
The Effect of Concentration Ratio of Gelatine and
Polyvinylpyrrolidone as Binders on the Physical Properties of Red
Ginger (Zingiber officinale Rosc.) Extract Lozenges
Inding Gusmayadi, Priyanto
Faculty of Pharmacy and Sciences Universitas Muhammadiyah Prof. DR. HAMKA, Indonesia
Keywords: Red ginger extract, Lozenge.
Abstract: Lozenges require the tablet hardness of 7-14 Kgf to be a suitable binder. Gelatine can be used to meet the
requirement as it creates granules with bad flow time. PVP produces granules with better flow time but it
takes a large quantity to reach the lozenges hardness desired. This study aimed to determine the effect of the
concentration ratio of gelatine and PVP as binders on the physical properties of red-ginger extract lozenges.
Lozenges made employing the wet granulation method following the ratio of gelatine and PVP of 1:1, 1:2,
1:3, 1:4 and 1:5. The hardness test results of the formula 1 to 5 are 9, 14, 16, 13 and 12 Kgf respectively and
the friability test results were 0,4%, 0,7%, 0,2%, 0,3% and 0,6% accordingly. The combination of gelatine
and PVP as binders provide a significant difference in the hardness and friability of the tablets.
1 INTRODUCTION
Ginger, one of the most commonly used herbs in
food worldwide, has a wide range of medicinal uses
including carminative, antiemetic, spasmolytic,
antiflatulent, antitussive, hepato-protective, anti-
platelet aggregation and hypolipidemic effects.
Ginger has a strong odour generated by a mixture of
phenolic compounds that can stimulate thermogenic
receptors leading to the antiemetic effect (Heinrich
et al. 2009). Generally, herbal preparations of red
ginger are consumed as the instant powder. The use
of instant powder in the treatment is less practical
and therefore the dosage form needs to be developed
to be more practical and effective (Badan
Pengawasan Obat and Makanan Republik Indonesia
2012).
The lozenge is a practical choice to develop a
dosage form of red ginger. The lozenge is a solid
preparation that will dissolve or break down slowly
in the mouth (Agoes 2008). Two types of lozenges
are widely used, i.e. hard candy and compressed
tablet lozenges (Peters 1989). Compressed tablet
lozenges can be created using direct compression,
dry granulation or wet granulation. The wet
granulation method benefits to facilitate the
agglomeration process in the formula so that it will
result in the excellent physical properties and mass
of the tablets (Siregar and Wikarsa 2010).
The differences in physical properties of
lozenges and conventional tablets are the hardness of
7-14 Kgf, the diameter of 0.625-0.75 inches and the
weight range of 1.5-4.0 grams. The pre-formulation
of lozenge excipients should be filler, sweetener,
lubricant, glidant, flavoring agent and binder to
produce the good quality of lozenge physical
properties required (Hadisoewignyo and Fudholi
2013, Siregar and Wikarsa 2010). The binder is an
excipient in a tablet formula providing a cohesive
force between particles thus compromising a
compact and robust structure (Anwar 2012). The
binding effect in the lozenge component is essential
to produce greater hardness compared to the level of
conventional tablet hardness. The bonding agent is
divided into two groups: the synthetic and the
natural binder.
The natural binding agents that can be used are
starch, gum, tragacanth and gelatine. Gelatine has a
characteristic that inhibits the disintegration time
and thus it is suitable to be used as a binder on a
lozenge (Voigt 1995). Gelatine solution of 2-10%
can be used as a binder of the tablet formula (Anwar
2012). The use of gelatine as a binder on a wet
granulation can produce poor granule flows (Hamed
Gusmayadi, I. and Priyanto, .
The Effect of Concentration Ratio of Gelatine and Polyvinylpyrrolidone as Binders on the Physical Properties of Red Ginger (Zingiber officinale Rosc.) Extract Lozenges.
DOI: 10.5220/0008240901470153
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 147-153
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
147
et al. 2005). The viscous properties of natural-
sourced gelatine can increase the size of granules
which in turn increases the granular flow time. This
property can be improved by combining a natural
and a synthetic binder (Agubata et al. 2012).
One of the synthetic binders that can be used in
lozenge compression is polyvinylpyrrolidone (PVP)
in the range 0.5-5% (Rowe et al. 2009). A large
amount PVP is needed to be used as a lozenge
binder. Previous research reported that 3% of PVP in
the formula yielded a hardness of 6.23 Kgf (Sari and
Astuti 2010). It takes 10% of PVP to produce the
optimum lozenge ginger extract hardness of 14.63
kg (Mutmainah 2005), while the combination of
PVP (4.7%) and gelatine (9.3%) creates the
physical properties of tablets fitting the tablet
hardness and fragility requirements of 13.04 Kgf and
0.215% respectively (Liauw 2012). This research
examined the combination of PVP and gelatine for
ginger extract as an active ingredient. It aimed to
obtain the optimum combination to achieve the
maximum hardness and the minimum fragility on
ginger extract lozenges.
