize energetically more favorable electron donors in the process of Cr (VI) reduction [24]. The
commonly of known electron donors are low molecular weight carbohydrates, organic acids, and
fatty acids. Since microbes will give priority to the use of electron donors that are more conducive to
growth, it is extremely important to select the appropriate electron donor to improve the reduction
capacity of Cr (VI). Hence the influence of electron donors such as glucose, sucrose, sodium lactate
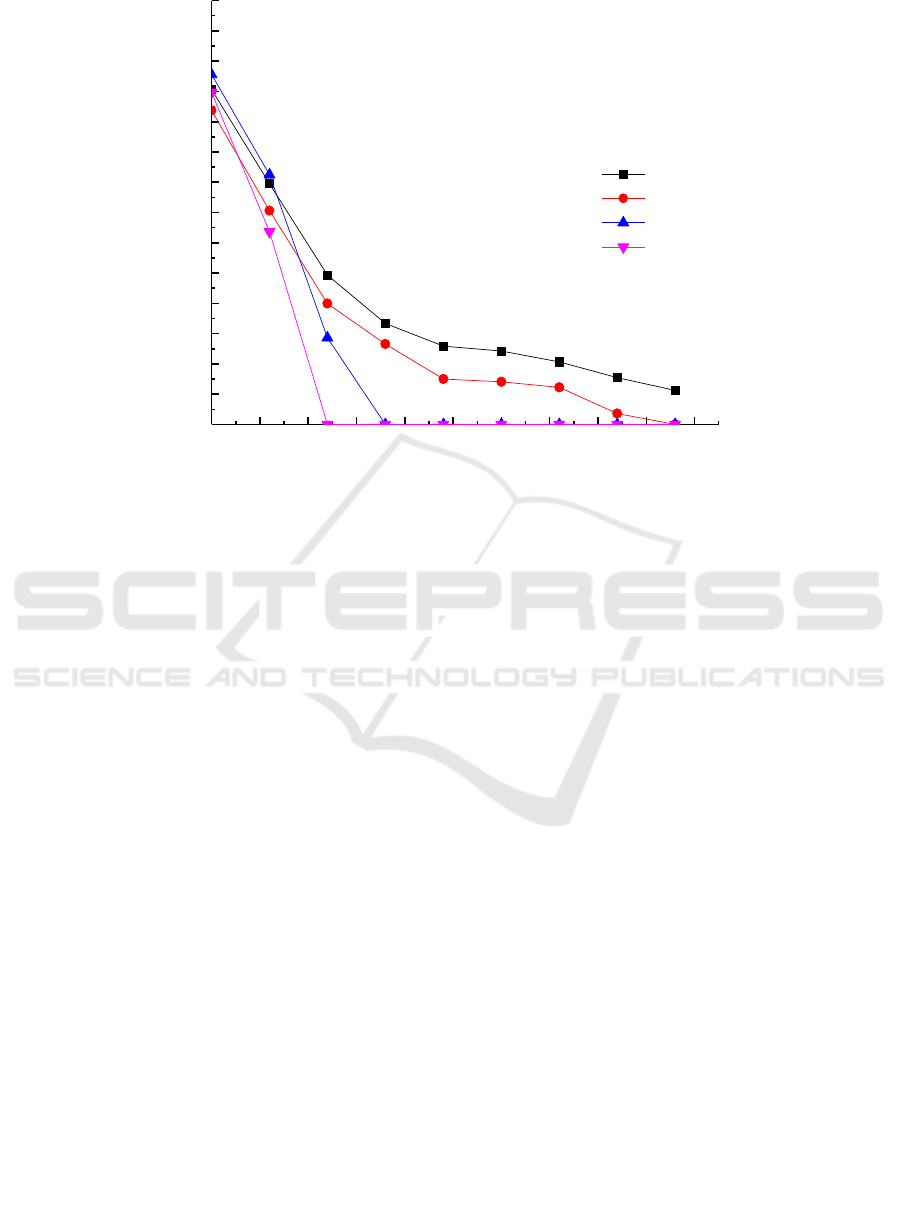
and glycerin on chromium reduction of the strain G-13 were studied (Figure 6).
