
Molecular Characterization and Antibiotic Resistance of
Yersinia Entrocolitica Strains Isolated from Fish
X L Gou
1,2
, J Li
3,4
, L Wang
1, *
, H L Zhao
5
and Y Q Su
2
1
College of Life Science and Technology, Southwest University for Nationalities,
Chengdu, P. R. China.
2
Tongwei Group Company Limited, Chengdu, P. R. China.
3
College of Environment and Ecology, Chengdu University of Technology,
Chengdu, P. R. China.
4
State Environmental Protection Key Laboratory of Synergetic Control and Joint
Remediation for Soil & Water Pollution, Chengdu University of Technology,
Chengdu, P. R. China.
5
Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, P. R.
China.
The first two authors are the joint first authors.
Corresponding author and e-mail: L Wang, qinxin916@aliyun.com
Abstract. Yersinia enterocolitica, a widespread food and water-borne pathogen, is
responsible for diseases in humans and animals. The aims of this study were to determine the
molecular epidemiology of Y. enterocolitica strains isolated from, as well as, to investigate
their pathogenic potential and assess the antibiotic resistance. Twelve Y. enterocolitica strains
isolated from diseased fish were studied. Genotypic diversity was analyzed by ERIC-PCR
and 16S rDNA-RFLP, virulence genes were assessed by PCR method. Kirby-Bauer disk
diffusion method was applied for the antibiotic resistance profile. The results indicated that (i)
The 12 strains were grouped into five clusters by ERIC-PCR with similarity ranging from
81.8% to 100%, while four RFLP types were identified by 16S rDNA-RFLP with similarity
ranging from 83.3% to 100%. (ii) Genes ail and intB were detected in all strains, whereas,
virF and ystB were found at high probability of 83.3%, 75%, respectively, yadA was 41.6%.
(iii) The isolated strains showed antibiotics resistance as follows, lincomycin (100%),
sulfafurazole (41.6%), cephalothin V (66.7%), rifampicin (75.0%) compound
sulfamethoxazole (8.3%), streptomycin (8.3%) and rocephin (8.3%), but all were sensitive to
gentamicin, kanamycin, tetracycline and enrofloxacin. It will benefit the further study of
pathogenesis and prevention of Y. enterocolitica in.
1. Introduction
Yersinia entrocolitica is a widespread zoonotic pathogen that connected to yersiniosis disease in
humans and animals. It is responsible for the intestinal diseases including acute terminal ileitis,
enterocolitis with an inflammatory diarrhea and the extra-intestinal manifestations such as reactive
654
Gou, X., Li, J., Wang, L., Zhao, H. and Su, Y.
Molecular Characterization and Antibiotic Resistance of Yersinia Entrocolitica Strains Isolated from Fish.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 654-662
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

arthritis, erythema nodosum, infected mycotic aneurysm, axillary abscessesbux, respiratory ract
infection, urinary tract, and endocarditis [1-2].
Epidemiology studies of Y. entrocolitica strains previously have largely depended on biochemical,
serotyping, antibiotic susceptibility and phage typing. However, these techniques are limited by their
low reproducibility and discrimination power. Therefore, alternative methods have been attempted,
most of them are DNA-based molecular techniques. They give the information on the source of
infection, cross-transmission, as well as the geographical and host distributions. Random amplified
polymorphic DNA(RAPD), analysis restriction enzyme analysis of plasmids/chromosomes
(REAP/REAC), ribotyping were used to characterize the Y. enterocolitica strains [3-4]. In previous
studies, pulsed-field gel electrophoresis(PFGE), enterobacterial repetitive intergenic
consensus-PCR(ERIC-PCR) and repetitive intergenic palindromic sequence(REP -PCR) have been
reported for typing the Y. enterocolitica isolates [5-7].