Based on the previous review, this research
investigated the effect of gelatine (4.0-6.7%) and
PVP (1.3-4%) combination on the physical
properties of red ginger extract lozenge employing
wet granulation method. The combination of
gelatine and PVP was performed in various
concentrations of 1: 1 (formula I), 1: 2 (formula II),
1: 3 (formula III), 1: 4 (formula IV) and 1: 5
(formula V). The combination of Gelatine and PVP
was not 0:1, 1:1, and 1:0 due to the trial results of
the combination of gelatine less than 4% did not
meet the requirement of lozenges hardness. The
quality of lozenges was assessed based on the
physical properties: (1) the evaluation of the tablet
mass includes the compressibility test, the flow time,
the angle of repose, the particle size distribution; and
(2) lozenge evaluation includes the organoleptic, the
weight uniformity, the size uniformity, the hardness
and friability test (Siregar and Wikarsa 2010).
2 MATERIALS AND METHOD
2.1 Materials
Materials used included the dry red ginger extract
(PT Haldin Pacific Semesta), PVP K-30 (Kimia
Farma), gelatine (Kimia Farma), dextrose (Kimia
Farma), mannitol (Kimia Farma), Talc (Kimia
Farma), and Magnesium Stearate (Kimia Farma).
2.2 Methods
2.2.1 Lozenges Formula
Lozenges were made in five formulas namely F1,
F2, F3, F4 and F5. The five formulas had different
ratios of gelatine and PVP combinations as the
binding agents of 1000 mg tablet (see Table 1).
2.2.2 Lozenges Production
All materials were prepared and weighed. The red
ginger extract was put into the container, the
mannitol and dextrose were added and stirred
resulting in a homogeneous mixture. The PVP
solution was prepared by dissolving it in the 70%
ethanol (1: 5) and the gelatine solution was prepared
by hydrating the gelatine in cold water (1:2) for 24
hours before mixing and heating. PVP solution and
gelatine solution were added in warm conditions
slowly and stirred to be homogeneous and mass was
wet enough could be formed into granules.
The mass was sieved using a 12-mesh sieve and
then put into an oven at ± 50 ° C for ± 24 hours. The
granules were then sieved back with an 18-mesh
sieve. The magnesium stearate and talc were added
and mixed homogeneously. The granule evaluation
was then conducted. The granules were prepared and
put into the hopper. The tablet weight and hardness
were set. The lower punch was set if the hardness
was less than 7.0-14 Kgf. The upper punch was set if
the tablet weight was less than 1000 mg. The engine
ran until all granules transform into tablets.
2.2.3 The Evaluation of Extract, Granules,
and Tablets
The evaluation of dried red ginger extract included
the organoleptic, loss on drying, ash residue,
solubility, particle size and phytochemical extract
tests.
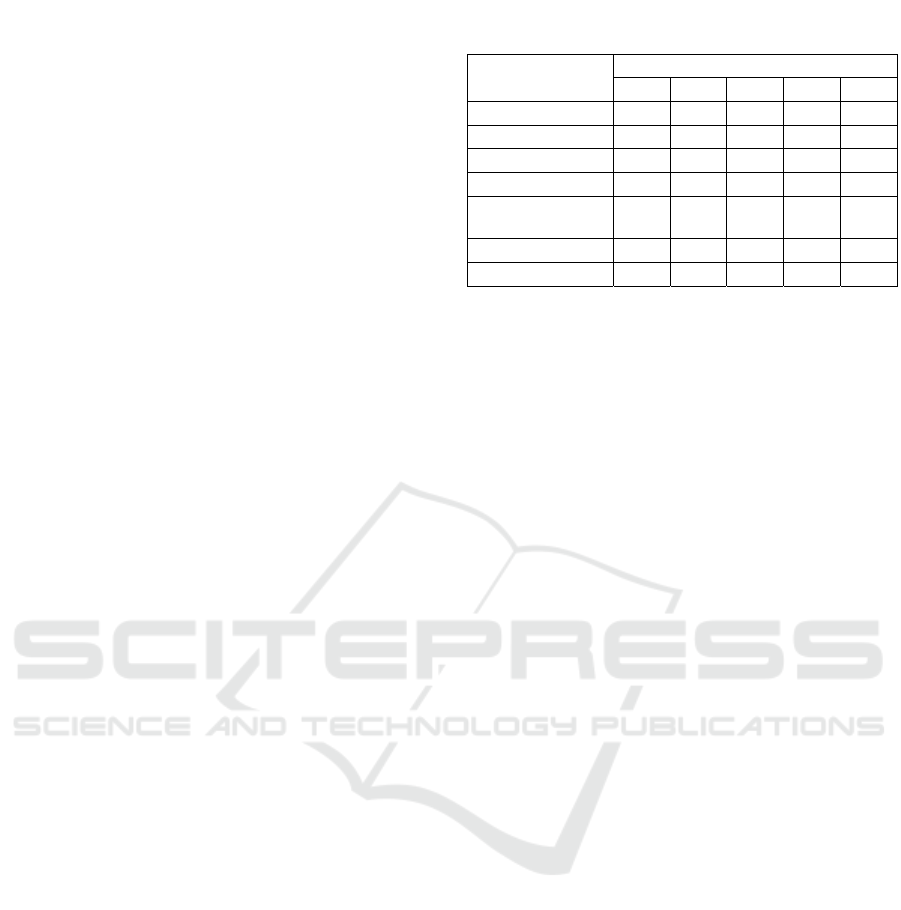
Table 1: Lozenges Formula.
