
Selective Separation of Cu (II) and Cd (II) from Aqueous Solution by
Shear Induced Dissociation and Ultrafiltration Using Rotating Disk
Membrane
Shuyun Tang and Yunren Qiu
*
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Keywords: Complexation-ultrafiltration, Rotating disk membrane, Shear induced dissociation, Dynamic filtration,
Selective separation, Regeneration.
Abstract: Selective separation of Cu (II) and Cd (II) from aqueous solution by shear induced dissociation and
ultrafiltration have been investigated using rotating disk membrane (RDM) and polyacrylic acid sodium
(PAAS) as complexing agent. The shear rate distribution on the membrane surface has been calculated at a
certain rotating speed. The polymer-metal complex can dissociate when the shear rate exceeds the critical
shear rate (γ
c
), the smallest shear rate at which the polymer-metal complex starts to dissociate. The
difference of the critical shear rates of the polymer-metal complexes can be used to separate different metal
ions from aqueous solution. The critical shear rate of PAA-Cd complex (γ
c,Cd
) is greater than that of PAA-
Cu complex (γ
c,Cu
) at pH 6.0. Cu (II) and Cd (II) can be separated when shear rate is greater than 8.0×10
4
s
-1
at pH 6, P/M 27.5. Moreover, the regeneration of PAAS has been also finished at γ>1.31×10
5
s
-1
. Compared
with the acidification, shear induced dissociation, is a novel and green technology for recovery of heavy
metal ions and polymer from aqueous solutions without the consumption of acid and alkali.
1 INTRODUCTION
Electroplating wastewater streams contain much Cu
(II) and Cd (II), which are harmful to human (Manis,
et al., 2013). Complexation-ultrafiltration is
an excellent way to remove heavy metals due to its
high efficiency and no secondary pollution (Qiu and
Mao, 2013). It is necessary to separate various heavy
metal ions in the treatment of wastewater for the
recovery of metal ions.
The inorganic acids are used to acidify the
complexes solution and then diafiltration
experiments is performed to recover polymer and
heavy metal in conventional acidification
decomplexation method (Shao, et al., 2013), but it is
easy to cause secondary pollution due to the use of
the chemicals of acid and alkali, and is not
convenient for the recovery of heavy metals.
In our previous work, polyacrylic acid sodium
(PAAS) is applied to remove various heavy metal
ions from aqueous solutions by complexation-
ultrafiltration using rotating disk membrane (RDM).
RDM filtration consists in creating the shear rate on
the membrane surface by a relative motion between
the fixed membrane and a disk rotating (Jaffrin,
2008). The stabilities of polymer-metal complexes in
the shear field have been studied and the results
show that the polymer-metal complexes can
dissociate when the shear rate is higher than the
critical shear rate γ
c
, the smallest shear rate
at which
the polymer-metal complex starts to dissociate (Gao
et al., 2018; Chen and Qiu, 2018; Tang and Qiu,
2018). In this work, shear induced dissociation and
ultrafiltration, is applied to separate Cu (II) and Cd
(II) and regenerate PAAS from the mixed aqueous
solution, which is novel and green technology
without the consumption of acid and alkali.
2 EXPERIMENTAL
2.1 Chemicals, Membrane and Set-up
PAAS with average molecular weight 250 kDa was
purchased from Wako Pure Chemical Industries,
Japan. The PAAS solutions were pretreated by
diafiltration to remove small molecular weight
PAAS. Cadmium nitrate tetrahydrate and copper
Tang, S. and Qiu, Y.
Selective Separation of Cu (II) and Cd (II) from Aqueous Solution by Shear Induced Dissociation and Ultrafiltration Using Rotating Disk Membrane.
DOI: 10.5220/0008189503190322
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 319-322
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
319
nitrate hydrate were used to prepare the aqueous
solution. Hydrochloric acid and sodium hydroxide
were used to adjust aqueous pH. All solutions in the
experiment were prepared with deionized water.
Polyether sulphone (PES) flat ultrafiltration
membrane with MWCO 10 kDa was supplied by
Shanghai Yuling Filter Equipment Co, Ltd.
