
Preparation of a -N-bridged Binuclear Fe(III) Schiff-base Complex
as Catalyst for the Degradation of Emerging Contaminants
Dibutylphthalate (DBP)
Haoyu Shen
*
, Yufei Wang, Leyi Weng, Zhehao Jiang and Qi Jiang
Ningbo Institute of Technology, Zhejiang University; Ningbo, Zhejiang, 315100, China
Keywords: Fe(III) Schiff base complex; Emerging contaminants (ECs); Dibutyl phthalate (DBP); Degradation;
Fenton-like reaction.
Abstract: Schiff base ligand (ST = N, N'-tetraethylenepentaminebis (salicylideimine)) was prepared by condensation
reaction of salicylaldehyde (SA) and tetraethylenepentamine (TEPA), and further coordinated with iron (III)
via coordination reaction to form a binuclear iron (III) complex, [Fe
2
(ST)(H
2
O)
4
]Cl
4
(abbreviated as,
Fe
2
(ST)). It was characterized by elemental analysis (EA), Fourier transform infrared spectrometer (FTIR),
and ultraviolet-visible spectroscopy (UV-vis), etc. It was used as catalyst for the degradation of emerging
contaminants, e. g., dibutyl phthalate (DBP) under visible light with hydrogen peroxide solution as oxidant.
The results showed that at pH 3.5-8.0, with the initial concentration of Fe
2
(ST) complex larger than 5.50
mol·L
-1
, and that of H
2
O
2
larger than 8.16 mmol·L
-1
, the degradation of DBP at initial concentration less than
20.0 mg·L
-1
can be reached to almost 100% within 10 min. The catalytic reaction system has been monitored
by electronic spectrum before or after adding H
2
O
2
at different time intervals. The result showed the catalytic
activity site may be a -N-bridged binuclear Fe(III) centre and a di-Fe(III)-H
2
O
2
transition-state might be
formed, which was favourable to the activation of the H
2
O
2
under visible light. The Fe
2
(ST) is a potential
effective and green catalyst for the degradation of DBP.
1 INTRODUCTION
Phthalate esters (PAEs) are commonly used synthetic
materials and plasticizers, pesticides, etc. They are
typical environmental hormones and Emerging
contaminants (ECs) (Vrijheid et al., 2016). In recent
years, PAEs residue was detected in the
environmental samples, and even in food. The
pollution of PAEs is becoming more and more
serious (Shen, 2005). How to effectively remove the
PAEs in the environment is a major challenge in the
field of environmental science and technology. At
present, the most effective methods for removing
PAEs are advanced oxidation processes (AOPs)
(Legrini et al., 1993). Among AOPs, Fenton reaction
is an attractive method for effective degradation of
PAEs because of its low cost, the lack of toxicity of
the reagents (Hu et al., 2012). Recently, it has proved
that the Fe
3+
transition metal complex-H
2
O
2
system
has the advantages of high utilization rate of H
2
O
2
than the conventional Fenton oxidation method (Hu
and Xu, 2014). Among them, the metal Schiff-base
complex has attracted much attention due to the
peculiar electronic characteristics, stable structure,
and tailoring the electronic and space effect of
complex accurately and easily by changing the ligand
or metal ion. The iron Schiff-base can act as catalyst
in lots of oxidation reactions, which not only
overcome the shortcomings of high acidity
requirements, but also exhibit some characteristics of
biomimetic reactions. At present, iron Schiff-base
complexes have been reported about the selective
catalytic oxidation of different group mainly in
homogeneous reaction, but few research pays
attention to the heterogeneous photocatalytic
performance in water treatment of toxic organic
pollutants under visible light.
In this work, a novel binuclear iron (III)
complexes with Schiff base [Fe
2
(ST) (H
2
O)
4
]Cl
4
(abbreviated as: Fe
2
(ST)) was synthesized and
characterized. It was used for the catalytic
degradation of DBP. The effects of the solution pH
value, the concentration of H
2
O
2
in the system,
Fe
2
(ST) and the initial concentration of DBP were
Shen, H., Wang, Y., Weng, L., Jiang, Z. and Jiang, Q.
Preparation of a -N-bridged Binuclear Fe(III) Schiff-base Complex as Catalyst for the Degradation of Emerging Contaminants Dibutylphthalate (DBP).
DOI: 10.5220/0008184900290033
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 29-33
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
29

investigated. The presumed degradation mechanism
was deeply investigated in present work.