Materials
Formula (%)
1 2 3 4 5
Ginger Extract 30 30 30 30 30
Dextrose 20 20 20 20 20
PVP 4 2,7 2 1,6 1,3
Gelatine 4 5,3 6 6,4 6,7
Magnesium
Stearate
1 1 1 1 1
Talc 2 2 2 2 2
Mannitol ad 100 100 100 100 100
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
148
The evaluation of granules included the flow
time, the angle of repose, the compressibility, the
granule size distribution and the loss on drying.
The lozenge evaluation included the
organoleptic, uniformity size, weight uniformity,
tablet fragility and tablet hardness tests.
3 RESULTS AND DISCUSSION
The dry extract obtained from PT. Haldin was then
determined. The result of the extract determination
from LIPI Cibinong showed that the dry extract
observed was Red Ginger which belongs to the
Zingiberaceae tribe. The result of the organoleptic
test on the dry red ginger extract is the fine yellow-
brown powder with spicy taste and red ginger
specific smell. The LOD test to examine the
moisture of the dry extract of red ginger was
conducted to prevent the powder become moist
which can accelerate the microbial growth. The
LOD test result was 5,61%. This shows that the
moisture of the extract meets the requirements, i.e.
no more than 10% (Departemen Kesehatan RI
1980).
The LOD extract test used a moisture balance
employing the gravimetric principle. This tool
measured the moist in the extracts that evaporated
from the heat generated by the appliance. The
extract moisture may be due to the water or organic
solvents used during the extraction process. So, it is
not specific to measure the water in the extract. If
the extract is made using an organic solvent, the tool
can detect the remaining solvent in the extract as the
amount produced. The results showed that the water
in the extract was 5.6%. Based on Hadisoewignyo
and Fudholi (2013), the extract classifies into the
non-hygroscopic category (<10%).
The residual test of dry red ginger extract ash
aimed to investigate the inorganic impurities in the
extract. The larger ash in the material shows the
higher mineral in the material. According to
Departemen Kesehatan RI (1980), the residual ash
requirement of excellent red ginger rhizome extract
should be no more than 5.0%. The results showed
that the ash residue obtained was 4.7%, so it can be
concluded that the dry ginger extract meets the
requirements of the excellent ash content.
The results of the phytochemical screening test
showed that the red ginger extract contained the
alkaloid, flavonoid, saponin, terpenoid, and
glycoside compounds. The purpose of the test is to
examine the active substance of the gingerol
compound, a phenol-derived compound. This
compound does not break when it is processed at
temperatures below 70℃ but it will be converted
into shogaol compounds that increase the spicy
flavour of the red ginger extract (Heinrich et al.