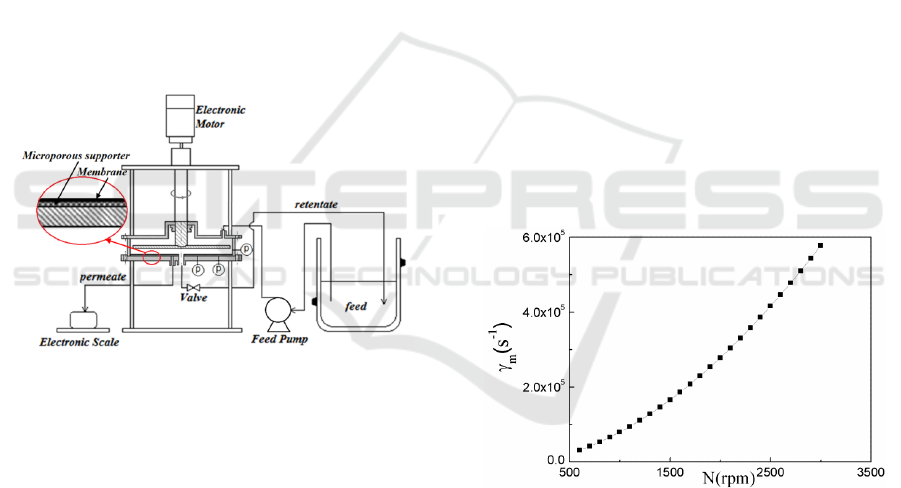
The device consists of a cylindrical housing
containing a metal disk. The rotating shaft which
connects with the motor outside the housing is
located at the centre of feed inlet side of the housing
and can provide the rotating speed of 0~3000 rpm.
The flat PES ultrafiltration membrane is fixed on the
retentate outlet side of the cavity. The depth of
housing is 30 mm, and the inner radius of the
housing is 88 mm which is same as the radius of the
membrane. The detailed structure is shown in Figure
1. The radius of the rotating disk is 80 mm, the
thickness is 4 mm, the six blades are evenly
distributed around the centre of the disk and the
blade height is 2 mm and the distance from the
membrane surface is 14 mm.
Figure 1: Schematic of filtration device.
2.2 Experimental Procedure
The metal ions fully reacted with polymers after two
hours under the specified pH and P/M (mass ratio of
polymer to metal ions) conditions, and then pumped
into the device by peristaltic pump. The
concentrations of metal ions were 10 mg/L.
According to our previous studies (Chen and Qiu,
2018; Tang and Qiu, 2018), the separation efficiency
of Cu (II) and Cd (II) was investigated using 550
mg/L PAAS and pH=6.0 to make heavy metal
completely complexed in a static filtration. At pH
6.0, the critical shear rates of PAA-Cd complex (γ
c,Cd
)
and PAA-Cu complex (γ
c,Cu
) were 8.0×10
4
s
-1
,
1.31×10
5
s
-1
, respectively. The initial operating
pressure of the device was controlled at 10 kPa.
Then, the diafiltration experiments were carried out
at different rotating speeds to separate Cu (II) and
Cd (II) and regenerate PAAS. The concentration of
PAAS was measured with chemical oxygen demand
(COD). The concentration of heavy metal was
measured by atomic absorption spectrophotometry.
All experiments were carried out at 25°C.
3 RESULTS AND DISCUSSION
3.1 The Shear Rate on the Membrane
Surface
The distributions of shear rate on the membrane
surface was explored by the recent research of the
present authors(Tang and Qiu, 2018).
γ
ml
=0.77υ
-0.5
(
kω
)
1.5
r (1)
γ
mt
=0.0296υ
-0.8
(
kω
)
1.8
r
1.6
(2)
where γ
ml
and γ
mt
represent the shear rates at laminar
and turbulent state, respectively (s
-1
). υ is kinematic
viscosity of test fluid (m
2
/s). ω is the angular
velocity of the disk (rad/s). r is the radius (m). The
velocity factor k of the six rectangular blades is
0.79(Chen and Qiu, 2018; Tang and Qiu, 2018).
Figure 2 shows the variation of the shear rate on
the edge of membrane surface with rotating speed.
The shear rate increases with the rotating speed.
Figure 2: Variation of shear rate with rotating speed.