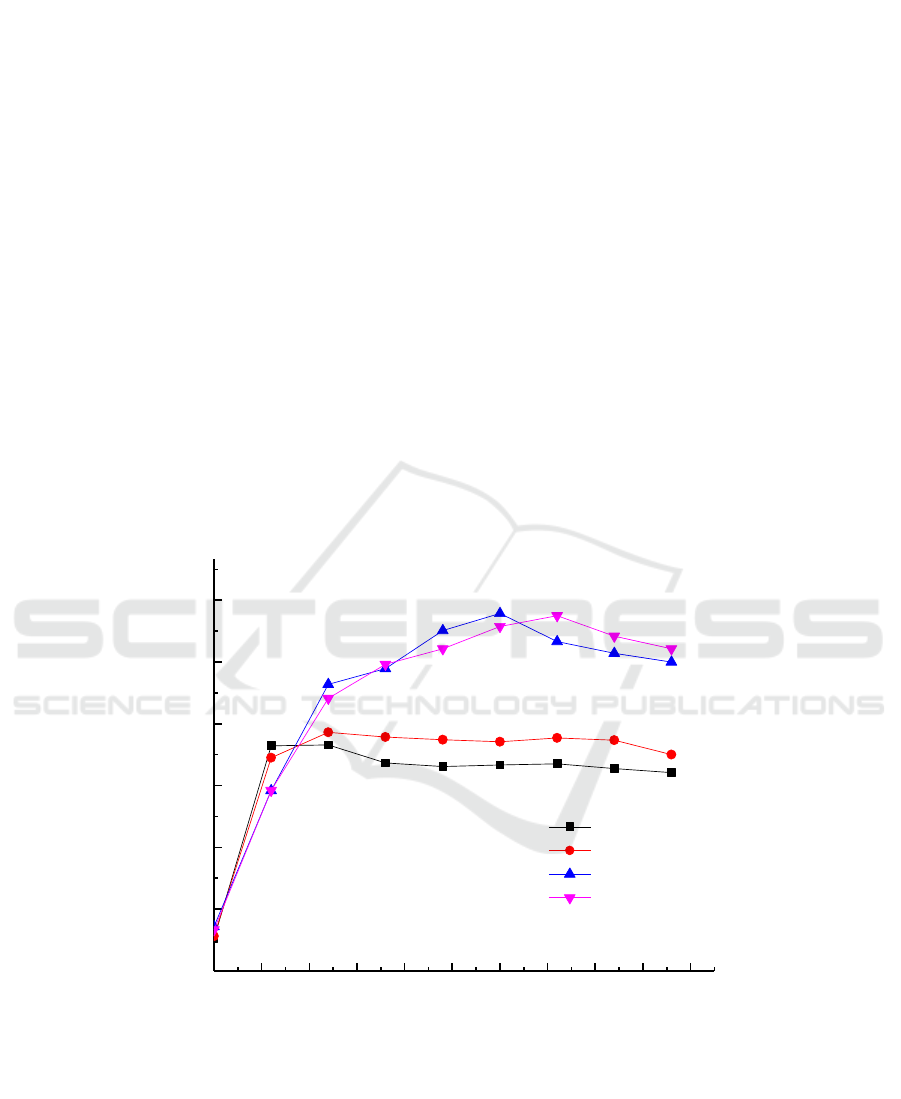
The study found that the growth of the cells was affected in the medium of different carbon source
types with the increase of time and the growth trend was: sodium lactate> glycerol> sucrose> glucose.
The results showed that the growth tendency of the Micrococcus sp.G-13 strain was the best in the
culture medium containing sodium lactate and glycerol. Simultaneously, the results obtained from the
Figure 7 that the reduction rate of Cr (VI) in the Micrococcus sp.G-13 strain was significantly
increased under the condition of glycerol and sodium lactate as the electron donor, which can be 50
mg/L Cr (VI) reduced to 0 in 36 h, the reduction rate of 100%.In the presence of four electron donors,
the chromium reduction rate was glycerol> sodium lactate> sucrose> glucose. The results indicated
that low molecular weight carbohydrates as electron donors may inhibit the growth of the strain,
thereby inhibiting the ability of Cr (VI) reduction.
0 10 20 30 40 50 60 70 80 90 100
0.0
0.3
0.6
0.9
1.2
1.5
1.8
glucose
surcose
sodium lactate
glycerol
OD
600
Time(h)
Figure 4. Growth curve of the Micrococcus sp. G -13 strain at different electron donor.
Isolation and Characterization of Chromium Reducing Bacteria
611
0 10 20 30 40 50 60 70 80 90 100
0
5
10
15
20
25
30
35
40
45
50
55
60
65
70
glucose
surcose
sodium lactate
glycerol
Cr(Ⅵ) (mg/L)
Time(h)
Figure 5. Chromium reducing ability the Micrococcus sp. G -13 strain at different electron donor.
4. Conclusions
At present, chromium is widely used in industrial activities, resulting in a large area of environmental
chromium contamination. Therefore, search for alternative techniques for treatment of Cr (VI)
contaminated environment came into existence. Microbial remediation as a technology with
enormous potential that has attracted much attention to remedy chromium contaminated environment,
So in the present study, the microbial reduction of Cr (VI) using Micrococcus sp.G-13 strain isolated
from chromium-contaminated environment was investigated. Simultaneously, the effects of
parameters such as temperature, pH, initial chromium concentration and carbon sources on the
growth and chromium reduction ability of the Micrococcus sp.G-13 strain were studied and the
optimum conditions for the reduction of chromium were achieved. This study found that the
optimum temperature and pH for the strain were found to be 30°C and 7.0 or 8.0, respectively.