2009). Due to the stability of this compound
indicated by the spicy flavour and the same spot on
the TLC test, it can be concluded that the extract as
an active substance does not suffer damage during
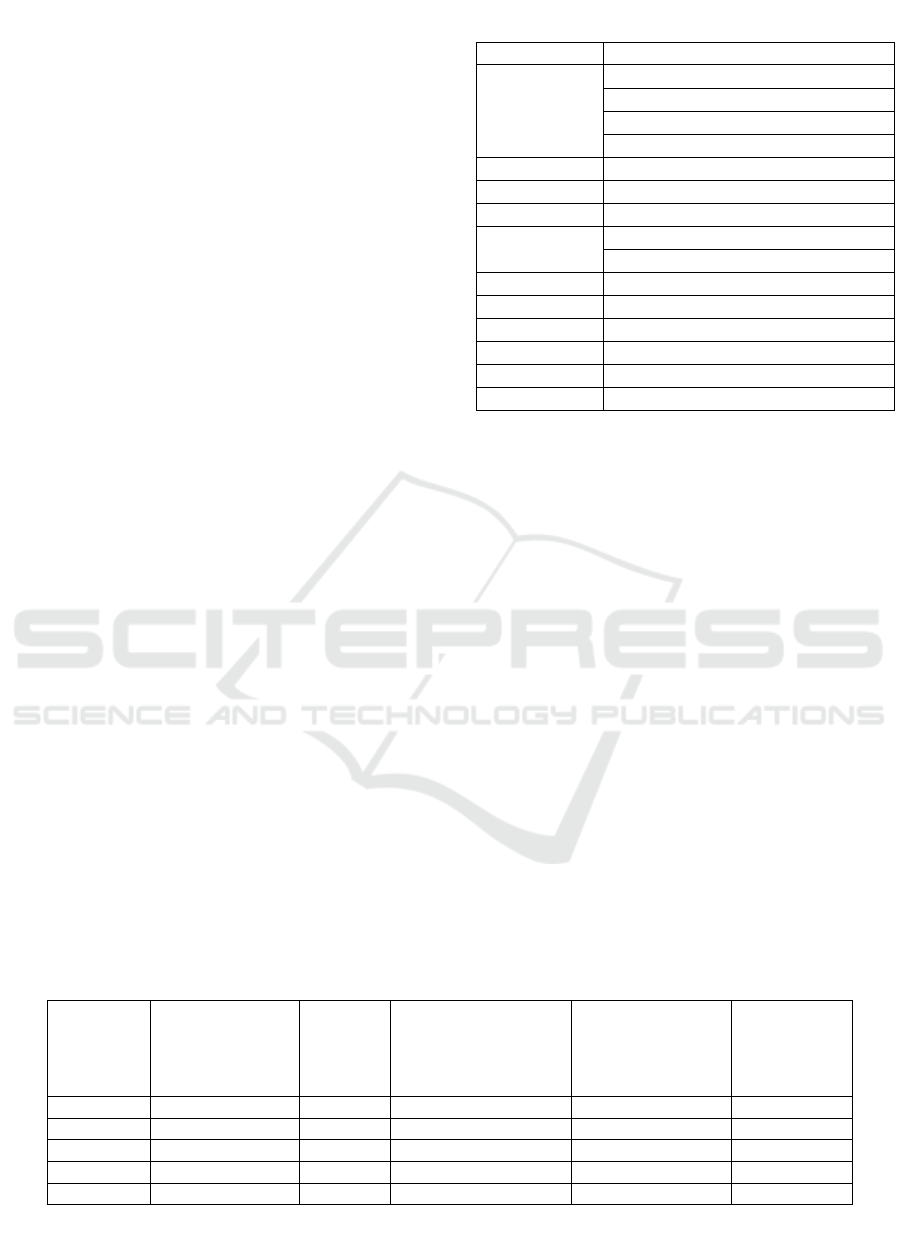
Table 2: The results of evaluation of red ginger extract.
Parameter Results
Organoleptic
Color : Yellow-Brown
Odor : Specific Ginger Odor
Taste : Spicy
Form : Fine Powder
LOD 5,61 %
Ash Residue 4,6912%
Solubility 100 mg dissolve in 1,73 mL of water
Particle size
93,79% passing the 80-mesh sieve
80,47% passing the 100-mesh sieve
Flavonoid +
Saponin +
Tannin -
Phenol +
Triterpenoid -
Steroid -
Table 3: The results of the granule evaluation.
Formula
Flow Time
(g/sec)
Angle of
Repose
Compressibility (%) LOD (%)
Granule Size
(μm)
F1 10,21 ± 0,17 28º58" 2,7 ± 0,36 3,70 ± 0,16 826
F2 9,51 ± 0,37 27º01" 2,6 ± 0,09 3,45 ± 0,29 817
F3 9,09 ± 0,14 27º38" 2,5 ± 0,43 3,27 ± 0,23 798
F4 9,74 ± 0,18 27º51" 2,6 ± 0,26 3,28 ± 0,15 813
F5 10,76 ± 0,67 30º41" 2,8 ± 0,51 4,38 ± 0,45 859
The Effect of Concentration Ratio of Gelatine and Polyvinylpyrrolidone as Binders on the Physical Properties of Red Ginger (Zingiber
officinale Rosc.) Extract Lozenges
149

the granule drying process and can be used to
produce tablet employing the wet granulation
method.
The solubility test was performed to determine
the solubility of the extract. The solubility test was
carried out using water as a solvent resulting in 100
mg of ginger extract dissolved in 1.73 mL of water.