The pathogenicity of Y. enterocolitica strains has been attributed to various genes presented in a
highly conserved 70-kb virulence plasmid (pYV/pCD) and the chromosome, as well as the high
pathogenicity island (HPI). The pYV plasmid virulence genes, such as yadA, virF, ysa and tccC,
contribute to the bacteria survival and proliferate in host cell [8]. Chromosomally borne genes ail and
inv allow Y. enterocolitica to adhesive, invade and translocate across the intestinal epithelium, while
the yst gene encoding the Yersinia stable toxins might be contributed to diarrhea associated with
yersiniosis. Furthermore, high-path ogenicity island (HPI) genes such as ybt, irp, intB play a
significant role in iron acquisition in pathogenic strains.
In China, some virulence genes have been reported in Y. enterocolitica strains isolated from
diarrhea patients, animals, foods and natural environment [9-11]. Epidemiology of Y. enterocolitica
strains was mainly performed with pulsed-field gel electrophoresis to investigate the molecular
subtypes, which presented a high discrimination power, but expensive and time-consuming [12].
Nevertheless, there was few data about Y. enterocolitica strains isolated from fish. In this study, we
analyzed the strains of Y. enterocolitica isolated from various types of fish in Sichuan province,
inspect the genotype diversity, virulence genes and antibiotic resistance of Y. enterocolitica strains. It
will benefit the further understanding of epidemiology of Y. enterocolitica strains.
2. Materials and methods
2.1. Bacteria strains
Twelve strains of Y. enterocolitica, isolated from fish with typical symptoms, were analyzed in this
study. The isolates were directly cultured on Cefsulodin-Irgasan-Novobiocin Agar-1 (CIN)
supplemented with ampicillin, chloramphenicol and diphenyl or on Modified Y medium, the
inoculated media were incubated at 28°C for 18~24 h. These strains were confirmed as Y.
enterocolitica by morphological appearance and biochemical tests [13], and 16S rDNA gene
sequencing [14-15]. All the diphenyl identified strains were belonging to serotype O:8 which were
frozen at -80°C with 20% glycerol and conserved in CIN-1 Agar (plates) at 4 °C for further study.
All experiments involving live fish were approved by the Animal Ethics Committee at Southwest
University for Nationalities.
2.2. Extraction of genomic DNA
Genomic DNA was extracted by using the TIANamp Bacteria DNA Kit (QIAGEN). The purity and
concentration of DNA preparations were estimated spectrophotometrically at 260 and 280 nm.
2.3. 16S rDNA-RFLP typing
The 16S rDNA gene sequences were amplified from isolated strains using universal primer: Reverse
primer (5'-AGAGTTTGATCATGGTCAG-3') and Forward primer
Molecular Characterization and Antibiotic Resistance of Yersinia Entrocolitica Strains Isolated from Fish
655

(5'-ACGGCTACCTTGTTACGACTT-3'). The PCR reaction system contained 10 pmM primers, 1
×QIAGEN PCR mixture (QIAGEN) and 50 ng genomic DNA. The amplification program were
described by Charbonneau et al.(2012). The PCR products were digested with Taq I (TaKaRa) for
16S rDNA-RFLP typing
2.4. ERIC-PCR typing
The PCR reaction system contained 10 pmM primer, 1.0 µL Taq DNA polymerase (TaKaRa), 2.5 µL
10×PCR Buffer (Mg
2+
Free), 2.5 mM MgCl
2
, 1.0 µL dNTP Mixture and 50ng genomic DNA. The
primers used were ERIC-Forward (5'-AAGTAGTGACTGGGGTGAGCG-3') and ERIC-Reverse
(5'-ATGTAAGCTCCTGGGGATTCAC-3')[16]. All PCR reactions were repeated twice for each Y.
enterocolitica strain.
2.5. Computer data analysis
The gel photographs of ERIC-PCR and 16S rDNA-RFLP were analyzed by Gel-Pro analyzer 4.0.
Dendrograms were constructed by the unweighted pair group method (UPGMA) using NTSYS-pc
software (version 2.10e).
2.6. PCR amplification of virulence genes
The PCR reaction contained 10 pmM primers, 1×QIAGEN PCR mixture (QIAGEN) and 50 ng
genomic DNA. PCR reactions were 94 °C for 4 min, followed by 35 cycles of 94 °C for 45 s, graded
temperature (45 °C to 65 °C ) for 45 s, and 72 °C for 40 s, and incubated at 72 °C for 5 min.The
primer sequences, size of products, annealing temperature and the references sequences were
summarized in Table 1.
