
Utilization of Lanthanum Carbonate and Bentonite for Phosphorus
Removal from Domestic Sewage Effluent
Yifan Lu, Huawei Wu, Yan Xia and Mei Huang
*
College of Chemical & Biochemical Engineering, Zhejiang University, Zheda Road, Hangzhou, China
Keywords: lanthanum carbonate, bentonite, adsorption, phosphorus removal, domestic sewage effluent
Abstract: lanthanum carbonate and bentonite were used as adsorbents to remove phosphorus from domestic sewage
effluent. Three batch-mode adsorption experiments (using La
2
(CO
3
)
3,
Bentonite/La
2
(CO
3
)
3
and
Bentonite+La
2
(CO
3
)
3
as adsorbents) were carried out to investigate the effect of adsorbents for the removal
of phosphorus. The experimental results show that Bentonite/La
2
(CO
3
)
3
and Bentonite+La
2
(CO
3
)
3
of
320mg/L can reduce phosphate concentration down to 0.06 mg P/L and 0.04 mg P/L from an initial value of
0.609 mg P/L at the contact time of 48 h, respectively.
1 INTRODUCTION
Excess phosphorus (P) in freshwater bodies is one of
the major causes of eutrophication (Zhu et al., 2016).
The regulations for removing phosphate from
water/wastewater treatment applications are
becoming more stringent (Zhao et al., 2014).
Therefore, phosphorus removal from
water/wastewater has attracted considerable research
interest in the last few decades (Zhang et al., 2011).
And a range of methods have been developed,
mainly including biological, chemical, and physical
treatments. Among these methods, the adsorption
process is promising for phosphate removal,
attributed to its attractive advantages of simple
operation, high removal efficiency and fast
adsorption rate, especially at low phosphate
concentration (Mor et al., 2016). Many inorganic
and organic adsorbents as well as industrial by-
products, and biological wastes have been used in
the treatment of phosphorus in water/wastewater.
Lanthanum-based adsorbents are gaining
attention for the adsorption of phosphorus from
water/wastewater in terms of their high affinity for
phosphate and the lanthanum–phosphate complex
forms, even when present in low concentrations of
phosphorus. Various salts of lanthanum, such as
La
2
(SO
4
)
3
, La(NO
3
)
3
, La(OH)
3
and La
2
O
3
, have
attracted intensive research interest in practical
applications (Zhang et al., 2011; Yang et al., 2011;
Zhang et al., 2016). However, some typical
problems still exist which limit the wide application
of these adsorbents. For example, it is found that
most of their adsorption capacity is greatly declined
in neutral and basic conditions. And the incomplete
accessibility of La to phosphate in bulk samples is
considered to be another reason for the reduced
efficiency of La removal. Benefiting from the low
leaching of lanthanum carbonate during phosphate
adsorption, lanthanum carbonate can exhibit much
higher safety and environmental friendly
characteristics. Moreover, the pH buffering of
carbonate salts is helpful for lanthanum carbonate
show the observed small increase in pH value.
Unfortunately, lanthanum carbonate is mainly used
in the treatment of hyperphosphatemia of chronic
kidney disease (Aaseth et al., 2018), very limited
work has been done for dephosphorization agent
outside the medical field.
The object of present paper is to provide
lanthanum carbonate and bentonite for the treatment
of phosphorus in domestic sewage effluent.
Experimental results clearly indicate that the
combined effect of lanthanum carbonate and
bentonite significantly improved the removal
efficiency of phosphorus in domestic sewage
effluent.
Lu, Y., Wu, H., Xia, Y. and Huang, M.
Utilization of Lanthanum Carbonate and Bentonite for Phosphorus Removal from Domestic Sewage Effluent.
DOI: 10.5220/0008189603230326
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 323-326
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
323

2 MATERIALS AND METHODS
2.1 Materials
All the chemicals used in this study were of
analytical grade and were used without further
purification. Lanthanum chloride hexahydrate
(LaCl
3
·6H
2
O) was provided by Desheng New
Material Co., Ltd. (Shandong, China). Sodium
bicarbonate (NaHCO
3
) and Sodium bicarbonate
heptahydrate (MgSO
4
·7H
2
O) were obtained from
Zhanyun Chemical Co., Ltd. (Shanghai, China).
Sodium hydroxide (NaOH) and potassium
dihydrogen phosphate (KH
2
PO
4
), were purchased
from Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China). Bentonite was supplied by
Zhejiang Fenghong New Material Co. Ltd, Zhejiang,
China.
