
A Simple Method for Isolation of Citral using Column
Chromatography
Adlina Savira,
1
Achmad Syahrani,
1
Marcellino Rudyanto
1,2*
1
Department of Pharmaceutical Chemistry,
Faculty of Pharmacy, Airlangga University, Jl. Dharmawangsa Dalam,
Surabaya, Indonesia.
2
Institute of Tropical Disease, Airlangga University, Jl. Mulyorejo, Surabaya, Indonesia.
Keywords: citral, Cymbopogon citratus, chromatograhy, separation.
Abstract: Citral is the main component of lemongrass (Cymbopogon citratus) oil. This compound has biological
activities such as antibacterial, antifungi, analgesic, and antiinflammation. Citral also has importance for
its use as starting material for the synthesis of Vitamin A. Due to the broad utilisation of citral, it is
important to develop method of isolation which is relatively simple, low cost, but able to give pure citral
in high yield. Materials and method. Citral was isolated from commercially available lemongrass oil by
simple column chromatography using silica gel as stationary phase. Elution was carried out in isoctaric
mode. Mobile phase was chosen among hexane – diethyl ether, hexane – ethyl acetate, and hexane –
ethanol based on separation factor and Rf value on thin layer chromatography. Optimum ratio of
sample and stationary phase was also optimized based on isolation yield. Isolated citral was analyzed by
gas chromatography – mass spectroscopy. The best separation factor on TLC was obtained from hexane
– ethyl acetate (97:3) as eluent. The best yield (49,61 ± 2,59 %) was obtained when stationary phase
was used at ratio 20:1 to sample.
1 INTRODUCTION
Lemongrass (Cymbopogon citratus) is a fast-
growing aromatic grass, growing to about 1 meter (3
feet) high with long, thin leaves (
Joga Rao et al.,
2015). It is native to Sri Lanka and South India and is
now widely cultivated in the tropical areas of
America and Asia, including Indonesia (
Ravinder et
al., 2010).
Lemongrass is also one of the main
essential oil producing plants in Indonesia (
Marques
& Kaplan, 2013)
. In Indonesia, it is commonly known
as sereh dapur and its stem is used as a spice
because of its distinctive lemon-like aroma. This
lemony odor is due to its high content of the
aldehyde citral, which ranges from 65% to 85%
(
Ravinder et al., 2010).
Citral is a component of essential oils that can be
found in a variety of plants of the genus Citrus. It is
volatile, has a lemon-like odor and a form of light
yellowish oil (
Pushpakumari & Vatakencherry, 1985).
Citral is a mixture of two compounds, namely
geranial (Citral-a or trans-citral) and neral (Citral-b
or cis-citral) (
Carbajal et al., 1989). Besides in plants
of the genus Citrus, citral is also contained in
essential oils of lemon myrtle (90-98%), Litsea
citrate (90%), Litsea cubeba (70-85%), and
Cymbopogon citratus (65-85%) (
Purwanto et al.,
12
Savira, A., Syahrani, A. and Rudyanto, M.
A Simple Method for Isolation of Citral Using Column Chromatography.
DOI: 10.5220/0008356900120020
In Proceedings of BROMO Conference (BROMO 2018), pages 12-20
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2016). This compound has various benefits, such as
antibacterial, antifungal, antiprotozoal, ascaricidal,
analgesic, antiinflammatory, and antioxidant effects
as well as hypoglycemic, hypolipidemic and
hypochesterolamic effects (
Ravinder et al., 2010).
Citral is also an important starting compound for the
synthesis of vitamin A (retinol) (
Purnamasari et al.,
2016)
. Due to the broad utilisation of citral, it is
important to develop method of its isolation which is
relatively simple, low cost, but able to give pure
citral in high yield.
One of the oldest isolation methods of citral is by the
reaction of adducting with sodium bisulfite (
Carbajal
et al., 1989)
. Unfortunately, it is not the purest
method because sodium bisulfite can adduct other
aldehydes and methyl ketones available in
lemongrass oil. Therefore, other compounds which
are also present in lemongrass oil such as aldehyde
compounds (e.g. citranelal), or methyl ketone
compounds (e.g. methyl heptenone), can also be
adducted together with citral (
Carbajal et al., 1989).
