
Synthesis of 1-Nitronaphthalene under Homogeneous Conditions
Yuriy G. Khabarov
*
, Viacheslav A. Veshnyakov, Ivan A. Snigirev and Ilya I. Pikovskoy
Northern (Arctic) Federal University, Northern Dvina Emb., 17, Archangelsk, Russian Federation
Keywords: 1-nitronaphthalene, naphthalene nitration, nitration, dioxane
Abstract: Various reagents can be used for the nitration of naphthalene ranging from the classical nitrating mixture to
unusual reagent systems. In this study, a simple and effective method of nitration of naphthalene using a
classical nitrating mixture in 1,4-dioxane is presented. The reaction occurs under homogeneous conditions
and GC-MS analysis of the product showed 2 peaks corresponding to 1-nitronaphthalene (96%) and 2-
nitronaphthalene (4%). Melting point, UV and IR spectra of the product (isolated in 96-97% yields)
correspond to 1-nitronaphthalene.
1 INTRODUCTION
Nitration of naphthalene is often the first stage in the
synthesis of such naphthalene derivatives as
naphthylamine (Chen et al., 2016), naphthol and
aminonaphthalenesulfonic acids which are used in
the synthesis of dyes (Booth, 2012).
1-Nitronaphthalene can be synthesized from
naphthalene using a classical nitration mixture
(HNO
3
+H
2
SO
4
) in a yield of 95% (Fierz-David and
Blangey, 1949; Duvalma et al., 1964). In addition,
90% HNO
3
can be used to carry out the reaction in
acetonitrile with 85% yield (Wright, 1965). Instead
of H
2
SO
4
, indium(III) triflate (Yin and Shi, 2006) or
lanthanum(III) nitrobenzenesulfonates (Parac-Vogt
et al., 2004) can be used as catalysts. Nitration of
naphthalene with practically complete conversion
can also be carried out on high silica zeolite in
petroleum ether with 70% HNO
3
(Bakhvalov and
Ione, 1993). The use of zeolites with the NO
2
/O
2
system in acetonitrile leads to moderate yields (52-
80%) (Shi et al., 2013). A moderate yield of 1-
nitronaphthalene (63%) is also obtained with 95%
HNO
3
and a mixed catalyst on silica gel without the
use of solvents (Shi and Cui, 2003).
Bi(NO
3
)
3
, KNO
3
, (NH
4
)
2
Ce(NO
3
)
6
and even
NaNO
2
were added into a suspension of silica gel in
THF for the nitration of naphthalene, while the
yields of 1-nitronaphthalene were 76-88% (Badgujar
et al., 2007). Cerium ammonium nitrate was also
used for nitration of naphthalene with a conversion
ratio of more than 98% in a ionic liquid
(Dleersnyder et al., 2009). 1-Nitronaphthalene is
formed in a yield of 99% when naphthalene is
nitrated with an equimolar mixture of
benzyltriphenylphosphonium nitrate and anhydride
of methanesulfonic acid without the use of solvents
(Hajipour and Ruoho, 2004).
Nitration of naphthalene with acetyl nitrate in a
phosphonium ionic liquid gives a 74% yield (Powell
et al., 2005). Usually, direct nitration of naphthalene
with various reagents leads to the formation of a
mixture of 1- and 2-nitronaphthalenes. However, the
reaction is sufficiently regioselective, the content of
1-nitronaphthalene in products, as a rule, is 90-92%.
Ipso-nitration of naphthalene derivatives makes
it possible to obtain individual isomers. Reaction of
1- and 2-naphthylboronic acids with tert-butyl nitrite
in 1,4-dioxane without catalysts leads to the
preparation of 1- and 2-nitronaphthalenes with
yields of 75% and 76%, respectively (Wu et al.,
2011). A higher yield, 88% for 2-nitronaphthalene,
gives nitration with (CH
3
)
4
NNO
2
and Cu
2
O as a
catalyst in acetonitrile (Yan et al., 2012).
Mononitrotanaphthalenes in yields of 70-82% are
formed as a result of catalytic ipso-substitution of
bromo- or iodo-derivatives of naphthalene with
KNO
2
in the presence of copper(II) triflate in DMSO
(Amal Joseph et al., 2012). 1-Nitronaphthalene was
unexpectedly obtained from 5-nitronaphthylamine
by deamination (Srivastava et al., 2011).