2.2 Adsorbate Solution
The phosphorus solutions were collected from rural
domestic sewage effluent in Zhuji, Zhejiang
province, China. And the initial total P
concentrations was 0.609 mg P/L.
2.3 X-Ray Diffraction (XRD) Analysis
The prepared lanthanum carbonate was analyzed
using different equipments to reveal their physical
properties. And the crystal structure of the
adsorbents was analyzed using Bruker D8 Advance
diffractometer (X’Pert Powder, PANalytical,
Netherlands) with Cu-Ka radiation (40 kV, 40 mA)
over the 2θ range of 10-60°.
2.4 Preparation of Lanthanum
Carbonate
Lanthanum carbonate was synthesized via
conventional coprecipitation method. The Mg
2+
solution (MgSO
4
) was added into the 0.4M La
3+
solution (LaCl
3
) at room temperature. Then 1.2M
NaHCO
3
was added droplet under stirring and
allowed to react for another 2hr. After the reaction
mixture was stood for additional 1 hours, the mixed
compounds were filtered and subsequently washed
thoroughly with ethanol and deionized water until
the pH of the effluent solution was neutral. Finally,
the washed samples were oven-dried at 65°C for 12h
to obtain the corresponding lanthanum carbonate
adsorbents.
2.5 Batch Experiments
All batch adsorption experiments were performed in
duplicate at room temperature using 25 ml glass
tubes. Various concentrations (20-320 ppm) of
adsorbents were introduced into 25 ml of adsorbate
solution with an initial total P concentration of 0.609
mg P/L and a pH∼7. The mixing of adsorbents and
adsorbate solution was performed by resting there
for specified time. After that, the suspension was
immediately centrifuged for phase separation. The
supernatant was collected through filtration by using
0.45μm syringe filter, and analyzed to determine the
residual P concentration by Mo-Sb anti-
spectrophotometer method using a UV-Vis
spectrophotometer (HACH, DR900, America).
The removal rate (R) of P was calculated from
the following relation:
100
C
)C-C
R
0
e0
(
(1)
In the above equation C
0
represents initial
concentration whereas C
e
as final level of phosphate
(mg P/L) in aqueous solution.
3 RESULTS AND DISCUSSION
3.1 The X-Ray Diffraction Analysis of
Lanthanum Carbonate Adsorbents
The structure of lanthanum carbonate adsorbents
was confirmed by the XRD analysis. As seen from
Figure 1, the XRD patterns of the as-synthesized
lanthanum carbonate (La
2
(CO
3
)
3
) are typical
structure of lanthanum carbonate with sharp peak at
10.27° corresponding to the (002) plane, which are
highly crystalline structure of La
2
(CO
3
)
3
·8H
2
O (PDF
#25-1400), and broad peaks located at 18.51°,
20.79°, 21.24°, 27.08°, 29.06°, corresponding to the
crystal surfaces of La
2
(CO
3
)
3
·8H
2
O (020), (004),
(022), (220) and (222) planes.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
324
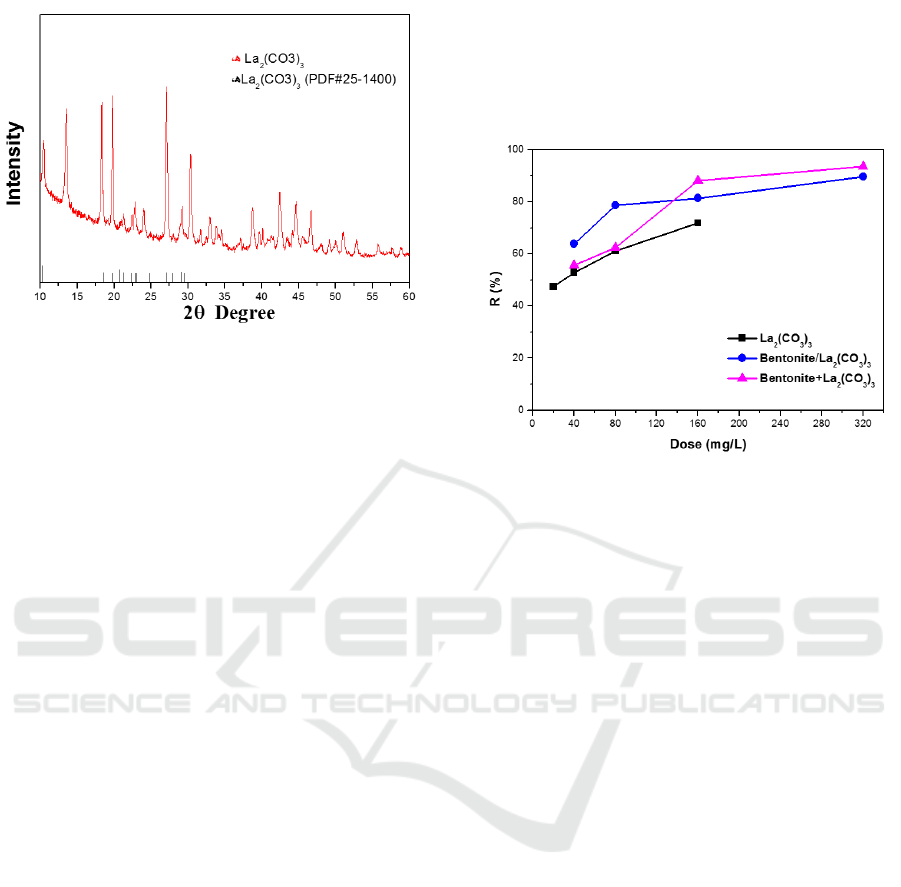
Figure 1: X-ray diffraction analysis of lanthanum
carbonate.
3.2 Adsorption Experiments
Adsorption experiments were conducted using the
synthesized adsorbents to evaluate their affinity
towards phosphorus. In batch experiments, they