3.2 Effect of Rotating Speed on the
Rejection of Cu (II) and Cd (II)
In the previous studies, the rejection of Cu (II) was
99.5% when the rotating speed was lower than 1000
rpm at pH 6.0, P/M=25, and the rejection of Cd (II)
could reach 99.7% when the rotating speed was less
than 1320 rpm at pH 6.0, P/M=30(Chen and Qiu,
2018; Tang and Qiu, 2018). Effect of rotating speed
on the rejection of Cu (II) and Cd (II) was
investigated using 550 mg/L PAAS and pH 6.0 to
make heavy metal ions completely complexed in a
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
320
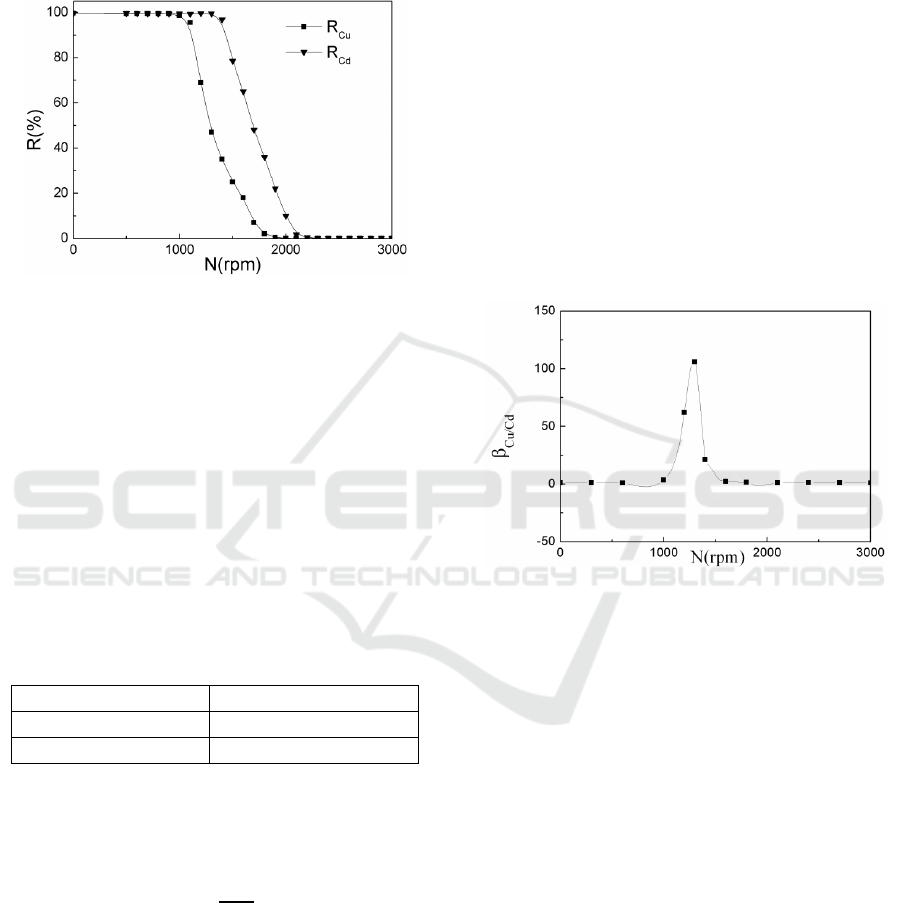
static filtration. As shown in Figure 3, the R
Cu
remains stable when the rotating speed is lower than
1000 rpm, while the R
Cu
has a sudden drop when the
rotating speed exceeds 1000 rpm. Similar variations
can be found in R
Cd
.
Figure 3: Effect of rotating speed on the rejection of Cu
(II) and Cd (II) (pH 6.0, P/M=27.5).
The critical shear rate of complexes is the
smallest shear rate at which the complex begins to
dissociate and the value is only related to the
geometry of the device at a certain pH. So, the
critical shear rates (γ
c
) of PAA-Cu and PAA-Cd at
pH 6.0 can be calculated by Eqs. (1) and (2), and the
calculation results are shown in Table 1. The critical
shear rate of PAA-Cd is greater than that of PAA-
Cu, indicating that the shear stability of PAA-Cd is
stronger than that of PAA-Cu at pH 6.0. The
experimental results provide support for the
following shear induced separation.
Table 1: The γ
c
of PAA-Cd and PAA-Cu at pH 6.0.
Complex
γ
c
/ s
-1
PAA-Cu complex
8.0×10
4
PAA-Cd complex
1.31×10
5
3.3 Selective Separation Coefficient
The selective separation coefficient(Uludag, et al.,
1998) (β
Cu/Cd
) of the both metals can be defined as:
β
Cu/Cd
=
1-R
Cu
1-R
Cd
(3)
where R
Cu
and R
Cd
are Cu (II) and Cd (II) rejections,
respectively.