Steam distillation as well as partial fraction
distillation methods have also been used. In these
methods, isolation occurs successfully and produces
high-purity citral (
Joga Rao et al., 2015; Carbajal et al.,
1989). However, these methods have their
shortcomings. Separation of components such as
geraniol and nerol from citral is difficult because
they have a boiling point which differ only a few
degrees Celsius from citral. Furthermore, citral is
labile to high temperature, hence overheating can
lead to rearrangement, polymerization, and even
destruction of the citral (
Oxtoby et al., 2008).
Other methods already used include preparative thin
layer chromatography and column chromatography
(
Carbajal et al., 1989; Oxtoby et al., 2008). Preparative
thin layer chromatography is only able to produce
isolates in very small amounts (
Pushpakumari &
Vatakencherry, 1985; Oxtoby et al., 2008). On the
contrary, column chromatography can be used on a
large scale. Column chromatography is very
important in industrial use because its methods can
easily be adopted from the laboratory scale to the
production scale (
Bidlingmeyer, 1989).
Therefore, in this study, column chromatography
method was selected to isolate citral from
lemongrass oil. For the optimization of mobile phase
to be used for the isolation method, different
mixtures of mobile phase used in other experiments
were compared, such as mixtures of hexane-ether
based on Pushpakumari and Vatakencherry (1986),
mixtures of hexane-ethyl acetate based on Scott et
al. (1989) , and mixtures of hexane-ethanol based on
Purnamasari et al. (2016) of different ratios. The
goal was to determine which mobile phase mixture
at which ratio can produce the best resolution to
isolate citral. In addition, silica gel as stationary
phase and different sample-to-silica ratios were
compared to determine which ratio is the most
efficient for sample-loading based on the isolate’s
yield percentage obtained from each ratio. The
isolation results were further tested qualitatively and
quantitatively using gas chromatography -mass
spectrometry (GC-MS) and compared with
commercial citral as a standard.
2 MATERIALS AND METHODS
2.1 Materials
Lemongrass (C. citratus) oil was obtained from CV
M & H Farm. Commercial citral was obtained from
Aldrich. Hexane p.a., ethanol p.a., ethyl acetate p.a.
A Simple Method for Isolation of Citral Using Column Chromatography
13

as well as silica gel 60 (0.063-0,200 mm) were
obtained from Merck. Ether p.a. were obtained from
Riedel-de Haen AG. The anisaldehyde reagents were
obtained from Merck. Iodine used to stain the spot
on TLC plate was obtained from Kimia Farma.
Filter paper, thin layer chromatography (TLC)
chamber, TLC silica gel 60 F254 were obtained
from Merck. To apply the lemongrass oil and the
fraction onto the TLC plate for the determination of
mobile phase mixture to be used for column
chromatography, 2 μl capillary pipes were used.
Chromatography was then performed in a 2,5 x 50
cm column and the fractionation results were
collected in 10 ml vials. The products were
subjected to gas chromatography (GC). GC was
performed on an Agilent Model 6890N, equiped
with mass spectrometer (MS) Agilent 5973 with
inert mass spectrum detector (MSD) and Head Space
Sampler Model 7697A HSS
2.2 Methods
2.2.1 Determination of Mobile Phase
Mixture for Column Chromatography
The solvent mixtures to be compared were hexane -
ether (97:3, v/v), hexane - ethyl acetate (97:3, v/v),
and hexane - ethanol (97:3, 98:2, 99:1, v/v).
Both the essential oil of lemongrass and the
commercial citral were applied on to the TLC plate.
The plate was then inserted into the saturated
chamber to be eluated with 5 ml of each solvent
mixture. Spots from the eluation process were
observed under UV lamps of λ 254 nm and also
stained by spraying anisaldehyde reagents or by
putting the TLC plate into chamber containing
iodine.