Furthermore, the reduction of Cr (VI) increased with increased biomass and decreased with increase
in initial Cr (VI) concentration. At the same time, research found that the great increase reduction rate
of Cr (VI) for the Micrococcus sp.G-13 strain when the addition of glycerol and sodium lactate to do
electronic donor. All the results indicated the necessity to study the various parameters of the
chromium reduction rate of the strain. Therefore, it can be concluded that future studies can focus on
how to use advanced biotechnology to optimize the culture conditions of strains and reduction
mechanism of chromium bacteria to improve the reduction rate of Cr (VI).
Acknowledgements
This work was financially supported by the Major Science and Technology Program of Hunan
Province, China (2016SK2046); Special Funds for Fundamental Scientific Research Business of
Central South University (Changsha, China) (2017zzts362)
References
[1] Mahmood Q, Raja I A, Khan A and Sultan S 2012 Ecological Engineering vol 42 (Amsterdam:
Elsevier) pp 256-259
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
612

[2] Iyengar C A and Usha M S 2016 Biologija vol 62 (Germany: researchgate)
[3] Zahoor A and Rehman A 2009 Journal of Environmental Sciences vol 21 (Amsterdam:
Elsevier) pp 814-820
[4] Malik A 2007 Environment International vol 33 (New York: Wiley–Interscience) p 122
[5] Wang Y, Chai L, Liao Q,Tang C,Liao Y and Peng B 2016 Geomicrobiology Journal vol 33
(United Kingdom: Taylor & Francis) pp 222-229
[6] Gheju M and Balcu I 2011 Journal of Hazardous Materials vol 196 (United Kingdom: Europe
PMC) pp 131-138
[7] Alidokht L, Khataee A R, Reyhanitabar A and Oustan S 2011 Desalination vol 270
(Amsterdam: Elsevier) pp 105-110
[8] Liu Y G, Zeng X G M, Li X and Gao H 2006 Process Biochemistry vol 41 (Amsterdam:
Elsevier) pp 1981-1986
[9] Yang G and Xia J 2011 Environmental Science & Technology vol 45 (United Kingdom:
Europe PMC) pp 8605-6
[10] Park D, Yun Y S and Park J M 2005 Process Biochemistry vol 40 (Amsterdam: Elsevier) pp
2559-2565
[11] Choppala G K, Bolan N S, Megharaj M, Chen Z and Naidu R 2012 Journal of Environmental
Quality vol 41 (Bethesda: National Center for Biotechnology Information) pp 1175-1184
[12] Park D, Lim S R, Yun Y S and Park J M 2008 Bioresource Technology vol 99 (Bethesda:
National Center for Biotechnology Information) pp 8810-8
[13] Sundar K, Mukherjee A, Sadiq M and Chandrasekaran N 2011 Journal of Hazardous
Materials vol 187 (Amsterdam: Elsevier) p 553
[14] Zhong Y, Qiu X, Chen D, Li N, Xu Q and Li H 2016 Scientific Reports vol 6(London: Nature)
p 31090
[15] Santos E D C D, Silva I S, Simões T H N, Simioni K C M, Oliveira V M and Grossman M J
2012 International Biodeterioration & Biodegradation vol 70 (Amsterdam: Elsevier) pp
104-110
[16] He Z, Li S, Wang L and Zhong H 2014 Water Air & Soil Pollution vol 225 (Heidelberg:
Springer) pp 1-10
[17] Ravikumar S, Baylon M G, Si J P and Choi J I 2017 Microbial Cell Factories vol 16
(Bethesda: National Center for Biotechnology Information) p 62
[18] He Z G, Gao F L, Tao S, Hu Y H and Chao H 2009 Journal of Hazardous Materials vol 163
(United Kingdom: CAB Direct) pp 869-873
[19] Wani R, Kodam K M, Gawai K R and Dhakephalkar P K 2007 Applied Microbiology &
Biotechnology vol 75 (Netherlands: Europe PMC) pp 627-32
[20] Narayani M and Vidya Shetty K 2013 Science & Technology vol 43 (United Kingdom: Taylor
& Francis) pp 955-1009
[21] Ilias M, Rafiqullah I M, Debnath B C, Mannan K S B and Hoq M M 2011 Indian Journal of
Microbiology vol 51 (Heidelberg: Springer) pp 76-81
[22] Stasinakis A S, Thomaidis N S, Mamais D, Papanikolaou E C, Tsakon A and Lekkas T D 2003
Water Research vol 37 (Amsterdam: Elsevier) pp 2140-8
[23] Mangaiyarkarasi M S M, Vincent S, Janarthanan S, Rao T S and Tata B V R 2011 Saudi
Journal of Biological Sciences vol 18 (Amsterdam: Elsevier) p 157
[24] Okeke B C 2008 Journal of Industrial Microbiology & Biotechnology vol 35 (Michigan:
ProQuest) pp 1571-9
Isolation and Characterization of Chromium Reducing Bacteria
613