Table 1. Primers of the virulence genes.
Genes
Primers
Primers sequence (5'-3')
Amplicon
Size (bp)
GenBank
accession
No.
Annealing
ail
Forward
taa tgt gta cgc tgc gag
gac gtc tta ctt gca ctg
351
M29945
50
Reverse
ystB
Forward
gta cat tag gcc aag aga cg
gca aca tac ctc aca aca cc
200
GU229276
60.3
Reverse
virF
Forward
ggc aga aca gca gtc aga cata
561
NC004564.
45
Reverse
ggt gag cat aga gaa tac gtc g
yadA
Forward
ctt cag ata ctg gtg tcg ctg t
800
NC004564
58.5
Reverse
atg cct gac tag agc gat atc c
intB
Forward
tgc gcc atg cgg tcc atc
ggt gca taa gat tct cgg
714
NC008800
50
Reverse
2.7. Antibiotics resistance of strains
Drug resistance of Y. enterocolitica strains were evaluated by Kirby-Bauer’s disk diffusion method
[17]. The tested antimicrobial drugs and concentration were as follows, kanamycin(30 μg),
gentamicin(10 μg), streptomycin (10 μg), tetracycline (30 μg), enrofloxacin (5 μg), rocephin (30 μg),
cephalothin V (30 μg), polymyxin (300 μg), lincomycin (2 μg), rifampicin (5 μg), compound
sulfamethoxazole (75 μg), sulfafurazole (300 μg).
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
656
3. Results
3.1. 16S rDNA-RFLP analysis
The digestion of 16S rDNA PCR products by Taq I yielded different fragments sizes from 100 to 700
bp and a common band of 350 bp appeared in all strains (Figure 1). Dendrogram showed that four
groups were clustered for 12 Y. enterocolitic isolates (Figure 2). The first group included Y.
enterocolitic strains YE01, YE04, YE07, YE08, YE09, YE11, YE12, and the similarities were 100%
among them. The second group included YE06 and YE10, with similarity of 100%. The third group
contained YE02 and YE03 with similarity of 100%. But the isolate YE05 comprised the last group
only shared 83.3% similarity to other groups. Based on these results, it inferred that strains of the
same serotype showing different PCR-RFLP profiles.
Figure 1. RFLP of 16S rDNA segments of Y. enterocolitic isolates using Taq I
restriction enzyme. M:DL2000,1-12: Strains isolated from different fish.
Figure 2. Dendrogram based on RFLP of 16S rDNA segments of Y. enterocolitic isolates
using Taq I restriction enzyme.
Molecular Characterization and Antibiotic Resistance of Yersinia Entrocolitica Strains Isolated from Fish
657
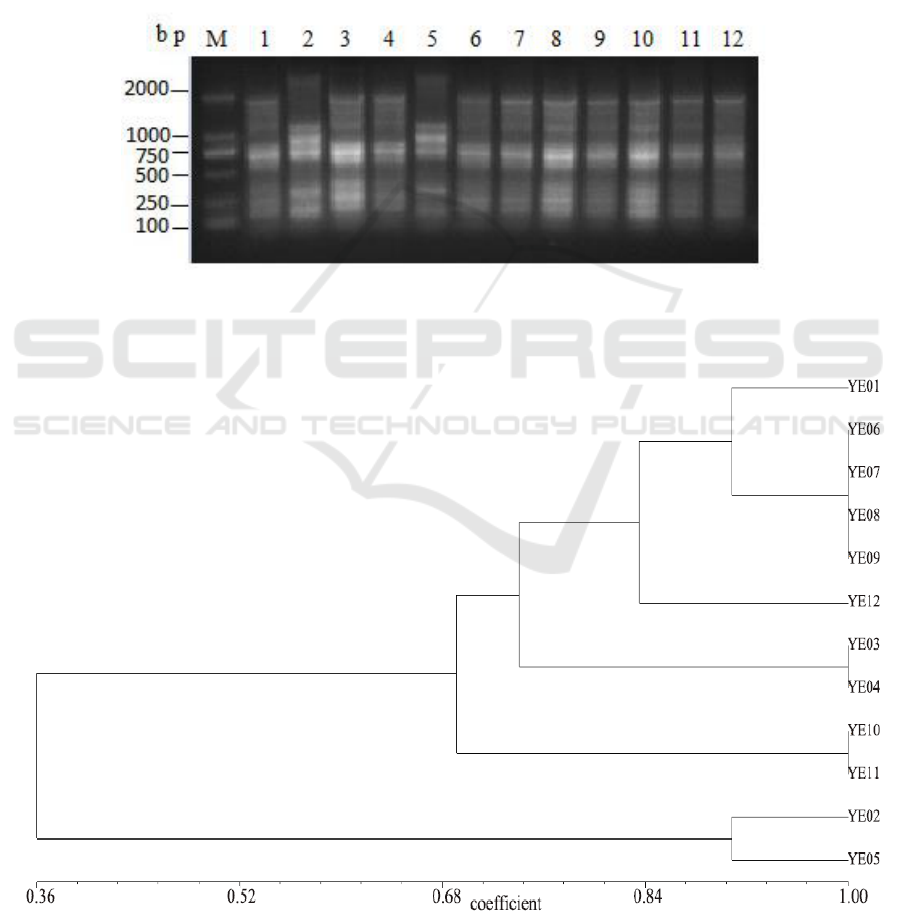
3.2. ERIC-PCR analysis
DNA fragments generated by ERIC-PCR were composed of 6 to 10 amplification bands ranging
from 200 to 2,500 bp (Figure 3). All isolated strains were differentiated into five electrophoretic
patterns (Figure 4). The vast majority of isolates were divided into cluster I with 33.3% of the 12