The purpose of this research was mononitrtaion
of naphthalene using a classical nitrating mixture
under homogeneous conditions with 1,4-dioxane as
a solvent.
Khabarov, Y., Veshnyakov, V., Snigirev, I. and Pikovskoy, I.
Synthesis of 1-Nitronaphthalene under Homogeneous Conditions.
DOI: 10.5220/0008185800730076
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 73-76
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
73
2 EXPERIMENTAL
2.1 Reagents
The following reagent grade commercial chemicals
were used: naphthalene, 65% nitric acid, 96%
sulfuric acid, 1,4-dioxane and 96% ethanol.
2.2 Synthesis of 1-Nitronaphtalene
Naphthalene (0.50 g, 3.9 mmol) was dissolved in
1,4-dioxane in a 50-ml Erlenmeyer flask. The
nitrating mixtures were prepared by mixing
concentrated nitric (0.50-1.00 ml, 7.2-14.3 mmol)
and sulfuric (0.50-1.00 ml) acids with cooling. The
acid mixtures were added to a solution of
naphthalene in dioxane and the reaction mixtures
were heated under reflux with stirring for various
periods of time. Each reaction mixture was
transferred to a test tube, cooled, diluted to 25 ml
with ice-cold distilled water. The resultant
precipitate was isolated by vacuum filtration,
washed with ice-cold water until the washings were
neutral and dried in a vacuum desiccator at room
temperature to produce yellow crystals.
2.3 Analytic Methods
Melting points were determined in open capillaries
on a Stuart SMP30 melting point apparatus. UV-Vis
spectra of products in ethanol solutions were
recorded over the wavelength range of 200-500 nm
with a Shimadzu UV-1650PC spectrophotometer in
1 cm path length quartz cuvettes relative to ethanol.
The IR spectra were recorded over the wavelength
range of 4000-600 cm
-1
on a Shimadzu FTIR-8400S
spectrophotometer equipped with a Pike
Technologies MIRacle single reflection horizontal
ATR accessory with a ZnSe crystal plate.
For GC-MS analysis, a product sample (1 mg)
was dissolved in 20 ml HPLC-grade
dichloromethane, injection volume was 1 µl with
split 5:1. Mass spectra were recorded on a Thermo
Scientific Exactive GC Orbitrap mass spectrometer
coupled to a TRACE 1310 Gas Chromatograph
equipped with TriPlus RSH autosampler.
Chromatographic separation was performed on a
Thermo Scientific Trace GOLD TG5-SilMS
capillary column, 30 m×0.25 mm×0.25 µm. Helium
(99.9999%) was used as a carrier gas with flowrate
1.2 ml/min. The inlet temperature was 280°C. The
following thermostat program was used: initial
temperature 40°C, hold 2 min, ramp 10°C/min to
280°C, hold 5 min. The mass spectrometer operated
in fullscan mode with 60 000 FWHM mass
resolution. Electron ionization (70 eV) was used.
Transfer line and ion source temperatures were 280
and 230°C respectively. The control of the mass
spectrometer, the collection and preprocessing of the
data were performed using Thermo Scientific
Xcalibur software. The obtained mass spectra were
evaluated using the NIST 14 spectral library by the
similarity index (SI).
3 RESULTS AND DISCUSSION
In this study nitration of naphthalene was carried out
in homogeneous conditions. Naphthalene was
dissolved in 1,4-dioxane and remained in solution
when the nitration mixture of various compositions
was added and remained homogeneous when heated.
During the nitration nitrogen dioxide was evolved in
the first 10 min of heating. The product was isolated
from the reaction mixture after cooling and addition
of water. The yields were the same under the
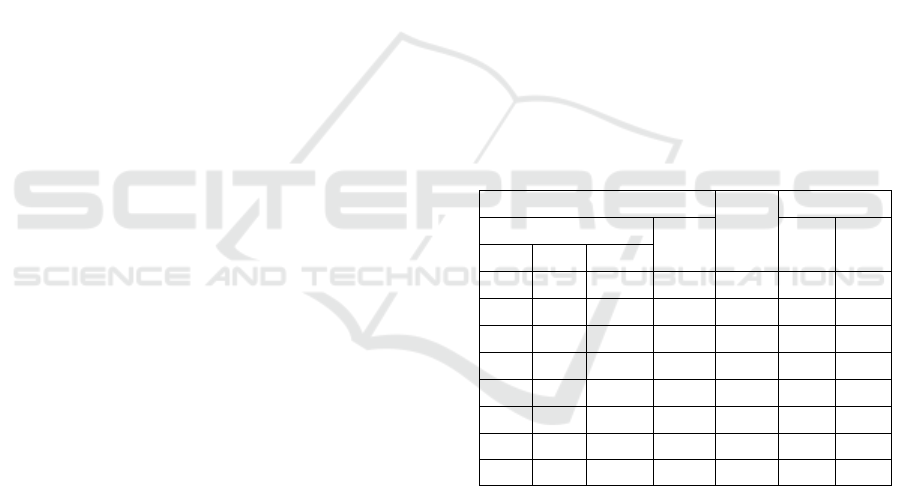
selected synthesis conditions (Table 1).