2 EXPERIMENTAL
2.1 Preparation of Fe
2
(ST)
0.2 mmol (37.9mg) of TEPA and 0.4 mmol (48.8mg)
of SA was dissolved in 10 mL methanol,
respectively. SA methanol solution was added to the
TEPA solution dropwise under vigorous stirring. The
reaction was continued at 60
o
C for 2 hrs. After the
mixture solution was cooled down, the yellow colour
Schiff base ligand, in which ST is the N,
N'-tetraethylenepentamine-bis (salicylide-imine),
was obtained. The yellow crystals of ST were
collected by filtering under vacuum, washed with a
small amount of water and methanol for three times,
and dried under vacuum at 60
o
C for 12 hrs, yield:
96.5%. 0.2 mmol (54.6 mg) of FeCl
3
6H
2
O and 0.1
mmol (35.2 mg) of ST was dissolved in 10 mL
methanol, respectively. FeCl
3
6H
2
O methanol
solution was added to the ST solution dropwise under
vigorous stirring. The 0.1 mol·L
-1
NaAc solution at
was then added dropwise to adjust the mixture
solution pH to 8-9. The reaction was continued at 60
o
C for 2 hrs. After the mixture solution was cooled
down, the brown microcrystalline product was
obtained, and collected by filtering under vacuum,
washed with a small amount of water and methanol
for three times, and dried under vacuum at 60
o
C for
12 hrs, yield: 89.6%. The overall preparation
procedure was shown in Scheme 1.
CHO
OH
CH
3
OH
FeCl
3
/NaAc
60
o
C
CH
3
OH
60
o
C
Cl
4
+
NH
2
NH
N
H
HN
H
2
N
Fe
Fe
CH
O
HC
O
N
N
N
N
N
H
2
O
OH
2
H
2
O
OH
2
CH
OH
HC
HO
N
N
H
N
H
N
H
N
Scheme 1: Preparation of Fe
2
(ST).
2.2 Catalytic Degradation of DBP by
Fe
2
(ST)
Catalytic degradation experiments were carried out
in 150 mL stoppered flasks, each of which contained
25.00 mL of DBP acetonitrile solution at
concentration of 20 mg·L
-1
, 40 L of
Fe
2
(ST)
acetonitrile solution at concentration of 4.0 mg·L
-1
was added, followed adding 20 uL of 30% H
2
O
2
. The
mixture was shaken at 150 rpm in a thermostatic
shaker, sampling at every 1 min, followed by adding
1 drop of 10% Na
2
SO
3
solution to stop the reaction.
The experiments of traditional Fenton system was
carried out similarly by replacing Fe
2
(ST) with
FeSO
4
acetonitrile solution of at concentration of 4.0
mg·L
-1
. HPLC method was applied for the
determination of the residue concentrations of DBP.
The degradation rates of DBP under different loading
amount and different pH conditions were calculated
according to Eq. (1):
%100
0
0
A
AA
D
t
(1)
Where D
is the degradation rate of DBP; A
t
and A
0
are the HPLC peak area of DBP at time t and at time
0, respectively.
The effects of initial pH value, usage amount of
H
2
O
2
, initial concentration of Fe
2
(ST) DBP
concentration on the degradation of DBP were
investigated.
3 RESULTS AND DISCUSSION
3.1 Characterization of Fe
2
(ST)
Fe
2
(ST) was characterized by EA, TG/DTG, UV/Vis
and FTIR, etc. The EA results showed that the
elemental percentage of Fe
2
(ST) were (experimental
(theoretical), %): C: 36.58 (36.65), H: 5.25 (5.17), O:
13.52 (13.31), N: 9.52 (9.71) and Fe: 15.22 (15.49),
respectively. The molar conductivity of Fe
2
(ST) was
determined by using acetonitrile as a solvent. It was
found that Fe
2
(ST) is a 1: 4 type electrolyte (Geary,
1971). The concentration of chlorine ion (Cl
-
) was
obtained by titration of the Fe
2
(ST) solution in
acetonitrile with AgNO
3
solution, and found to be
19.96% (19.67%, theoretically). TG/DTG (Figure 1)
shows that Fe
2
(ST) has 10.2% weight lose at ~120℃,
which would be relevant to 4 molecular of water
(theoretical data: 9.99%). Combined with the results
of its conductivity and Cl
-
content, it would be
deducted that the four water molecules might be in
the inner boundary of the Fe
2
(ST) and coordinated
with Fe (III), while the four Cl
-
ions are in the outlay
of the complex.