strains, Strains YE06, YE07, YE08 and YE09 in cluster I had exhibited 100% similarity, and YE01
(90% similarity). Cluster II was comprised by YE12 showed a different banding pattern to the other
isolates. YE03 and YE04 in cluster III isolated from Yellow catfish in the same farm were grouped
together with 100% similarity. In cluster IV, YE10 and YE11 isolated from different host in Xindu
aquafarm presented similar banding patterns. Particularly, YE02 and YE05 collected from Carassius
auratus and Yellow catfish in different aquafarms exhibited 90% similarity, which were clustered to
cluster V.
Figure 3. Amplification of ERIC genes for twelve Y. enterocolitica strains
M:DL2000,1-12: Strains isolated from different fish.
Figure 4. Dendrogram produced by UPGMA based on Jaccard coefficient representing genetic
relationships between Y. enterocolitica isolates based on ERIC-PCR analysis.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
658

3.3. Virulence genes
All isolated strains were screened for the virulence genes (Table 2). Genes ail and intB harbored in
all strains (100%), while genes virF, ystB and yadA were respectively 83.3% (10/12 strains), 75%
(9/12 strains) and 41.6% (5/12 strains). We also found that 83.3% of Y. entrocolitica strains
presented at least two virulence genes. Interestingly, in this study, gene ystB was found correlated to
gene ail, virF and yadA.
3.4. Antimicrobial resistance
Drug resistance of Y. entrocolitica isolates analyzed in this study showed that all strains were
resistant to lincomycin. sulfafurazole (41.6%), cephalothin V (66.7%) and rifampicin (75.0%). While,
a slightly antibiotic resistance was observed in compound sulfamethoxazole (8.3%), streptomycin
(8.3%) and rocephin (8.3%). Intermediate susceptible to polymyxin B was distributed among all the
isolated strains. No resistance was observed in gentamicin, kanamycin, tetracycline and enrofloxacin.
More than 33.3% of the strains were resistant to two classes of antimicrobial agents in this study.
4. Discussion
It is well known that Y. enterocolitica is an emerging food-borne pathogen that is widespread
throughout the world. This bacterium is acquired primarily through the consumption of contaminated
food and water. Therefore, epidemiological investigations play an important role in eluc idating
contamination routes and establishing the implementation of control and prevention measures [8,18].
Elaborated the relationship of Y. enterocolitica strains isolated from animals, humans and
environment, but no molecular typing study has been reported in aquaculture fish.