It can be concluded that one part of the extract is
soluble in 17 parts of waters (1:17). The solubility
nature of the extract is soluble in water (Departemen
Kesehatan RI 1979). Based on the result of particle
size analysis (table 2), it was found that 93,79% of
the dried red ginger extract can pass through the 80-
mesh sieve and 80,47% of the extract can pass
through the 100-mesh sieve. The amount of extract
that can pass through 80-mesh sieve in the test is
larger than the certificate analysis of 80%.
In this study, the dry ginger extract is used.
Based on the amount of rendemen dry extract that is
49.5%. The dose of the red ginger extract according
to Zick et al. (2008) is 150 mg. Thus, this study uses
300 mg per tablet.
3.1 The Results of Granule Evaluation
The purpose of the granule evaluation is to examine
the quality of granules in each formula concerning
the excellent granule requirements meeting the
requirements for the compression process to tablets.
The granule loss on drying (LOD) test aims to
investigate how many volatile materials include
water in the drying process and to determine the
moisture of the granules. The results (table 3)
showed that the same ratio (1:1) in F1 resulting a
greater LOD value than F2, while F5 had the biggest
LOD value. This is due to the amount of solvent in
each formula. In a ratio of 1:1, the amount of PVP
used was more than the other formulas, requiring
more ethanol. Although the nature of ethanol was
more volatile than water as a gelatine solvent, a
large amount of gelatine in F5 may affect the PVP
character to be more sensitive to moisture (Siregar
and Wikarsa 2010).
Therefore, F5 with the highest gelatine
concentration had the highest LOD value. The LOD
values in F2, F3 and F4 were not significantly
different since the PVP concentration was not as
large as F1, and the gelatine concentration was not
as large as F5. The results of the LOD value test of
granules in all formulas meet the requirements of 3-
5% (Voigt 1995). The LOD value of the granules
may affect the nature of the tablet produced. It is
concerned that the large LOD value may contribute
to the attachment of granules on the punch at the
time of printing which in turn can affect the weight
and size of the tablet produced (Siregar and Wikarsa
2010).
The results of the granule flow time test of the
five formulas met the requirements of the flow time.
While the ratio of 1:1 and 1:5 did not meet the
requirements of flow time because of the
inappropriate amount of the binding material, F1
was lack of gelatine and F5 contained too much of
gelatine. The inappropriate amount of the binder will
reduce the bonding between the granules particles
(cohesive force), consequently the particle size is not
good enough and the granules are difficult to flow
(Anwar 2002). The results showed that F3 has the
best flow properties, indicating that the 1:3 binder
ratio is the best ratio to obtain the optimal granule
cohesive force so that the granules can flow
smoothly. Flow time is also affected by the moisture
of the granules.
The repose of the angle test aimed to examine
the flow properties of the granules when subjected to
the tableting process. The angle of repose is the
fixed angle between the cone-shaped particles and
the horizontal plane. The results presented that the
angle of repose in the five formulas were different.
The difference may be affected by the cohesiveness
of the granules caused by the binder. The shape, size
and moisture of the granules influence the
magnitude of the repose angle. The value of repose
angle ranges from 25
o
to 45
o
(Siregar and Wikarsa
2010).
The five formulas met the requirements of the
repose angle. It can be concluded that the binder
ratio of F1 and F2 had decreased up to F3. However,
there was an increase in the granular repose angles
in F4 and F5 because of the lack of cohesiveness
among granules affected by the comparison of the
binder concentration. The measurement of the
granular particle size distribution to determine the
granule size and depth was necessary because it can
affect the mixing process. Based on the results of the
study, the granules left in the 18-24 sieve was the
heaviest. According to Agoes et al. (2008), the use
of gelatine solution in the formula affects the size of
the granules. The amount of gelatine solution
negatively influences the size of the granules. A
relatively small size granule has smaller internal
porosity contributing to the greater cohesion force
and causes the granules to pass the mesh size of the
larger sieve hardly. The larger particles of granules
tend to separate from the smaller particles and move
downward while small particles will rise (Lachman
et al. 2003).
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
150

The addition of gelatine concentration to F4 and
F5 did not result in smaller granule size, as shown in
Figure 5, the number of granules left increased in
18-mesh sieves. It can be concluded that the addition
of the gelatine concentration to F4 and F5 increases
the granular size caused by an unbalanced binder
ratio. The larger gelatine concentration in the binder
combination can lead to an increase in the sensitivity
of PVP as a binder (Anwar 2012), to decrease the
performance of the binder combination. The size of
the granules that generally falls on the 12-20 sieve is
840-1680 μm (Agoes 2012).