The best eluent for column chromatography was
chosen by taking Rf value and the separation factor
(α) of the standard spot (Rf 1) against the nearest
spot (Rf 2) into account. The separation factor (α) is
calculated by the formula:
=
10
2.2.2 Preparation of the Column for Column
Chromatography
The amount of silica gel needed for fractionation
depends on the results of the solvent mixture
optimization. The sample- to - silica gel ratios were
selected according to the guideline from Reichsstein
et al., (1960), as follows: 1:20, 1:35 and 1:50 if the
obtained separation factor (α) ≥1,5 ; 1:65 and 1:80 if
the obtained α ≤1,5.
2.2.3 Fractionation of Citral from
Lemongrass Oil Using Column
Chromatography and the
Determination of the Optimum
Sample-to-Adsorbent Ratio
Selected eluent was prepared for column
chromatography and 1 gram of lemongrass oil was
weighed before the fractionation. The flow rate was
set to approximately 1 drop per second. The droplets
of the eluent from the column were collected in
vials, each would contain 10 mL of droplets.
The TLC test was performed on every fifth vial.
The TLC test used the mobile phase of the column
chromatography. Spots from the elution were
observed under UV light 254 nm and stained by
anisaldehyde reagent or iodine vapor.
Fractions having the same Rf as the standard were
compared. This citral fraction was evaporated in the
rotary evaporator, until a constant weight was
obtained. Then the yield percentage of citral
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
14
obtained from fractionation was calculated with the
formula:
% =
100
The optimal sample-to-adsorbent ratio for column
chromatography was determined based on the yield
percentage of the obtained citral.
2.2.4 Identification of Obtained Citral Using
Gas Chromatography-Mass
Spectrometry
Citral obtained from fractionation with column
chromatography was injected into GC-MS. The gas
chromatography was equipped with a 30 m 0.25
mm, 0.25 mm film thickness column. Helium was
used as mobile phase with an average flow of 1.0
ml/min. The condition of the GC-MS was according
to the study from Bayala et al. (2018): Oven
temperature program was from 50° C (3.2 min) to
300° C at 8° C/min, 5 min post run at 300° C.
Sample was injected in split mode, injector and
detector temperature being at 250° C and 280° C
respectively. The peaks generated in the total ion
chromatogram are identified by comparing the mass
spectra obtained with the mass spectra found in the
GC-MS libraries.
3 RESULT AND DISCUSSION
3.1 The Optimum Mobile Phase
Mixture for Column
Chromatography
The data obtained from the TLC test with various
mixture of mobile phase (as described beforehand)
are listed in Table 1 below.
The best eluent for column chromatography was
chosen by taking Rf value and the separation factor
(α) of the spots on the TLC plate into account.
The optimal Rf value is 0.15-0.30.
10
The selected
solvent mixture is therefore hexane-ethyl acetate
(97: 3, v/v), due to its separation factor of ≥ 2.00
meaning that the separation between two compound
occured easily and its Rf value of 0.24 which lied
within the accepted range.
10
Table 1: Rf Values of Lemongrass Oil and Comercial Citral on TLC Using Various Mobile Phases
Mobile phase
mixture and its
ratio (v/v)
Rf value of essential oil Rf value of citral standard α
1 2 3 1 2
Hexane - ether
(97:3)
0,06 0,18 - 0,06 0,18 3,44
Hexane - ethyl
acetate (97:3)
0,03 0,24 0,39 0,03 0,24 2,02
Hexane -
ethanol (97:3)
0,09 0,79 0,88 0,09 0,79 1,94
Hexane -
ethanol (98:2)
0,05 0,49 0,68 0,05 0,49 2,21
Hexane -
ethanol (99:1)
- 0,17 - - 0,17 -
A Simple Method for Isolation of Citral Using Column Chromatography
15
3.2 Fractionation of Citral from
Lemongrass Oil Using Column
Chromatography and the
Determination of the Optimal
Sample-to-Adsorbent Ratio
After fractionation of citral from the lemongrass oil
as described before, the results obtained are listed in
Table 3.