In this study, the relationship between twelve isolates was analyzed by ERIC-PCR and 16S
rDNA-RFLP methods. Majority of isolates from different farms manifested different ERIC profiles,
which indicated its potential usefulness in epidemiological studies for Y. enterocolitica, which was
consistent with the study of Wojciech [7], who used ITS profiling, REP-PCR and ERIC-PCR to
assess the genomic diversity among 35 Y. enterocolitica strains isolated from humans, pigs and foxes.
ERIC-PCR was regard as an effective method to discriminate Y. enterocolitica strains in a study of
81 biovar 1A strains isolated from clinical and nonclinical trails and in another study of 81 strains
isolated from India, Germany, France and USA[1-2]. Similarly, in the study carried out by
Paixão[19], 61 strains of Y. enterocolitica isolated among pigs and slaughterhouses in Brazil were
characterized by SE-AFLP, ERIC-PCR and PFGE techniques, in which ERIC-PCR appeared more
useful for separating the isolates among the different serotypes, though with a slightly lower
discriminatory power than the other techniques.
The universal primers of 16S rDNA/rRNA were conservative molecules, universally distributed
and functionally constant. Thus, the PCR-restriction fragment length polymorphism analysis by 16S
rDNA/rRNA technique had been used to discriminate the Bradyrhizobium and Rhizobia strains
isolated from soybean and to assess the genetic diversity of Aeromonas veronii, Thermophilic
bacterial and Arcobacter spp. [20-23]. In this study, most strains isolated from the same farms were
grouped together. The results were almost consistent with ERIC, suggesting that these strains were
not only genetically related, but also associated with their living environment and feeding conditions.
Strains belonging to RFLP cluster did not identically belong to the same ERIC cluster, it might be
caused by high sequence divergence of 16S rDNA genes among different strains. Additionally, it was
interesting to note that all isolated strains were shared a common band of 350 bp in RFLP profiles.
This region would be a favourable clue for the development of genetic amplification assay for
identification and diagnostic purpose. Both ERIC-PCR and 16S rDNA-RFLP methods gave a high
level of homogeneity, implying that these twleve Y. enterocolitica strains might have descended from
a common ancestor, which indicated they were useful techniques for studying the prevalence of Y.
Molecular Characterization and Antibiotic Resistance of Yersinia Entrocolitica Strains Isolated from Fish
659

enterocolitica from diverse fishes. While, these techniques presented a distinct advantage with
efficiency, simplicity, lower costs and universality of primers used [24].
Y. enterocolitica invasion factor encoding gene ail is presently stable in chromosomal that
attributed to pathogenic biotypes Th reported ystB gene to be presented us, some researchers have
established detect methods based on ail gene for Y. enterocolitica detection [25]. The result of this
study indicated that all Y. enterocolitica strains tested were all positive for ail gene. The result was
consistent with a previous study in which 172 Y. enterocolitica strains isolated from conventional and
antimicrobial-free pig production systems from different geographic regions [26]. However, the ystB
gene, producing an enterotoxin, presented in 75% (10 of 12) of the isolates which was much higher
than the results reported by Tadesse et al., and in contrast to other studies, which reported that ystB
gene was presented only in biotype 1A strains [27]. Y. enterocolitica strains in this study were
isolated from aquatic fish, while other studies were from animals and humans, it might be related to
the distribution of isolated species.
Plasmid-borne genes yadA and virF were selected as markers in this study. Interestingly, most of
the isolated strains were positive for virF (83.3%), while only 5 of them positive for yadA (41.6%).
This was accord with previous studies [28], in which Y. enterocolitica strain virF was positive while
yadA was negative. It suggested that perhaps virulence genes virF and yadA were not located on the
same plasmid every time. Moreover, the virulence plasmid could be easily lost because of repeatedly
cultivation or storage in room temperature for a long time. On the other hand, plasmids acted as
mobile elements and were transferable between bacterial strains which could be likely gained or lost
under selective pressure. In addition, amount of virulence genes were clustered in a genomic island
called Yersinia high-pathogenicity island (HPI) in Y. enterocolitica 1B isolates. Genes harbored on
the island, including the mobility module locus termed asn-int, might play a role in encoding for
biosynthesis and transportation of yersiniabactin (Ybt), and mediated iron acquisition [29]. Gene intB,
a unidirectional site-specific recombinase, was the main part of the genetic dissemination machinery.