β
Cu/Cd
obtained at rotating speed from 0 to 3000
rpm at pH 6.0 is shown in Figure 4. It is clearly seen
that β
Cu/Cd
firstly keeps invariable when the rotating
speed is lower than 1000 rpm, but after this it shows
a remarkable increase followed by a significant
drop. This can be explained as follows: when the
shear rate on the membrane surface is lower than the
critical shear rate of PAA-Cu complex and PAA-Cd
complexe, both of the PAA-Cu complex and PAA-
Cd complex remain stable. When the rotating speed
exceeds 1000 rpm, the shear rate on the membrane
surface is higher than the critical shear rate of PAA-
Cu complex, causing the dissociation of the PAA-Cu
complex and the decrease of R
Cu
, while PAA-Cd
complex remains stable and R
Cd
keeps constant.
Therefore, β
Cu/Cd
shows an enlargement trend. When
the shear rate is higher than the critical shear rate of
PAA-Cd complex, PAA-Cd complex also begins to
dissociate, R
Cd
declines and β
Cu/Cd
also decreases.
Therefore, it may be preferable for selective
separation to choose a suitable rotating speed, such
as 1300 rpm, at which the shear rate is between
8.0×10
4
s
-1
and 1.31×10
5
s
-1
. The greatest selection
separation coefficient can be obtained at 1300 rpm,
as shown in Figure 4.
Figure 4: Effect of rotating speed on selective separation
coefficient β
Cu/Cd
.
3.4 Selective Separation of Cu (II) and
Cd (II) and Regeneration of PAAS
by Shear Induced Dissociation and
ultrafiltration
The selective separation and the regeneration
experiments were carried out at pH 6.0 and
P/M=27.5. In rotating disk diafiltration experiments,
an amount of de-ionized water was add to the feed
tank simultaneously and continuously to keep the
volume of the feed remain constant. The rotating
speed was adjusted to 1300 rpm for diafiltration
experiments to separate Cu (II) and Cd (II).
The PAA-Cu complex easily dissociates due to
its smaller critical shear rate, and the free Cu (II)
permeates the membrane into the permeate. Figure 5
is the variation of the concentration of
residual copper (C
Cu
) and the concentration
of cadmium (C
Cd
) in the retentate with the volume
of make-up water (V
m
) at 1300 rpm. It can be seen
that C
Cu
decreases obviously but C
Cd
keeps constant,
Selective Separation of Cu (II) and Cd (II) from Aqueous Solution by Shear Induced Dissociation and Ultrafiltration Using Rotating Disk
Membrane
321
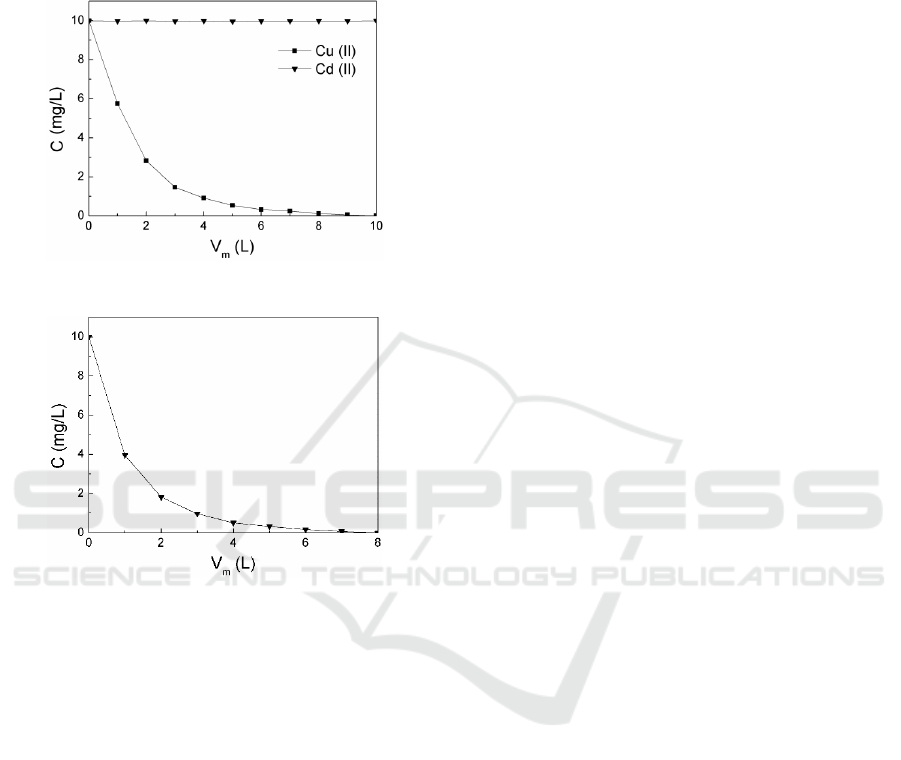
because PAA-Cu complex dissociates and PAA-Cd
complex keeps stable at this rotating speed. When
V
m
increases to10.0 L, the content of residual copper
in the retentate is very little, and the separation of Cu
(II) and Cd (II) is achieved.