Table 1: Nitration conditions for naphthalene and product
yields.
Reaction condition
Yield
(%)
Isomer, %
Volume, ml
Time
(min)
1-
nitro
2-
nitro
HNO
3
H
2
SO
4
dioxane
1
1
3
60
97
96.4
3.6
1
1
4
60
96
–
–
1
1
5
60
96
95.8
4.2
0.75
1
5
60
96
95.7
4.3
0.5
1
5
60
97
–
–
1
1
5
50
95
–
–
1
1
5
40
96
–
–
1
0.5
5
60
96
–
–
The isolated products were characterized by their
melting point, UV and IR spectra, these results are
the same for all samples and correspond to the
literature data for 1-nitronaphthalene (Talukder and
Kates, 1995; Srivastava et al., 2011). GC-MS
analysis was carried out only on 3 samples. The GC-
MS analysis showed 2 detectable peaks in the gas
chromatographic separation and mass spectra
corresponding to 1-nitronaphthalene (retention time
11.38 min, m/z found: 173.04720, SI 797) and 2-
nitronaphthalene (retention time 11.89 min, m/z
found: 173.04714, SI 699). Calculation of the areas
of chromatographic peaks showed that the content of
1-nitronaphthalene in the samples of products is
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
74

about 96%, i.e. nitration of naphthalene with a
classical nitrating mixture in 1,4-dioxane passes with
good regioselectivity. In general, a high
regioselectivity of naphthalene nitration is typical in
the case of using the classic nitration mixture.
(Ikegami and Hiyama, 1954; Alcorn and Wells,
1965). Another advantage of the nitration is the
availability of reagents and simplicity of performing
the synthesis.
1-Nitronaphtalene: isolated yield 0.65 g (96%),
yellow crystalline solid, mp 52.5-53°C [lit. mp 52°C
(Talukder and Kates, 1995)]. IR, cm
-1
: 2831, 1631,
1600, 1568, 1514, 1458, 1439, 1354, 1344, 1261,
1216, 1200, 1173, 1163, 1148, 1141, 1079, 1030,
1000, 978, 961, 953, 930, 914, 872, 861, 815, 804,
787, 759, 726, 655, 628. EIMS: m/z for [M
+
] found:
173.04720. Calc. for C
10
H
7
NO
2
: 173.0471.
4 CONCLUSIONS
Nitration of naphthalene with a nitrating mixture in
1,4-dioxane occurs under homogeneous conditions.
The proposed method for the synthesis of 1-
nitronaphthalene is simple and effective. The yield
of the product reaches 96-97%. Nitration of
naphthalene in 1,4-dioxane has a high
regioselectivity. The content of 1-nitronaphthalene
and 2-nitronaphthalene in the product is 96 and 4%,
respectively.
ACKNOWLEDGEMENTS
The work was performed using the instrumentation
of Core Facility Center “Arktika” of Northern
(Arctic) Federal University (project
RFMEFI59417X0013).
REFERENCES
Alcorn, P.G.E., Wells P.R., 1965. Nitration of the
methylnaphthalenes. Australian Journal of Chemistry,
18(9), 1377-1389.
Amal Joseph, P.J., Priyadarshini, S., Lakshmi Kantam, M.,
Maheswaran, H., 2012. Copper catalyzed ipso-
nitration of iodoarenes, bromoarenes and heterocyclic
haloarenes under ligand-free conditions. Tetrahedron
Letters, 53(12), 1511-1513.