UV-Vis spectrum (Figure 2(a)) of ST and Fe
2
(ST)
showed that the characteristic peaks of -*
transition and n-* transition absorption of benzene
ring and imine in ST appeared at 252 nm and 315 nm.
After forming Fe
2
(ST) complex, these two peaks
were red-shifted to 260 nm and 325 nm, respectively,
along with the absorption peak at 325 nm
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
30
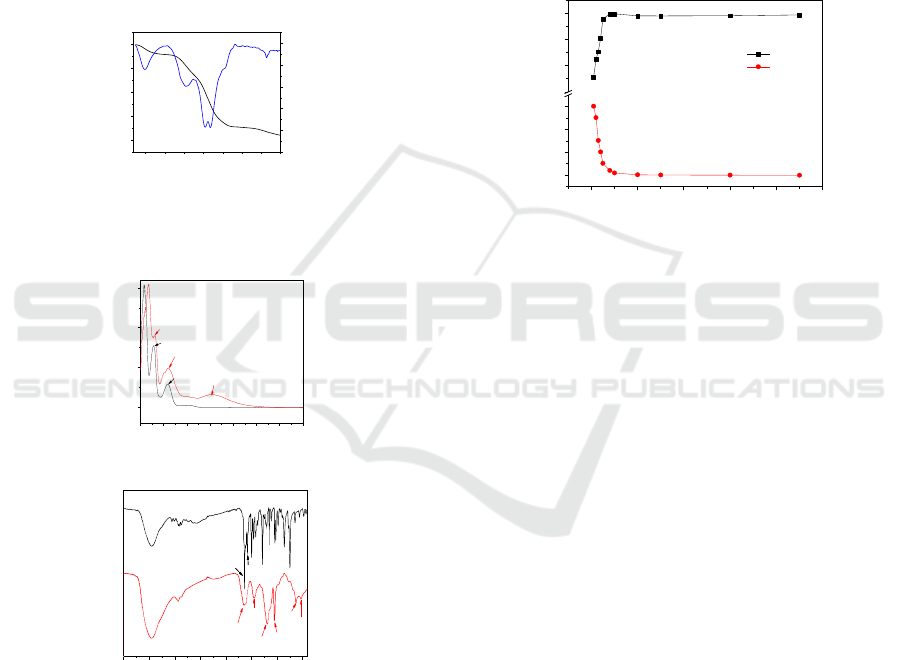
significantly broadening, which can be attributed to
the charge transfer of the p
x
(N) d
xz
(Fe) in Fe
2
(ST).
Simultaneously, the d-d transition absorption peak of
Fe(III) was observed at 512 nm. The FTIR of ST and
Fe
2
(ST) (Figure 2(b)) showed that typical peaks at
1642 cm
-1
and 1585 cm
-1
assigned to the absorption
of imine and amino groups of ST appeared. After
coordination with Fe(III), these two peaks were
red-shifted to 1635 cm
-1
and 1445 cm
-1
, respectively,
with a large broadening. New peaks at 620 cm
-1
and
525 cm
-1
, attributed to the characteristic absorption
peaks of Fe-N and Fe-O bonds appeared (Mao et al.,
2009), indicating the successful formulation of
Fe
2
(ST) complex.
100 200 300 400 500 600 700 800
20
40
60
80
100
-10
-8
-6
-4
-2
0
Temperature(
o
C)
TG/%
DTG/(%/min)
307.4
109.4
409.6
437.2
Figure 1: TG-DTG curves of Fe
2
(ST).
200 300 400 500 600 700 800 900
0.0
0.5
1.0
1.5
260 nm
252 nm
315 nm
512 nm
A
(nm)
325 nm
TS
Fe
2
(ST)
(a)
4000 3500 3000 2500 2000 1500 1000 500
Wave number (cm
-1
)
Fe
2
(TS)
TS
1445 cm
-1
1635 cm
-1
1185 cm
-1
1045 cm
-1
525 cm
-1
T/%
620 cm
-1
1642 cm
-1
1585 cm
-1
(b)
Figure 2: (a) UV and (b) FTIR spectra of ST and Fe
2
(ST).