Table 2: Fractionation Results of Citral From Lemongrass Oil
Sample-to-Adsorbent Ratio Volume of the eluent (ml) Volume of the collected fraction (ml)
1:20 400 200,00 ± 10,00
1:35 600 286,67 ± 5,77
1:50 700 326,67 ± 20,82
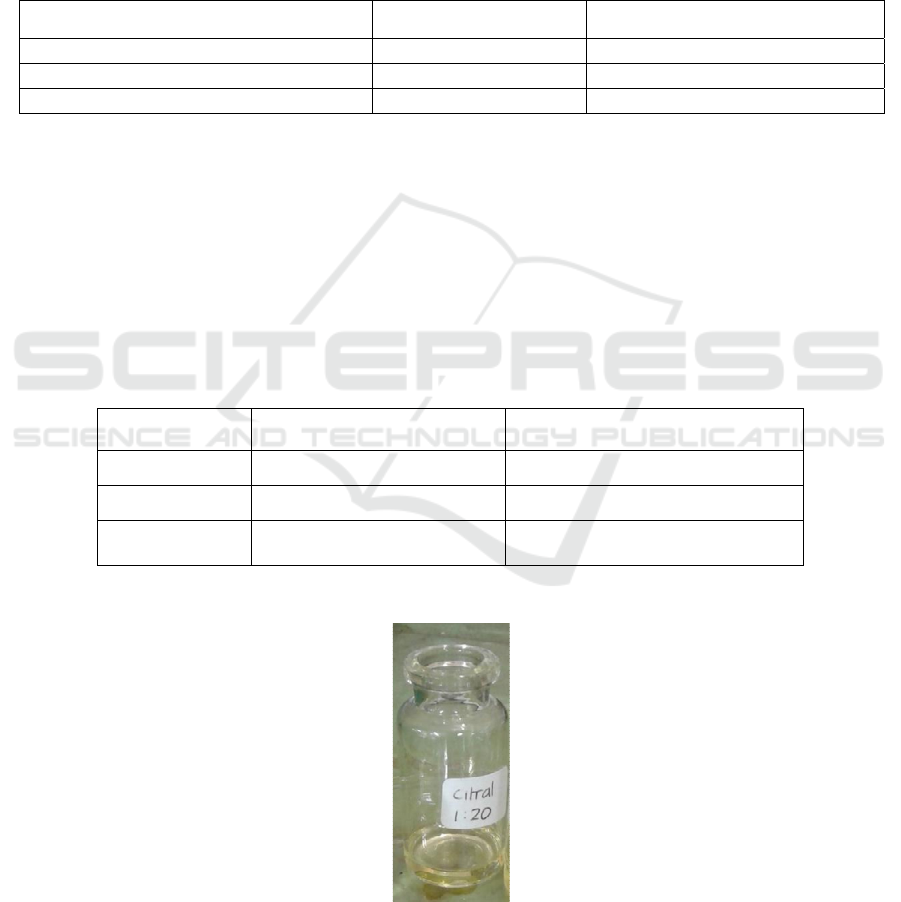
3.3 Organoleptical Analysis of the
Obtained Citral
The results from the organoleptical analysis were
compared with the data obtained from literature. The
organoleptical characteristics of the citral obtained
from the isolation are as follows.
Table 3: The Organoleptics of the Citral obtained from Fractionation Compared with Data from Literature
Data from literature (Marques
et al., 2013)
Obtained citral
Color Pale yellow Bright yellow
Form Thick oil Thick, oily
Odor Lemony odor Lemony/orange-like odor
Figure 1: Pure citral obtained from the fractionation
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
16
3.4 Analysis of Weight and the Yield
of the Obtained Citral
After the isolation of citral with different sample-
to-adsorbent ratios, the weight of pure citral and
the percentage of citral yield have been obtained as
indicated in Table 5. It was obtained from three
replications that the sample-to-adsorbent ratio of
1:20 can produce the highest yield percentage
when compared to the other ratios.