Badgujar, D.M., Talwar, M.B., Asthana, S.N., Mahulikar,
P.P., 2007. Environmentally benign synthesis of
aromatic nitro compounds using silica supported
inorganic nitrates. Journal of Scientific and Industrial
Research, 66(3), 250-251.
Bakhvalov, O.V., Ione, K.G., 1993. A method of nitration
of naphthalene. RU 2079482.
Booth, G., 2012. Naphthalene derivatives. In: Ullmann’s
encyclopedia of industrial chemistry. Wiley-VCH
Verlag GmbH & Co. KGaA. Weinheim, pp. 678-699.
Chen, Z., Zhang, W., Lu, M., Li, M., Zhang, S., 2016.
Preparation of naphthylamine by catalytic
hydrogenation over palladium/mesoporous carbon.
Shiyou Huagong/Petrochemical Technology, 45(12),
1449-1454.
Dleersnyder, K., Schaltin, S., Fransaer, J., Binnemans, K.,
Parac-Vogt, T.N., 2009. Ceric ammonium nitrate
(CAN) as oxidizing or nitrating reagent for organic
reactions in ionic liquids, Tetrahedron Letters, 50(32),
4582-4586.
Duvalma, M., Pirvulescu, I.V., Bota, T.D., 1964. Procédé
de préparation de nitro-1-naphtalène par nitration de
naphthalène. Fr 1352613.
Fierz-David, H.E., Blangey, L., 1949. Fundamental
processes of dye chemistry. Interscience Publishers,
Inc. New York.
Hajipour, A.R., Ruoho, A.E., 2004. A fast and mild
method for nitration of aromatic rings. Phosphorus,
Sulfur and Silicon and the Related Elements, 179(2),
221–226.
Ikegami, H., Hiyama, H., 1954. Relation of Nitration
Temperature of Naphthalene and β-Isomer Contents.
The Journal of the Society of Chemical Industry,
Japan, 57(4), 332-333.
Parac-Vogt, T.N., Pachini, S, Nockemann, P, Hecke, K.V.,
Meervelt, L.V., Binnemans, K., 2004. Lanthanide(III)
Nitrobenzenesulfonates as New Nitration Catalysts:
The Role of the Metal and of the Counterion in the
Catalytic Efficiency. European Journal of Inorganic
Chemistry, (22), 4560-4566.
Powell, B.D., Powell, G.L., Reeves, P.C., 2005.
Phosphonium ionic liquids as solvents for nitration
reactions of arenes. Letters in Organic Chemistry,
2(6). 550-553.
Shi, C., Peng, X., Tai, Y., Dong, X., Wang, H., 2013.
Zeolite-assisted regioselective mononitration of
naphthalene with nitrogen dioxide/molecular oxygen.
Research on Chemical Intermediates, 39(7), 3151-
3156.
Shi, M., Cui, S.-C., 2003. Electrophilic Aromatic Nitration
Using a Mixed Catalyst of Lithium, Molybdenum,
Ytterbium on Silica Gel. Advanced Synthesis and
Catalysis, 345(12), 1329-1333.
Srivastava, N., Shukla, M., Saha, S., 2011. An unusual
way to synthesize 1-nitronaphthalene from 1-amino-5-
nitronaphthalene. African Journal of Pharmacy and
Pharmacology, 5(4), 542-546.
Talukder, M., and Kates, C.R., 1995. Naphthalene
derivatives. In: Howe-Grant, M. (ed.) Kirk-Othmer
encyclopedia of chemical technology, 4th ed., Vol. 16.
Wiley, New York, p. 491.
Wright, O.L., 1965. Nitration process. US 3221062.
Synthesis of 1-Nitronaphthalene under Homogeneous Conditions
75

Wu, X.-F., Schranck, J., Neumann, H., Beller, M., 2011.
Convenient and mild synthesis of nitroarenes by
metal-free nitration of arylboronic acids. Chemical
Communications, 47(46), 12462+12463.
Yan, G., Zhang, L., Yu, J., 2012. Copper-Catalyzed
Nitration of Arylboronic Acids with Nitrite Salts
Under Mild Conditions: An Efficient Synthesis of
Nitroaromatics. Letters in Organic Chemistry, 9(2),
133-137.
Yin, W.-P., Shi, M., 2006. Indium triflate as a recyclable
catalyst for the nitration of aromatic compounds
without a halogenated solvent. Journal of Chemical
Research, (9), 549-551.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
76