3.2 Degradation of DBP by Fe
2
(ST)
Figure 3(a) shows the degradation of DBP by Fe
2
(ST)
in H
2
O
2
/visible light system. With the increasing of
the reaction time, the degradation rate of DBP
increased from 75.6% to 99.8% within 10 min. The
experimental results of traditional Fenton system are
shown in Figure 3(b). With the reaction time
increasing, the degradation rate of DBP decreases
from 30.2% to less than 0.5% in 8 mins. This might
be due to the fact that ·OH free radical is easily
deactivated generated by the traditional Fenton
system. It can be seen that the Fe
2
(ST)/H
2
O
2
/visible
light system has higher catalytic degradation
performance for DPB than that of the traditional
Fenton system. The high catalytic degradation
efficiency under visible light conditions might be
contributed to the -N-bridged binuclear Fe(III)
centre of the Fe
2
(ST).
0 20 40 60 80 100
0
10
20
30
70
80
90
100
a
b
Degradation percentage (%)
Reaction time (min)
Figure 3: Degradation extent of DBP under (a)
Fe
2
(ST)/H
2
O
2
/vis, (b) traditional Fenton catalytic systems.
The electronic spectra of DBP solution before and
after the addition of Fe
2
(ST)/H
2
O
2
are shown in
Figure 4. With the addition of H
2
O
2
(c, 0 min; d, 1
min; e, 5 min; f, 10 min), the intensity of the
characteristic peak of the charge transfer of the p
x
(N) d
xz
(Fe) of Fe
2
(ST) at about 325 nm gradually
increased and broadened. This might be due to the
fact that the formation of an -N-bridged binuclear
Fe(III)-H
2
O
2
transition states during the catalytic
process (Mao et al., 2009), shown in Figure 5. When
H
2
O
2
was added into the Fe
2
(ST), a H
2
O
2
bridged
transition state might be formed, which might be
favourable to the activation of H
2
O
2
, leading the
produce of ·OH free radicals, under visible light, the
DBP was mineralized to CO
2
and H
2
O. As shown in
Figure 4, the characteristic absorption peaks of DBP
at 230 nm and 275 nm disappeared, indicating a
significant degradation of DBP (Figure 4b). The
COD of the post-degradation solution was tested
according to HJ 828-2017 method and the result was
at 15 mg/L, indicating the mineralization of the DBP.
Preparation of a -N-bridged Binuclear Fe(III) Schiff-base Complex as Catalyst for the Degradation of Emerging Contaminants
Dibutylphthalate (DBP)
31
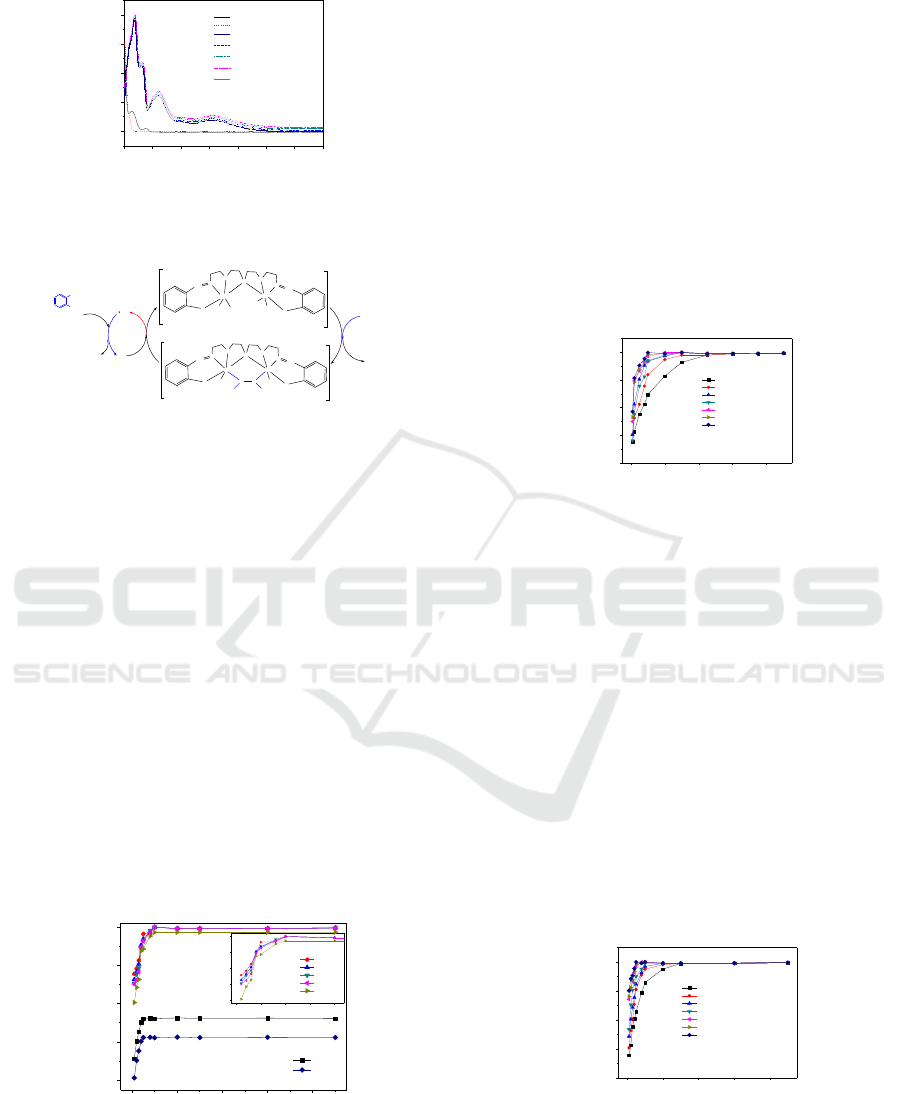
200 300 400 500 600 700 800 900
0.0
0.4
0.8
1.2
1.6