were classified into three main categories: batch
experiments I using only lanthanum carbonate as
adsorbent, denoted as La
2
(CO
3
)
3
; batch experiments
II using the mixture of lanthanum carbonate and
bentonite as adsorbent which is Bentonite/
La
2
(CO
3
)
3
; and batch III are batch experiments
which add bentonite first, then followed by
La
2
(CO
3
)
3
, denoted as Bentonite+La
2
(CO
3
)
3
. Their
results for phosphorus removal are shown in Figure
2. It can be seen that as the adsorbents dose
increases, percentage of phosphorus removal
increased in three batch experiments. This might be
due to the increased surface area of the adsorbent
with increased adsorbent dosage. Further, it is
noticed that the maximum removal of phosphorus,
which was achieved using Bentonite/La
2
(CO
3
)
3
or
Bentonite+La
2
(CO
3
)
3
as adsorbents, are 89.6% and
93.6%, respectively. These high phosphorus removal
rates indicate that addition of bentonite can promote
the adsorption of phosphate because of the more
positive charges on the adsorbents. In this way, after
phosphorus component being efficiently adsorbed by
nano-prepared lanthanum carbonate particles, effect
of flocculation by bentonite on nano-products made
the continued separation process efficient and
complete. Then, the improved phosphorus removal
can be obtained from domestic sewage effluent.
Meanwhile, it is found that feeding method also
affects the results of phosphorus removal. As
compare to batch experiments II using the mixture
of lanthanum carbonate and bentonite as adsorbent,
batch experiments III shows higher phosphorus
removal ability. This might be attributed to the
flocculation pretreatment of bentonite in batch
experiments III. So that lanthanum carbonate can
dephosphorize in a cleaner environment and exhibit
better treatment effect.
Figure 2: Effect of different types of adsorbents on
phosphorus removal efficiency.
3.3 Effect of Contact Time
In order to investigate the effect of adsorption and
desorption equilibrium on phosphorus treatment,
results of phosphorus removal were investigated at
the contact time of 24hr and 48hr, respectively. It
can be seen in Figure 3 that when using La
2
(CO
3
)
3
alone as the adsorbent, phosphorus removal rates is
positively correlated with the contact time for
different in feed La
2
(CO
3
)
3
concentration. However,
Inconsistent conclusions appeared for the other two
adsorbents, that is, as the initial amount of bentonite
and La
2
(CO
3
)
3
increase, phosphorus removal rate
increases first and then decreases when
Bentonite/La
2
(CO
3
)
3
and Bentonite+ La
2
(CO
3
)
3
were
added into the solution. This phenomena indicates
spontaneous nature of adsorption for phosphorus
removal. When the equilibrium was achieved, no
further increase in phosphorus adsorption was
observed. Furthermore, it seems that phosphorus
removal rate of Bentonite/La
2
(CO
3
)
3
and
Bentonite+La
2
(CO
3
)
3
decreased as the contact time
larger than equilibrium time. This may be due to the
different stability between the two adsorbent
components.