Figure 5: Variation of C
Cu
and C
Cd
with V
m
Figure 6: Variation of C
Cd
with V
m
After Cu (II) was removed, the rotating speed
increased to 2000 rpm, PAA-Cd complex would
dissociate and the dissociated Cd (II) was collected
in permeate, the PAAS remained in the retentate, as
shown in Figure 6. The concentration of Cd (II) in
the retentate decreases as the addition of make-up
water, the PAA-Cd complex is completely
dissociated when V
m
reaches 7.0 L, and the PAAS is
regenerated.
4 CONCLUSIONS
Selective separation of Cu (II) and Cd (II) from
aqueous solution by shear induced dissociation and
ultrafiltration have been investigated using rotating
disk membrane and PAAS as complexing agent. At
pH 6, P/M 27.5, the separation of Cu (II) and Cd (II)
has been achieved at 1300 rpm from simulated
aqueous solution, and the regeneration of PAAS has
been finished at 2000 rpm from polymer-metal
complex solution. Compared with acidification,
shear induced dissociation, is a novel and green
technology for recovery of heavy metal ions and
polymer from aqueous solutions without the
consumption of acid and alkali.
ACKNOWLEDGEMENTS
This work was supported by the National Natural
Science Foundation of China (NO. 21476265).
REFERENCES
Chen, L., Qiu,Y., 2018. Removal of Cd (II) from dilute
aqueous solutions by complexation– ultrafiltration
using rotating disk membrane and the shear stability of
PAA–Cd complex. Chinese J. Chem. Eng.
https://doi.org/10.1016/j.cjche.2018.06.026
Gao, J., Qiu, Y., Hou, B., Zhang, Q., Zhang, X., 2018.
Treatment of wastewater containing nickel by
complexation- ultrafiltration using sodium
polyacrylate and the stability of PAA-Ni complex in
the shear field. Chem. Eng. J. 334, 1878–1885.
https://doi.org/10.1016/j.cej.2017.11.087
Jaffrin, M.Y., 2008. Dynamic shear-enhanced membrane
filtration: A review of rotating disks, rotating
membranes and vibrating systems. J. Memb. Sci. 324,
7-25. https://doi.org/10.1016/j.memsci.2008.06.050
Manis, A., Soldenhoff, K., Jusuf, E., Lucien, F., 2003.
Separation of copper from sulfuric acid by
nanofiltration. Fifth Int. Membr. Sci. Technol. Conf.
Qiu, Y. R., Mao, L. J., 2013. Removal of heavy metal ions
from aqueous solution by ultrafiltration assisted with
copolymer of maleic acid and acrylic acid.
Desalination 329, 78–85.
https://doi.org/10.1016/j.desal.2013.09.012
Shao, J., Qin, S., Davidson, J., Li, W., He, Y., Zhou, H.S.,
2013. Recovery of nickel from aqueous solutions by
complexation-ultrafiltration process with sodium
polyacrylate and polyethylenimine. J. Hazard. Mater.
244-245, 472–477.
https://doi.org/10.1016/j.jhazmat.2012.10.070
Tang, S.Y., Qiu, Y. R., 2018. Removal of copper(II) ions
from aqueous solutions by complexation–
ultrafiltration using rotating disk membrane and the
shear stability of PAA–Cu complex. Chem. Eng. Res.
Des. 136, 712–720.
https://doi.org/10.1016/j.cherd.2018.06.030
Uludag, Y., Hilmi, O., Mu, J., 1998. Effect of operating
parameters on selective separation of heavy metals
from binary mixtures via polymer enhanced
ultrafiltration. J. Memb. Sci. 140, 251-266.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
322