a DBP 10 mg L
-1
b DBP after degradation
c Fe
2
(TS)/H
2
O
2
0 min
d Fe
2
(TS)/H
2
O
2
1 min
e Fe
2
(TS)/H
2
O
2
5 min
f Fe
2
(TS)/H
2
O
2
10 min
g Fe
2
(TS)/H
2
O
2
20 min
(nm)
A
.
Figure 4: UV spectra of DBP before (a) and after (b) adding
Fe
2
(ST)/H
2
O
2
at different reaction time (c-g).
Fe
III
H
O O
H
H
2
O
2
OH
H
2
O
Cl
4
Fe
III
Fe
III
CH
O
HC
O
N
N
N
N
N
H
2
O
OH
2
H
2
O
OH
2
Cl
4
Fe
III
CH
O
HC
O
N
N
N
N
N
H
2
O
OH
2
CO
2
+ H
2
O
COOCH
2
CH
2
CH
2
CH
3
COOCH
2
CH
2
CH
2
CH
3
Vis
Figure 5: Presumed mechanism of -N bridged binuclear
Fe(III)-H
2
O
2
transition-state and production of OH.
3.2.1 Effect of Solution pH Values
The effect of solution pH was investigated with the
pH values ranging from 3.0 to 9.0. The results (Figure
6) showed that at the pH range of 3.5-8.0, DBP can
degrade almost 100% within 10 min under the
Fe
2
(ST)/H
2
O
2
/visible light catalytic system. The pH
value of the DBP solution is about 6.0. Thus the
catalytic degradation can be carried out without pH
adjustment of the DBP solution. The reason for the
wider pH range of the present catalytic system than
that of the conventional Fenton reaction system may
be due to the fact that the Fe(III) coordinated to the
ST ligand might stable the Fe(III) active centre,
leading its resistance to the effect of the solution pH
varying on the degradation efficiency of DBP (Wang
et al., 2007).
0 20 40 60 80
20
40
60
80
100
0 5 10 15 20
60
70
80
90
100
pH=3.0
pH=9.0
pH=3.5
pH=4.0
pH=5.0
pH=7.0
pH=8.0
Reaction time (min)
Degradation percentage (%)
Reaction time (min)
Figure 6: Effect of the solution pH on the
Fe
2
(ST)/H
2
O
2/
DEP catalytic degradation system (insert:
pH=3.5-8.0 enlarged).
3.2.2 Effect of Usage Amount of H
2
O
2
The effect of usage amount of H
2
O
2
was investigated
with the concentration of H
2
O
2
in the catalytic system
was in the range of 2.04 to 40.8 mmol·L
-1
, with the
initial concentration of DBP at 20 mg·L
-1
. The results
(Figure 7) showed that when the concentration of
H
2
O
2
was at 2.04 mmol·L
-1
, nearly 45 mins were
needed for total degradation of DBP. When the
concentration of H
2
O
2
at 4.08 mmol·L
-1
, degradation
of DBP can be realized within 30 mins. With the
concentration of H
2
O
2
increasing, the degradation
rate increased gradually, and when the concentration
of H
2
O
2
at 8.16 mmol·L
-1
, the degradation of DBP
can be realized within 10 mins (Lee and Yoon, 2004).