In this study, all the strains were positive for intB, manifesting that this gene had been integrated
stability in the bacteria strains. Therefore, the isolates might be potential pathogenic to fish, while
further studies were needed for validating the pathogenesis in aquatic animals.
Antimicrobials are not usually recommended to treat uncomplicated yersiniosis, however, in more
severe cases, such as focal extra-intestinal infection or septicaemia, use of antimicrobials is required
[30]. In present study, the antimicrobial resistance of isolates was tested against some antibiotic drugs,
which were common used in human and aquatic treatments. The antimicrobial resistance profile
showed that 100% of the strains were resistant to lincomycin, which played a role as erythromycin.
Likewise, Estrella et al. found a very high resistance rate (100%) of this agent among pork and
chicken in Italy [21]. In previous studies, Y. enterocolitica strains were sensitive to polymyxin B [31].
However, in this study, all isolates were intermediately sensitive to polymyxin B. It insinuated that
the percentage of Y. enterocolitica strains resistant to polymyxin B had been increased over times.
A few strains were resistant to streptomycin agreed with previously published results, but differed
from the observation that the Y. enterocolitica organisms collected from pork and chicken in Italy,
during 2006 and 2007 [32-33]. Additionally, a lower resistance level to trimethoprim/
sulfamethoxazole (8.3%) was observed, in contrast to a study in Latvia, which reported a high
sensitivity of trimethoprim/sulfamethoxazole among Y. enterocolitica strains in slaughter pigs. The
resistance against sulfamethoxazole (41.6%) in this study was much higher than previous studies
observed by Bonke et al. [34]. and Meyer et al.[35]. Based on these data, it might be the use of
antibiotic agents varied in different countries and fish-raised farms.
5. Conclusions
The genotype diversity, virulence genes and antibiotic resistance of Y. ertrocolitica strains isolated
from fish were studied by molecular biology and pharmacological methods. Fish might become the
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
660

source of Y. enterocolitica infection. Y. enterocolitica may spread between fish and human, since fish
were important food for human. Moreover, the antibiotic resistance of Y. enterocolitica strains was
increasing. It was necessary to strengthen the monitoring of Y. enterocolitica infection in fish culture.
Further studied would be focused on the pathogenicity and pathogenesis of Y. enterocolitica in fish.
Acknowledgments
This work was supported by scientific research project from science & technology department of
Sichuan province (2016NZ0044).
References
[1] Bhagat N and Virdi J S 2007 FEMS Microbiol Lett. 266 pp 177-183
[2] Zhang L, Mei M, Yu C, Shen W, Ma L, He J and Yi L 2016 Pol J Microbiol 65 pp 5-12
[3] Blixt Y, Knutsson R, Borch E and Rådström P 2003 Int J Food Microbiol. 83 pp 15-26
[4] Estrada C S, Velázquez Ldel C, Escudero M E, Favier G I, Lazarte V and De Guzmán A M
2011 Food Microbiol. 28 pp 21-28
[5] Falcão J P, Falcão D P, Pitondo-Silva A, Malaspina A C and Brocchi M 2006 J Med Microbiol.