The large granule size will decrease the granule
mass density. Smaller granules can form a more
compact mass than larger granules (Banker and
Anderson 1994). The result of the granular
compressibility index test after the determination on
100 ml granule for F1 to F5 satisfied the requirement
of good flow property category, the compressibility
≤ 20% (Agoes 2012). The granular density
influences the compressibility of the granules
leading to the decreased internal porosity of the
granules to increase the hardness of the tablets
produced (Anwar 2012). The granules
compressibility of F1 was 2.7% after the addition of
4% gelatine concentration. The addition of gelatine
concentration at F2 decreased the compressibility
value to 2.6% and 2.5% in F3. The addition of
gelatine concentration in F4 and F5 further increased
the percentage of granular compressibility because
the addition of excess gelatine concentration can
disrupt the performance of PVP. Thus, the cohesive
forces between the granules and the decreased
porosity of the granules increased the
compressibility values in F4 and F5.
3.2 Results of Lozenge Evaluation
The purpose of the tablet evaluation is to examine
the quality of tablets in each formula concerning the
requirements of good tablets. The tablet evaluation
includes colour, shape, taste, weight uniformity,
uniformity size, tablet hardness and tablet fragility.
Details of tablet evaluation results can be seen in
Table IV.
The obtained lozenges of all formulas were
brownish white, oval and spicy-sweet. The oval
shape is adjusted to the availability of the punch for
a tablet weight of 1 gram. Tablets shape generally
are round but it can also be oval or other shapes. In
the pharmaceutical industry, tablet shape is used as a
product characteristic (Agoes 2012). Spicy taste on
lozenge was evident because of the lack use of
sweetener. In addition, the heating can change the
gingerol to be spicier yet it does not reduce the
pharmacological effects of the active substances
(Heinrich et al. 2009). Lozenges dissolve slowly
inside the mouth, so the formula having the highest
hardness lasts longer in the mouth. The spicy after-
taste of the tablets in the F3 with the hardness of
15.95 Kgf had the most unpleasant taste. F5
obtained the most delicious taste with a hardness of
12.31 Kgf and had the largest amount of gelatine.
Gelatine as a natural ingredient in the lozenge
formula can improve the characteristics and texture
of the lozenge surface when dissolving in the oral
cavity (Siregar and Wikarsa 2010).
The tablet produced showed color patches.
Striking and uniformly dispersed dark areas on the
surface of the tablet were due to the different color
of the active substance and other tablet excipients.
Such spots may arise due to the use of natural
materials in the lozenge formula (Badan Pengawas
Obat dan Makanan 2012). In addition, the use of
dextrose in the formula can contribute to the
brownish color if the temperatures given is above
37ºC (Siregar and Wikarsa 2010).
The tablet weight uniformity test was performed
by testing the weights of 20 tablets per formula. The
Table 4: The red ginger lozenge test results.
Evaluation F1 F2 F3 F4 F5
Organoleptic:
a. Shape Oval Oval Oval Oval Oval
b. Smell Specific Specific Specific Specific Specific
c. Color White Brownish
White
Brownish
White
Brownish
White
Brownish
White
Brownish
Thick
(
m
m
)
5,75 ± 0,01 5,75 ± 0,02 5,75 ± 0,01 5,75 ± 0,02 5,75 ± 0,02
Length (mm) 23,05 23,05 23,05 23,05 23,05
Width (mm) 10,45 10,45 10,45 10,45 10,45
Weigh (g) 1,008 ± 0,01 1,035 ± 0,01 1,030 ± 0,01 1,027 ± 0,01 1,025 ± 0,01
Friabilit
y
(
%
)
0,442 ± 0,01 0,674 ± 0,01 0,174 ± 0,01 0,337 ± 0,01 0,571 ± 0,01
Hardness
(
K
g
f
)
9,01 ± 0,71 14,15 ± 0,80 15,95 ± 0,76 13,45 ± 0,87 12,31 ± 0,68
The Effect of Concentration Ratio of Gelatine and Polyvinylpyrrolidone as Binders on the Physical Properties of Red Ginger (Zingiber
officinale Rosc.) Extract Lozenges
151

results of the test for F1 to F5 fulfilled the
requirement as no two tablets having a weight
deviation of 5% from the mean tablet weight and no
one tablet having weight deviation of 10% from the
average weight (Departemen Kesehatan RI 1979).
The tablet size uniformity test was performed by
measuring the width, length and thickness of the
tablet. There was no difference in length and width
of the lozenges as they were determined by the size
of the punch. If there was a difference in length and
width of the tablet, it might be due to the moisture of
granules causing a granule attached to the punch.
However, there were differences in the thickness of
the tablets due to the rise and fall of punch in the die
hole. This study used a single punch tablet machine
with only a pair of punch. The downward movement
of the bottom punch along with the up movement of
punch to a certain distance during the process of
filling the die hole resulted in the granule down due
to the gravitational effect. The distance between the
punch can be different, therefore there was a
thickness difference in the tablets produced yet it
was not significant.