0 20 40 60 80
20
40
60
80
100
2.04
4.08
8.16
16.3
24.5
32.6
40.8
mmol L
-1
Degradation percentage (%)
Reaction time (min)
..
mmol L
-1
.
mmol L
-1
.
mmol L
-1
.
mmol L
-1
.
mmol L
-1
.
mmol L
-1
.
Figure 7: Effect of the usage amount of H
2
O
2
on the
Fe
2
(ST)/H
2
O
2
/DEP catalytic degradation system.
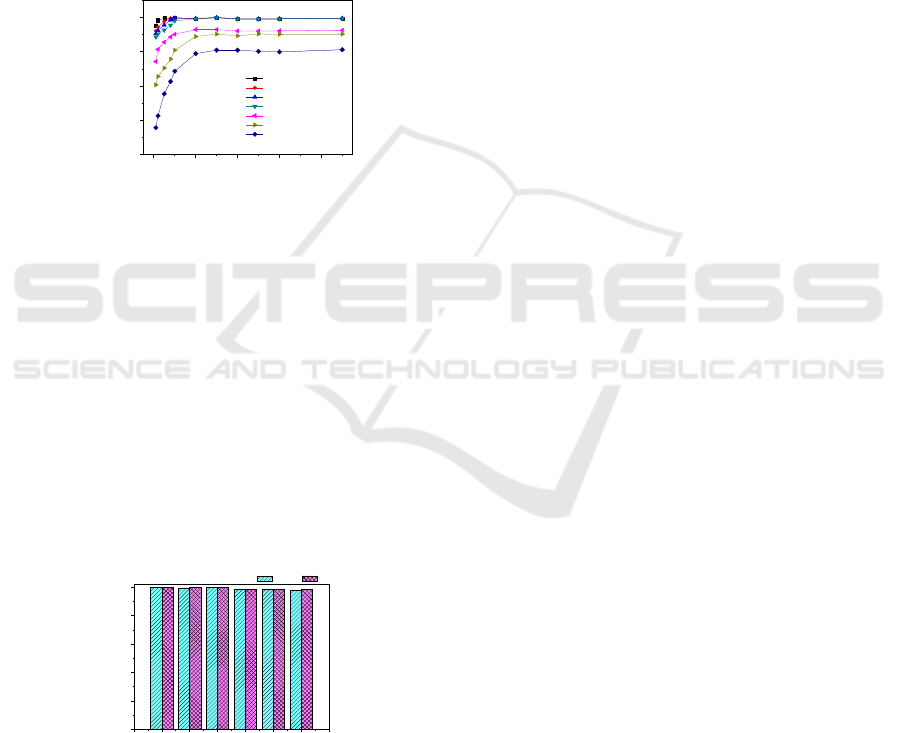
3.2.3 Effect of Usage Amount of Fe
2
(ST)
The effect of usage amount of Fe
2
(ST) was
investigated with the concentration of Fe
2
(ST) in the
catalytic system was at 1.10-11.0 mol·L
-1
. The
results (Figure 8) showed that Fe
2
(ST) was found to
be the key factor for the generation of the ·OH free
radicals. When the usage amount of Fe
2
(ST) was low,
the generation rate degradation rate of DBP was very
low. With the usage amount of Fe
2
(ST) increasing,
the generation rate of the ·OH free radicals increased,
leading a increasing of degradation rate of DBP.
When the usage amount of Fe
2
(ST) reached 5.50
mol·L
-1
, the degradation rate tends to be stable.
0 20 40 60 80
20
40
60
80
100
1.10
2.20
3.30
4.40
5.50
6.60
11.0
Degradation percentage (%)
Reaction time (min)
umol L
-1
..
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
Figure 8: Effect of the usage amount of Fe
2
(ST) on the
Fe
2
(ST)/H
2
O
2
/DEP catalytic degradation system.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
32
3.2.4 Effect of the Initial Concentration of
DBP
The effect of initial concentration DBP was
investigated with the concentration of DBP in the
catalytic system at 14.38-287.77 mol·L
-1
. The
results (Figure 9) showed that when the concentration
of DBP<71.6 mol·L
-1
(50mg·L
-1
), the DBP can be
totally degenerated in 10 mins. The residue
concentration of DBP was at g·L
-1
to mg·L
-1
levels
in the aqueous environment, thus the present
Fe
2
(ST)/H
2
O
2
/vis system can be used for the
treatment of the DBP in the environment.