Table 4: Weight and the Yield Percentage of the Obtained Citral
Weight and the Yield Percentage of the Obtained Citral
Sample – to Adsorbent ratio 1:20 1:35 1:50
Mean yield percentage 49,61 ± 2,59 % 45,66 ± 2,84 % 38,30 ± 1,72 %
3.5 Identification of Obtained Citral
Using Gas Chromatography-Mass
Spectrometry GC-MS
Further qualitative and quantitative identification of
the obtained citral from was done using gas-
spectrometry mass chromatography (GC-MS) to
determine the purity of the isolate. The results of the
normalization percentage of neral (1) was 11,50%
and geranial (2) compound was 77,95%. Thus, the
obtained citral from the fractionation using 1:20
(w/w) sample – to - adsorbent ratio had the purity of
89.45%.
2
1
A Simple Method for Isolation of Citral Using Column Chromatography
17

3.6 Discussion
In this research, the column chromatography method
of citral isolation from lemongrass oil (Cymbopogon
citratus) was optimized. The goal was to determine
which mobile phase mixture at which ratio can
produce the best resolution to isolate citral and
which sample-to-adsorbent ratio was the most
efficient for sample-loading. Mobile phase affects
the separation factor, while sample-to-adsorbent
ratio affects the effective theoretical plate number
of the chromatographic system.
10
Both separation
factor and the number of effective theoretical plates
are important parameters affecting the resolution of
a chromatographic system.
10,11
At the beginning of
the study, eluent mixture was selected using thin
layer chromatography (TLC) based on Rf value and
the separation factor (α) of the spots. The eluent
mixtures tested were hexane ether (95: 5, v/v),
hexane - ethyl acetate (97: 3, v/v), hexane - ethanol
(97: 3; 98: 2; and 99: 1, v/v). The optimal Rf value is
0.15-0.30, while the price of the optimal separation
factor is ≥ 1.5, meaning the separation between
compounds in essential oils was relatively easy.
Hence, a hexane-ethyl acetate solvent was selected
(97: 3, v/v) since the separation factor ≥ 2.00
classified as “easy separation” and the Rf value
within the range was 0.24.
10
After determining the
solvent mixture used as the mobile phase for
fractionation, fractionation process was performed
by column chromatography. In this study, an
isocratic chromatography method was used. The
chosen stationary phase was silica gel. To separate
polar compounds such as aldehydes (e.g., citral), a
polar stationary phase such as silica gel was required
since the surface comprises a highly polar hydroxyl
group and interacts with the dipole of a polar
solute,
12
hence silica gel was selected. At this stage,
the three sample-to-adsorbent ratios were compared
based on the yield percentage of the isolated citral.
The three sample-adsorbent ratios were 1:20, 1:35,
and 1:50 (w/w), respectively, chosen based on
guidelines from Reichstein et al. (1960).
11
With
each ratio, fractionation was performed with three
replication. The fractionation results were
evaporated until the weight stayed constant and the
yield percentage was calculated.
The results of the isolates obtained were yellow
liquid compounds smelling like lemon / orange. The
results of this organoleptic observation were
consistent with the data from the literature, which
states that the citral is a pale yellow liquid oil-like
compound smelling like lemon
4,8
.
After three replication, the largest yield percentage
of 49,61 ± 2,59 %. was obtained by the 1:20 (w/w)
sample – to - adsorbent ratio. This may occur
because the larger sample-to-adsorbent ratio, more
citral was retained on the surface of the silica gel.
The more the amount of silica, the greater the
surface area of the stationary phase and the greater
number of analyte interacting with the stationary
phase.
10
Therefore, it can be assumed that more
citral interacts with the polar hydroxyl group of
silica gel so that in larger amounts of silica the more
citral is left in the stationary phase. The variation in
the yield percentage of citral in one replication
compared to another could be caused by the drying
process, where the citral coalesced along with the
solvent.