The lozenge hardness requirement is 7-14 Kgf
(Hadisoewignyo and Fudholi 2013). The results
(tabble 4) reported that all formulas had different
hardness values; F2 and F3 did not meet the
requirements. The hardness of F2 and F3 exceeding
the requirement were 14.15 and 15.95 Kgf
respectively. The gelatine properties can draw water
into its bonds, resulting in a more spherical and
homogeneous granule and enhancing the cohesive
force between granular particles which in turn
increase the tablet hardness (Anwar 2012). The
characteristic of PVP is that the higher concentration
dissolved in alcohol, the stronger the liquid bridge
formed; so that the drying process of the solid bridge
formation is also stronger resulting in reduced
granular porosity increasing the greater granule
density and the tablet hardness (Siregar and Wikarsa
2010).
The addition of gelatine concentration on F4 and
F5 decreased the tablet hardness. The interaction
between PVP and gelatine in F4 and F5 can reduce
the performance of the binder because of the second
characteristic of the material. The gelatine properties
of having a low melting point, easily melt when
exposed to heat, causes the interaction with PVP
tending to be sensitive to water vaporization (Anwar
2012). When it is exposed to the pressure on the
machine, the tablet becomes moist and the bond
strength between granular particles is decreased
resulting in reduced tablet hardness (Siregar and
Wikarsa 2010).
The tablet fragility test was performed to
determine the tablet physical stability from
mechanical shock effects during the manufacturing,
packing and transportation process. The results of
tablet fragility test obtained from F1 to F5 fulfilled
the requirement that was below 0.8% (Voigt 1995)
due to the character of the binder components. The
properties of gelatine that can absorb water into its
bonds, result in a more spherical and homogeneous
granule and increase the cohesion force between
granular particles leading to increase tablet hardness
and decrease the tablet fragility value (Anwar 2012).
The PVP characteristic is that the higher the
concentration dissolved in alcohol, the stronger the
liquid bridge is formed; thus, the process of drying
solid bridge formation is also stronger which in turn
reduce the granular porosity and increase the granule
density leading to increase the tablet hardness and
reduce the tablet fragility (Siregar and Wikarsa
2010).
The addition of gelatine concentration on F4 and
F5 decreased the tablet fragility. The interaction
between PVP and gelatine in F4 and F5 decrease the
force of binding due to the gelatine (Anwar 2012).
The more gelatine leads to the less tablet fragility.
Therefore, tablet hardness decreases, and tablet
fragility increases (Siregar and Wikarsa 2010).
Based on the results of the data analysis, there is
a significant difference in each ratio of PVP and
gelatine concentration as a binder against the tablet
hardness and fragility. The results of the hardness
test identified that the increased hardness and
decreased fragility of the tablets were from the 1:1,
1:2 and 1:3 binding ratio. Also, there was a decrease
of the hardness and an increase of fragility in the 1:4
and 1:5 binder ratio.
4 CONCLUSIONS
The comparison of gelatine and PVP concentration
as the binding agent of red ginger lozenge provide
significant differences in the tablet hardness and
fragility. The ratio of gelatine and PVP
concentrations in F3 with a ratio of 1:3
concentrations identified as the highest hardness
value of 15.9 Kgf and the lowest vulnerability of
0.2%.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
152

ACKNOWLEDGEMENTS
We would like to express our gratitude to Faculty of
Pharmacy and Sciences of Universitas
Muhammadiyah Prof. DR. HAMKA for the
contribution in the Laboratory equipment and for
joining on MICH-PHD International Seminar.
REFERENCES
Agoes G. 2008. Seri Farmasi Industri-1. Pengembangan
Sediaan Farmasi, Edisi Revisi and Perluasan. Penerbit
ITB. Bandung. Hlm. 286-335.
Agoes G. 2012. Seri Farmasi Industri-6: Sediaan Farmasi
Padat. Penerbit ITB. Bandung. Hlm. 73-79,224-
234,245.
Agubata C, Onunkwo GC, Ugwu CE, Chime SA. 2012.
Physical and mechanical effects of starch-gelatin
binary binder mixtures on sodium salicylate tablets,
Journal of Chemical and Pharmaceutical Research, 4
(3), 1625-1628.
Agus S, Sudirman I, Feranses SP. 2007. Pengaruh Ukuran
Granul terhadap Kadar Solutio Gelatin sebagai Bahan
Pengikat terhadap Migrasi Vitamin B6. Jurnal
PHARMACY, Fakultas Farmasi Universitas
Muhammadiyah Purwokerto. Purwokerto. ISSN 1693-
3591. Hlm 3-4.
Departemen Kesehatan RI. 1979. Farmakope Indonesia.
