
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata
Presl. Usin
g
UPLC-QToF-MS/MS
Burhan Ma’arif*
1, 2
, Mangestuti Agil
3
1
Doctoral Program of Pharmaceutical Sciences, Department of Pharmacognosy and Phytochemistry,
Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia.
2
Department of Pharmacy, Faculty of Medical and Health Science, Maulana Malik Ibrahim
State Islamic University, Malang, Indonesia
3
Department of Pharmacognocy and Phytochemistry, Faculty of Pharmacy,
Universitas Airlangga, Surabaya, Indonesia
Keywords: Marsilea crenata Presl., Metabolite Profiling, UPLC-QToF-MS/MS, Phytoestrogens
Abstract: Marsilea crenata Presl. is a plant that widely used as traditional food in Surabaya, Indonesia. Although in
some research it was known contain phytoestrogens which have activity in bone formation, the
phytochemical properties of M. crenata has not been completely confirmed yet. The aim of this research
was to determine the metabolite profile of ethyl acetate extract of M. crenata using UPLC-QToF-MS/MS,
which can be used as a reference for further research and utilization of M. crenata. Dried powder of M.
crenata was extracted with n-hexane followed by ethyl acetate. The 100 ppm of ethyl acetate extract in
DCM and methanol then injected 5 µl each into the UPLC-QToF-MS/MS. The results were analyzed by
Masslynx 4.1 software, and showed various types of compounds, either detected compounds (36
compounds), or unknown compounds.
1 INTRODUCTION
Marsilea. crenata Presl. is an aquatic plant that
widely used as an ingredient for traditional food in
Surabaya, Indonesia (Nurjanah and Abdullah, 2012;
Ma’arif et al., 2016).
Figure 1: Marsilea crenata Presl.
Some of the research that had been done showed
that 96% ethanol extract, n-hexane extract, and ethyl
acetate extract of M. crenata leaves can inhibit
osteoporosis in female mouse (mus musculus) with
mechanism of bone formation improvement
(Laswati, 2011; Aemi, 2012; Adityara, 2017;
Widiasari, 2017). Other studies were also showed
that n-hexane extract of M. crenata leaves can
increase the alkaline phosphatase production in
MC3T3-E1 preosteoblast cell differentiation
process, which indirectly also play a role in bone
formation improvement (Ma’arif et al., 2018).
This activity appears to be suspected because of
the phytoestrogens content in M. crenata, where
phytoestrogens can bind to estrogen receptors (ERs)
in osteoblasts to increase their activity (Cos et al.,
2003; Villiers, 2009). Phytoestrogens are a group of
compounds contained in plants which have estrogen-
like structures or can replace the function of
estrogen, both in association with estrogen receptors
(ER-dependent) and not (ER-independent) (Ososki
and Kennelly, 2003; Yang et al., 2012; Cui et al.,
2013).
Although it has great potential as a medicinal
plants, the phytochemical properties of M. crenata
has not been completely confirmed yet. This
research was done to identify the metabolite profile
of ethyl acetate extract of M. crenata using UPLC-
QToF-MS/MS, which can be used as a reference for
further research and utilization of M. crenata.
50
Maâ
˘
A
´
Zarif, B. and Agil, M.
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata Presl. Using UPLC-QToF-MS/MS.