55 pp 1539-1548
[6] Sachdeva P and Virdi J S 2004 FEMS Microbiol Lett. 240 pp 193-201
[7] Wojciech Ł, Staroniewicz Z, Jakubczak A and Ugorski M 2004 J Vet Med B Infect Dis Vet
Public Health 51 pp 238-244
[8] Sabina Y, Rahman A, Ray R C and Montet D 2011 J Pathog, doi: 10.4061/2011/429069
[9] Ye Q, Wu Q, Hu H, Zhang J and Huang H 2015 FEMS Microbiol Lett. 71 pp 184-189
[10] Wang L 2016 Iranian Journal of Fisheries Sciences 15 pp 402-414
[11] Stachelska M A 2017 Pol J Vet Sci. 20 pp 477-484
[12] Wang X, Cui Z, Jin D, Tang L, Xia S, Wang H, Xiao Y, Qiu H, Hao Q and Kan B 2009 Eur J
Clin Microbiol Infect Dis. 28 pp 1237-1244
[13] Don J B, Noel R K and James T S 2005 Bergeys Manual of Systematic Bacteriology
(Springer)
[14] Charbonneau D M, Meddeb-Mouelhi F, Boissinot M, Sirois M, Beauregard M 2012 Indian J
Microbiol. 52 pp 41-47
[15] Santini A C, Santos H R, Gross E and Corrêa R X 2013 Genet Mol Res. 12 pp 655-664
[16] Versalovic J, Koeuth T and Lupski J R 1991 Nucleic Acids Res. 19 pp 6823-6831
[17] Matthew A, Franklin R, Karen B and Georgen M 2010 20th Informational Supplement
Document, M100-S20, CLSI, Wayne. (Clinical and Laboratory Standards Institute).
[18] Terentjeva M and Bērziņs A 2010 J Food Prot 73 pp 1335-1338
[19] Paixão R, Moreno L Z, Sena de Gobbi D D, Raimundo D C, Ferreira T S and Spindola M G
2013 J. Pathog. 1 pp 521510
[20] Bonardi S, Paris A, Bassi L, Salmi F, Bacci C, Riboldi E, Boni E, D'Incau M, Tagliabue S and
Brindani F 2010 J. Food Prot. 73 pp 1785-1792
[21] Estrella M J, Muñoz S, Soto M J, Ruiz O and Sanjuán J 2009 Appl Environ Microbio. 75 pp
1088-1098
[22] Figueras M J, Levican A and Collado L 2012 BMC Microbiol. 12 pp 292
[23] Nawaz M, Sung K, Khan S A, Khan A A and Steele R 2006 Appl Environ Microbiol. 72 pp
6461-6466
[24] Campioni F and Falcão J P 2014 APMIS 122 pp 215-222
[25] Huang Y, Wang X, Cui Z, Yang Y, Xiao Y, Tang L, Kan B, Xu J and Jing H 2010 BMC
Microbiol. 10 pp 211
[26] Lambertz S T, Nilsson C, Hallanvuo S and Lindblad M 2008 Applied and Environental
Microbiology 74 pp 6060-6067
Molecular Characterization and Antibiotic Resistance of Yersinia Entrocolitica Strains Isolated from Fish
661

[27] Karimova T V, Bogumil'chik E A, Voskresenskaia E A, Klimov V T, Tseneva G, Chesnokova
M V and Ivanov L I 2012 Zh Mikrobiol Epidemiol Immunobiol 1 pp 16-21
[28] Zheng D A, Bahnson P B, Funk J A, Morrow W E, Abley M J, Ponte V A, Thakur S, Wittum
T, DeGraves F J and Rajala-Schultz P J 2013 Foodborne Pathog Dis. 10 pp 80-86
[29] Zheng H, Sun Y, Mao Z and Jiang B 2008 FEMS Immunol Med Microbiol 53 pp 368-374
[30] Schubert S, Rakin A and Heesemann J 2004 Int J Med Microbiol 294 pp 83-94
[31] Bonardi -Ahomaa M, Cernela N, Hächler H and Stephan R 2012 Eur J Clin Microbiol Infect
Dis. 31 pp 1543-1550
[32] Baumgartner A, Küffer M, Suter D, Jemmi T and Rohner P 2007 Int J Food Microbiol 115 pp
110-114
[33] Novoslavskij A, Kudirkienė E, Marcinkutė A, Bajoriūnienė A, Korkeala H and Malakauskas
M 2013 J Sci Food Agric. 93 pp 1858-1862
[34] Bonke R, Wacheck S, Stüber E, Meyer C, Märtlbauer E and Fredriksson-Ahomaa M 2011
Microb Drug Resist. 17 pp 575-581
[35] Meyer C, Stolle A and Fredriksson-Ahomaa M 2011 Microb Drug Resist. 17 pp 479-484
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
662