Further qualitative and quantitative identification of
the obtained citral was done using gas-spectrometry
mass chromatography (GC-MS) to determine the
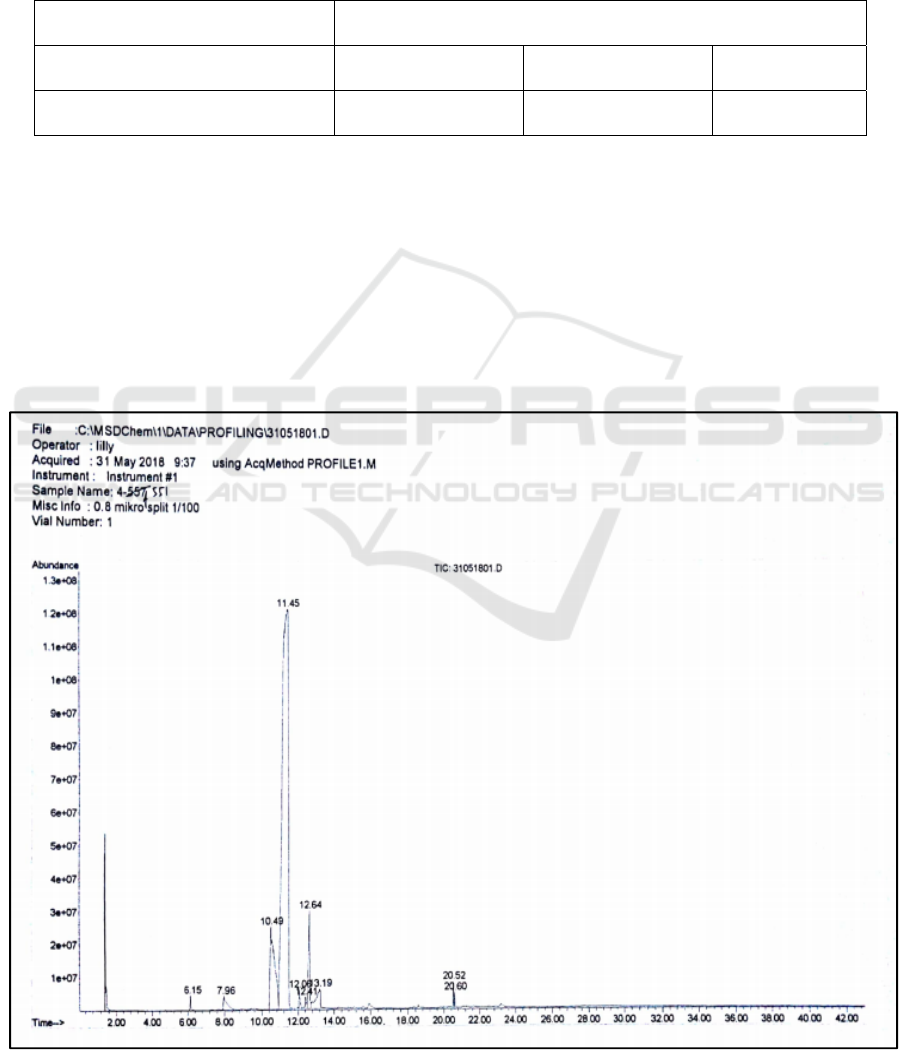
purity of the isolates. In chromatogram the peak of
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
18

neral can be seen at retention time of 10.49 min and
peak of geranial at retention time of 11.45 min.
Citral is a mixture of cis-citral compounds (also
called neral) and trans-citral compounds (also called
geranial).
5
The difference in retention time of both
compounds could be caused by the interaction of the
compound with the stationary phase in the gas
chromatographic system. The column used was
nonpolar, therefore the polar compound came out
first while the more nonpolar compounds would be
retained longer in the column. It can be concluded
that trans-citral compounds (neral) are more polar
than cis-citral (geranial) compounds.
8
The
normalization percentage of neral was 11,50% and
from geranial 77,95%. Thus, if added, the isolate
obtained by a 1:20 (w/w) sample – to - adsorbent
ratio had the purity of 89.45%.
All in all, the method of fractionation using column
chromatography optimized in this study has
provided pure isolate with high yield, despite using
an isocratic elution. Hence, the optimized method
has the advantages of an isocratic elution such as the
relatively fewer solvents needed if compared to
gradient elution. Another advantage of the optimized
method would be its suitability for preparative use in
large amounts. Yet further studies need to be done in
order to adapt this optimized method to larger scale.