0 20 40 60 80
20
40
60
80
100
14.4
28.8
54.0
71.6
143.9
215.8
287.8
Degradation percentage (%)
Reaction time (min)
umol L
-1
..
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
umol L
-1
.
Figure 9: Effect of the initial concentration of DBP on the
Fe
2
(ST)/H
2
O
2
/DEP catalytic degradation system.
3.3 Reusability Investigation
The reusable of the Fe
2
(ST)
was evaluated. Results
were shown in Figure 10, which indicated that
Fe
2
(ST) could be used for at least 10 cycles with
degradation rate higher than 95% upon recovery on
average. No obvious decrease in the degradation
efficiency and iron leaching were found. Compared
with literature reports, the Fe
2
(ST) is a potential
effective and reusable catalyst for the degradation of
DBP with high degradation effect.
-1 0 1 2 3 4 5 6
0
20
40
60
80
100
(a) (b)
Degradation percentage (%)
Cycle number
Figure 10: Recycle of the Fe
2
(ST) catalyst (a) Method 1:
re-adding DBP after rotating evaporation of the
post-degeneration solution, (b) Method 2: re-adding DBP
directly in the post-degeneration solution.
4 CONCLUSIONS
In this work, a binuclear iron (III) complex (Fe
2
(ST))
was prepared and characterized by EA, TG/DTG,
UV/Vis and FTIR, etc. It was used as a catalyst for
the Fenton–like reaction of the catalytic degradation
of the emerging contaminants di-butyl-phthalate
(DBP). It is found that under the condition of visible
light, the catalytic degradation of DBP could be
achieved by Fe
2
(ST)/H
2
O
2
system in aqueous
solution with the pH range from 3.5 to 8.0. Compared
with ordinary Fenton reaction system, a wide range of
pH value for the degradation of DBP achieved. The
degradation of DBP was more than 99% when the
concentration of Fe
2
(ST) was larger than 5.50
mol·L
-1
, the concentration of H
2
O
2
was larger than
8.16 mmol·L
-1
, and the concentration of DBP was
less than 20.0 mg·L
-1
. With the aid of the electronic
spectrum monitoring, it is found that the transition
state of -N bridged bi-nucleus Fe(III)-H
2
O
2
centre
may form in the catalytic process.
ACKNOWLEDGEMENTS
We would like to thank the National Natural Science
Foundation of China (51608479, 81502421), the
National Natural Science Foundation of Zhejiang
Province (LY14B04003), the National Natural
Science Foundation of Ningbo (2018A610206), the
National College Students’ innovation and
entrepreneurship training program (201813022009),
the Xinmiao Students’ innovation training program
of Zhejiang Province (2018R401181) for the
financial support.
REFERENCES
Geary, W. J., 1971. Coordination Chemistry Reviews, 7(1):
81–122.
Hu, M. Q. and Xu, Y. M., 2014. Chemical Engineering
Journal, 246: 299–305.
Hu, M. Q., Wang, Y., Xiong, Z. G., Bi, D. Q., Zhang, Y. H.,
Xu, Y. M., 2012. Environmental Science &
Technology, 46: 9005–9011.
Lee, C. H., Yoon, J., 2004. Chemosphere, 57:1449–1458.
Legrini, O., Olivers, E., Braun, A. M., 1993. Chemical
Review, 93(2): 671–698.
Mao, C. H., Zhao, Y., Li, Y. Q. et al., 2009. Chinese Journal
of Organic Chemistry, 29(6): 929–935.
Shen, H. Y., 2005. Talanta, 66: 734–739.
Vrijheid, M., Casas, M., Gascon, M. et al., 2016.
International Journal of Hygiene and Environmental
Health, 219: 331–342.
Wang, B. S., Zhang, J., Huang, J. L., 2007. Journal of
Harbin Institute of Technology, 39(2): 255-257.
Preparation of a -N-bridged Binuclear Fe(III) Schiff-base Complex as Catalyst for the Degradation of Emerging Contaminants
Dibutylphthalate (DBP)
33