DOI: 10.5220/0008357300500057
In Proceedings of BROMO Conference (BROMO 2018), pages 50-57
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
UPLC-QToF-MS/MS is a powerful technique used
for metabolite profiling which has improved in
performance of chromatographic resolution, speed
and sensitivity analysis, saves time, also reduces
solvent consumption (Patil et al., 2011),
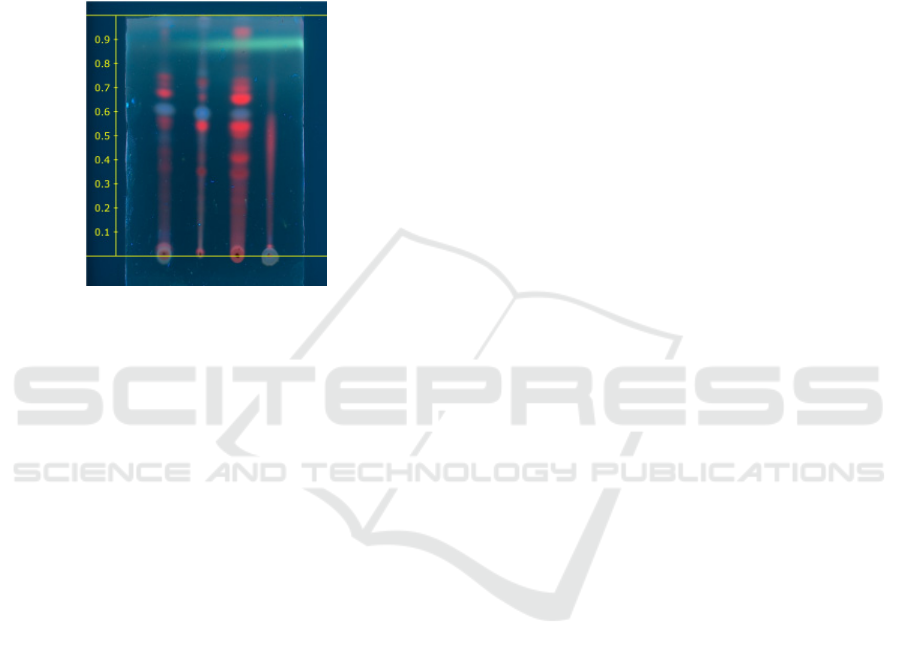
The ethyl acetate extract was selected because in
the preliminary study using TLC visualizer, this
extract showed the best TLC profile (Figure 2).
Whereas metabolite profiling of n-hexane extract has
been done before (Ma’arif et al., 2016).
Figure 2: TLC profile of : a. 96% ethanol extract; b. n-
hexane extract; c. ethyl acetate extract; and d. metanol
extract; from M. crenata leaves at λ 366 nm.
2 MATERIAL AND METHODS
2.1 Material
2.1.1 Plant Material
M. crenata were collected in Benowo, Surabaya,
Indonesia at November 2017, and identified in UPT
Materia Medica, Batu, Indonesia at December 2017
with specimen number 1a-17b-18a-1. The leaves
were prepared to get dry powder of M. crenata.
2.1.2 Chemical
All chemicals were grade of analytical reagent and
used as received. N-hexane, and ethyl acetate as
solvent were purchased from Pharmacy Department,
Faculty of Medical and Health Science, Maulana
Malik Ibrahim State Islamic University.
Dichloromethane, metanol, acetonitrile, and formic
acid as solvent and mobile phase on UPLC-QToF-
MS/MS were purchased from Central Forensic
Laboratory Badan Reserse Kriminal Kepolisian
Negara Republik Indonesia.
2.2 Methods
2.2.1 Extraction
Dry powder of M. crenata leaves were extracted
with n-hexane first. Its residue then re-extracted with
ethyl acetate. In the preliminary study, the 96%
ethanol extract was obtained by directly extracting
dry powder of M. crenata, while methanol extract
was obtained from re-extracting residue of ethyl
acetate extract with methanol. All extraction process
was using ultrasonic assisted extraction method
(Sonica 5300EP S3). This process was repeated,
collecting all the supernatants, which were finally
evaporated in a rotary evaporator (Heidolph) to get
ethyl acetate extract.
2.2.2 Analysis with UPLC-QToF-MS/MS
A simple, rapid, reliable and precise reversed phase
UPLC-QToF-MS/MS method has been developed
and validated according to the regulator guidelines.
The ethyl acetate preparation was done using solid
phase extraction, 100 ppm of ethyl acetate extract in
DCM and methanol then injected 5 µl each into the
an ACQUITY UPLC
®
H-Class System (Waters,
USA) coupled to an MS detector Xevo G2-S QToF
(Waters, USA). Sample were separated on an
ACQUITY BEH C
18
(1.7 µm 2.1x50 mm) with
acetonitril + 0.05 % formic acid and water + 0.05 %
formic acid as mobile phase, with flowrate 0.2
ml/min. The results of UPLC-MS analysis was
processed using the Masslynx Version 4.1 software,
to obtain the data of peak and m / z spectra of each
detected peak. The compound content can then be
predicted using the chemspider website.