Utilization of Lanthanum Carbonate and Bentonite for Phosphorus Removal from Domestic Sewage Effluent
325

Figure 3: Adsorption of phosphorus with respect to the
contact time.
3.4 Treatment Capacity
The correlation of treatment capacity and
concentration is given in Table 1. It can be seen that
the estimated effluent treatment capacity ranged
1.63-11.36 and 1.63–10.12 mg/g for
Bentonite/La
2
(CO
3
)
3
and Bentonite+La
2
(CO
3
)
3
,
respectively, which clearly indicates their removal
potential for phosphorus treatment. Owing to the
repulsion between the negatively charged PO
4
3−
species and negatively charged surface sites of
bentonite, it is difficult for bentonite itself to adsorb
phosphorus in domestic sewage effluent. Apart from
the samples without bentonite, the best performing
indicated the importance of cooperation between
lanthanum carbonate and bentonite for phosphorus
removal.
Table 1: Phosphorus treatment capacity at different
adsorbents concentration.
Dose
(mg/L)
Bentonite/La
2
(CO
3
)
3
Bentonite+La
2
(CO
3
)
3
40
11.36
10.12
80
6.30
5.58
160
3.41
3.15
320
1.63
1.63
4 CONCLUSIONS
The results of this study indicate that combination of
lanthanum carbonate and bentonite is very good
adsorbents for phosphorus disposal in domestic
sewage effluent. The results obtained for the
phosphorus removal were compared and the findings
showed that Bentonite/La
2
(CO
3
)
3
and
Bentonite+La
2
(CO
3
)
3
have good potential for
removing phosphorus from domestic sewage
effluent as compared to La
2
(CO
3
)
3
. In this work,
89.6% and 93.6% removal of phosphorus were
obtained by using Bentonite/La
2
(CO
3
)
3
and
Bentonite+La
2
(CO
3
)
3
at the dose of 320 mg/L for
48hr in the resting experiment.
ACKNOWLEDGEMENTS
This work was financially supported by National
Science and Technology Major Special Project,
China (2018ZX07208009).
REFERENCES
Aaseth, J., Bjorke-Monsen, A.L., 2018. Lanthanum
Carbonate - A New Phosphate Binding Drug in
Advanced Renal Failure. Curr. Med. Chem.,
25(1),113-117
Mor, S., Chhoden, K., Khaiwal, R., 2016. Application of
agro-waste rice husk ash for the removal of phosphate
from the wastewater. J. Clean. Prod. 129, 673-680.
Yang, J., Zhou, L., Zhao, L., Zhang, H., Yin, J., Wei, G.,
2011. A designed nanoporous material for phosphate
removal with high efficiency. J. Mater. Chem., 21(8),
2489-2494.
Zhao, F., Tang, W.Z., Zhao, D., Meng, Y., 2014a.
Adsorption kinetics, isotherms and mechanisms of
Cd(II), Pb(II), Co(II) and Ni(II) by a modified
magnetic polyacrylamide microcomposite adsorbent.
J. Water Process Eng. 4, 47–57.
Zhang, L., Wan, L., Chang, N., Liu, J., Duan, C., Zhou,
Q., Wang, X., 2011. Removal of phosphate from water
by activated carbon fiber loaded with lanthanum
oxide. J. Hazard. Mater. 190 (1–3), 848–855.
Zhang, J., Shen, Z., Shan, W., Mei, Z., Wang, W., 2011,
Adsorption behavior of phosphate on lanthanum(III)-
coordinated diamino-functionalized 3D hybrid
mesoporous silicates material. Journal of Hazard.
Mater. 186(1), 76-83.
Zhang, Y., Pan, B., Shan, C., Gao, X., 2016. Enhanced
phosphate removal by nanosized hydrated La(III)
oxide confined in crosslinked polystyrene networks.
Environmental Science & Technology, 50(3), 1447-
1454
Zhu, C., Tian, H., Cheng, K., Liu, K., Wang, K., Hua, S.,
Gao, J., Zhou, J., 2016. Potentials of whole process
control of heavy metals emissions from coal-fired
power plants in China. J. Clean. Prod. 114, 343–351.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
326