4 CONCLUSIONS
The optimal mobile phase mixture to isolate the
citral from lemongrass oil (Cymbopogon citratus)
using column chromatography method is hexane-
ethyl acetate with a ratio of 97: 3 (v/v), and the
optimal sample-to-adsorbent ratio to isolate citral
from the lemongrass oil (Cymbopogon citratus)
using column chromatography method is 1:20
(w/w).
REFERENCES
Bayala, B., Bassole, I.H., Maqdasy, S., Baron, S.,
Simpore, J. and Lobaccaro, J.M.A., 2018.
Cymbopogon citratus and Cymbopogon giganteus
essential oils have cytotoxic effects on tumor cell
cultures. Identification of citral as a new putative anti-
proliferative molecule. Biochimie. p. 1-9.
Bidlingmeyer, B. A. (ed.)., 1989. Preparative liquid
chromatography. Journal of Chromatography Library
38. Amsterdam: Elsevier.
Ella, M.U.E., Sumiartha, K.S., Suniti, N.W., Sudiarta, I.P.
and Antara, N.S., 2013. Uji Efektivitas Konsentrasi
Minyak Atsiri Sereh Dapur (Cymbopogon citratus
(DC.) Stapf) terhadap Pertumbuhan Jamur Aspergillus
Sp. Secara in vitro. E-Jurnal Agroekoteknologi
Tropika (Journal of Tropical Agroecotechnology),
2(1), p. 40-48.
Joga Rao, H., Kalyani, G., King, P., 2015. Isolation of
Citral from Lemongrass Oil Using Steam Distillation:
Statistical Optimization by Response Surface
Methodology. Int. J. Chem. Sci.: 13(3), p. 1305-1314
Marques, A.M. and Kaplan, M.A.C., 2013. Preparative
isolation and characterization of monoterpene isomers
present in the citral-rich essential oil of Pectis
brevipedunculata. Journal of Essential Oil Research,
25(3), p.210-215.
Oxtoby, D.W., Gillis, H.P., Nachtrieb, N.H., 2008.
Principle of Modern Chemistry. Sixth Edition.
Belmont: Thomson Brooks/Cole.
Pushpakumari, K.N. and Vatakencherry, P.A., 1985. A
new method of estimation of citral in lemon grass oil
by physical separation of citral. V International
Symposium on Medicinal, Aromatic dan Spice Plants
188, p. 241-246.
Carbajal D., Casaco A., Arruzazabala L., Gonzalez,
Tolon Z., 1989. Pharmacological study of
A Simple Method for Isolation of Citral Using Column Chromatography
19

Cymbopogon citratus leaves. JEthnopharmacol 25(1),
p. 103-107.
Purwanto, D. A., Rudyanto, M., Annuryanti, F. 2016.
Produksi Vitamin A untuk Fortifikasi Minyak Goreng
Sawit dengan Bahan Baku Minyak Sereh Dapur
(Cymbopogon citratus) Mengacu Permenperin
No.87/M-IND/PER/12/2013. Laporan Akhir Penelitian
Prioritas Nasional Masterplan Percepatan dan
Perluasan Pembangunan Ekonomi Indonesia 2011-
2025 (PENPRINAS MP3EI 2011-2025), Surabaya:
Universitas Airlangga.
Purnamasari, P., Nashrianto, H. and Rusman, M.S., 2016.
Isolasi dan Identifikasi Senyawa Citral dalam Sereh
Dapur (Cymbopogon citratus) Menggunakan
Kromatografi Lapis Tipis Preparatif (KLTP) Dan GC-
MS. Skripsi, Fakultas Matematika dan Ilmu
Pengetahuan Alam Universitas Pakuan, Bogor, p. 1-9.
Ravinder, K., Pawan, K., Gaurav, S., Paramjot, K., Gagan,
S. and Appramdeep, K., 2010. Pharmacognostical
investigation of Cymbopogon citratus (DC) Stapf.
Scholars Research Library, 2, p.181-189.
Scott, R. P. W., 1989. Journals of Chromatography,
Amsterdam: Elsevier.
Wilson, I.D (ed)., 2001. Encyclopedia of separation
science. Surrey: Academic Press.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
20