Edisi III. Jakarta: Departemen Kesehatan RI; Hlm. 6,
7, 93, 354, 378, 535, 807, 840.
Departemen Kesehatan RI. 1980. Materia Medika
Indonesia. Jilid IV. Jakarta: Departemen Kesehatan
RI; Hlm 78.
Badan Pengawasan Obat and Makanan Republik
Indonesia. 2012. Pedoman Tenologi Formulasi
Sediaan Berbasis Ekstrak. Volume 1. Jakarta: Badan
Pengawasan Obat and Makanan Republik Indonesia;
Hlm. 16- 18, 33 – 34.
Anwar E. 2012. Eksipien dalam Sediaan Farmasi
Karakterisasi and Aplikasi. Cetakan pertama. Jakarta:
PT. Dian Rakyat. Hlm.5, 26-92.
Banker GS and Anderson NR. 1994. Tablet. Editor:
Lahman L. Teori and Praktek Farmasi Industri. Edisi
III. Jilid II. Penerjemah: Suyatmi. UI Press. Jakarta.
Hlm. 643-703.
Hadisoewignyo L and Fudholi A. 2013. Sediaan Solida.
Yogyakarta: Pustaka Pelajar. Hlm.11,19,21,35.
Hamed E, Moe D. Khankari R. and Hontz J. 2005. Binder
and solvent dalam: Handbook of Pharmaceutical
Granulation Technology. Second edition. Taylor &
Francis Group. London; Hlm. 109-119.
Heinrich M, Barners J, Gibbons S, Williamson EM. 2009.
Farmakognosi and Fitoterapi. Alih bahasa Amalia H,
Hadinata. Penerbit EGC. Jakarta. Hlm. 235 – 236.
Lachman L, Lieberman HA. 2003. Pharmaceutical Dosage
Forms: Tablets Volume 2. United States of America,
New York. Hlm. 254, 299-300, 330, 714.
Liauw NR. 2012. Optimasi Formula Lozenge ekstrak
Rimpang Kencur (Kaempferia galanga L.)
menggunakan kombinasi PVP K-30 dengan Gelatin
Sebagai bahan Pengikat. Skripsi. Fakultas Farmasi
Unika Mandala, Surabaya. Hlm. 50, 51,52,96.
Moore, R., Lopes, J., 1999. Paper templates. In
TEMPLATE’06, 1st International Conference on
Mutmainah MD. 2005. Pengaruh PVP Sebagai Pengikat
Terhadap Sifat Fisik Lozenge Ekstrak Jahe (Zingiber
officinale Roxb). Skripsi. Fakultas Matematika and
Ilmu Pengetahuan Alam Universitas Islam Indonesia,
Jogjakarta.
Peters D. 1989. Medicated Lozenges, in: Lieberman HA,
Lachman L.(eds), Pharmaceutical Dosage Form.
Second edition. Volume 1. Marcel Dekker Inc. New
York. Hlm. 419, 420.
Rowe RC, Sheskey JP, Quinn ME. 2009. Handbook of
Pharmaceutical Exipient. Sixth Edition. The
Pharmaceutical Press. London. Hlm. xxviii – 917.
Sari E, Astuti IY. 2010. Formulasi Tablet Kunyah Ekstrak
Rimpang Jahe Merah (
Zingiber officinale Roxb)
dengan Bahan Pengisi Sorbitol and Laktosa and
kontrol kualitasnya. Dalam Jurnal Farmasi Indonesia.
Vol. 07 (02) ISSN 1693-3991. Hlm 67-75.
Siregar CJP, Wikarsa. 2010. Teknologi Farmasi Sediaan
Tablet Dasar-Dasar Praktis. Universitas Indonesia
Press. Jakarta. Hlm. 35, 193-195, 202, 505-523.
Voigt R. 1995. Buku Pelajaran Teknologi Farmasi. Edisi
V. Penerjemah Soendani Noerono. UGM
Press.Yogyakarta. Hlm. 160-161, 166, 168, 223, 564,
568, 570.
Zick S, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP,
Alrawi DP, Alrawi S, Feng MR, Brenner DE. 2008.
Pharmacokinetics of 6-gingerol, 8-gingerol, 10-
gingerol and shogaol and conjugate metabolites in
healthy human subjects. Cancer Epidemiol
Biomarkers Prev. 17 (8): 1930-35.
The Effect of Concentration Ratio of Gelatine and Polyvinylpyrrolidone as Binders on the Physical Properties of Red Ginger (Zingiber
officinale Rosc.) Extract Lozenges
153