3 RESULTS AND DISCUSSION
A total of 300 g dry powder of M. crenata leaves
were extracted with n-hexane and then ethyl acetate
to produce 2.82 g extract. The dry powder need to be
extracted first with n-hexane to remove impurities
which may interfere with the identification process,
such as fatty acid compounds. Ethyl acetate extract
of M. crenata were analysed by UPLC-QToF-
MS/MS to better interpret the diversity of available
phytochemicals.
a
b
c
d
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata Presl. Using UPLC-QToF-MS/MS
51
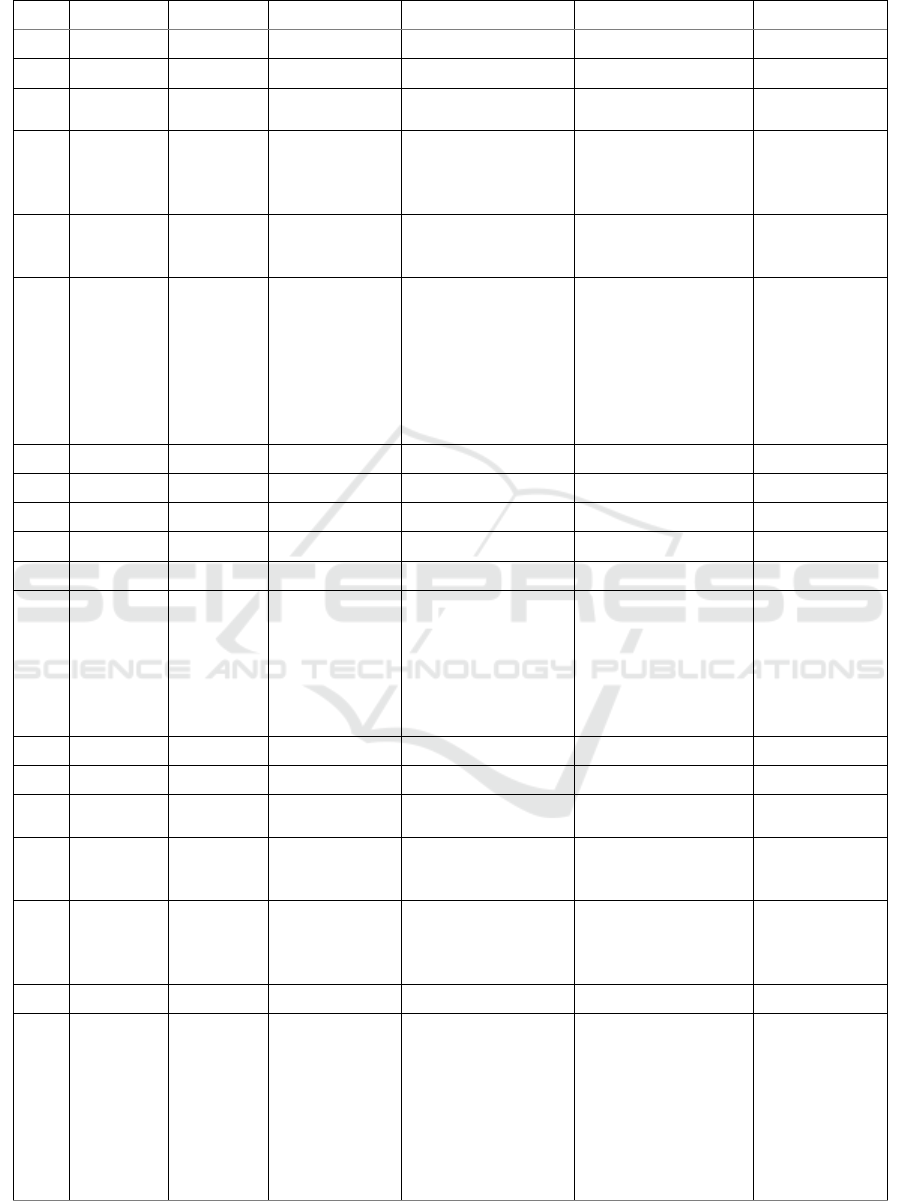
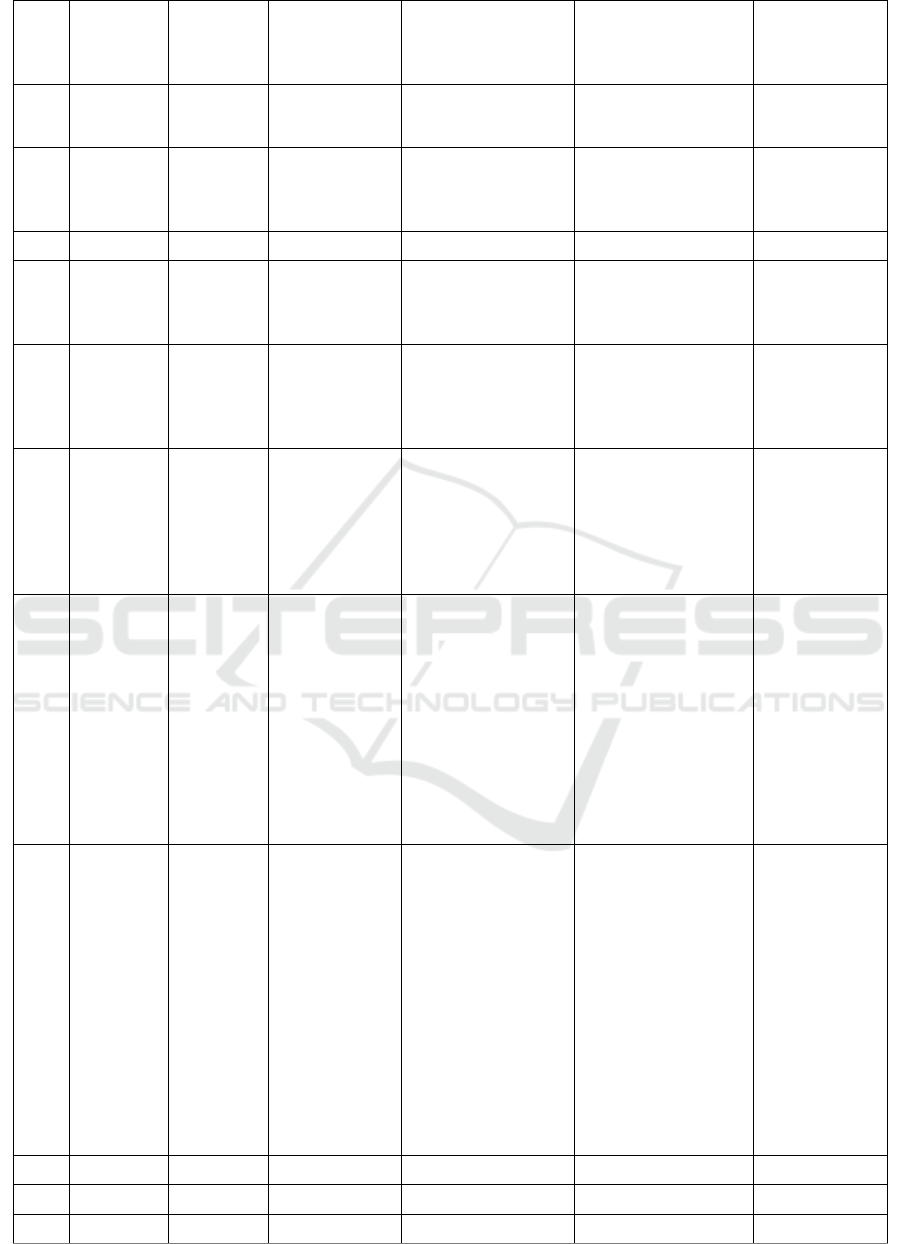
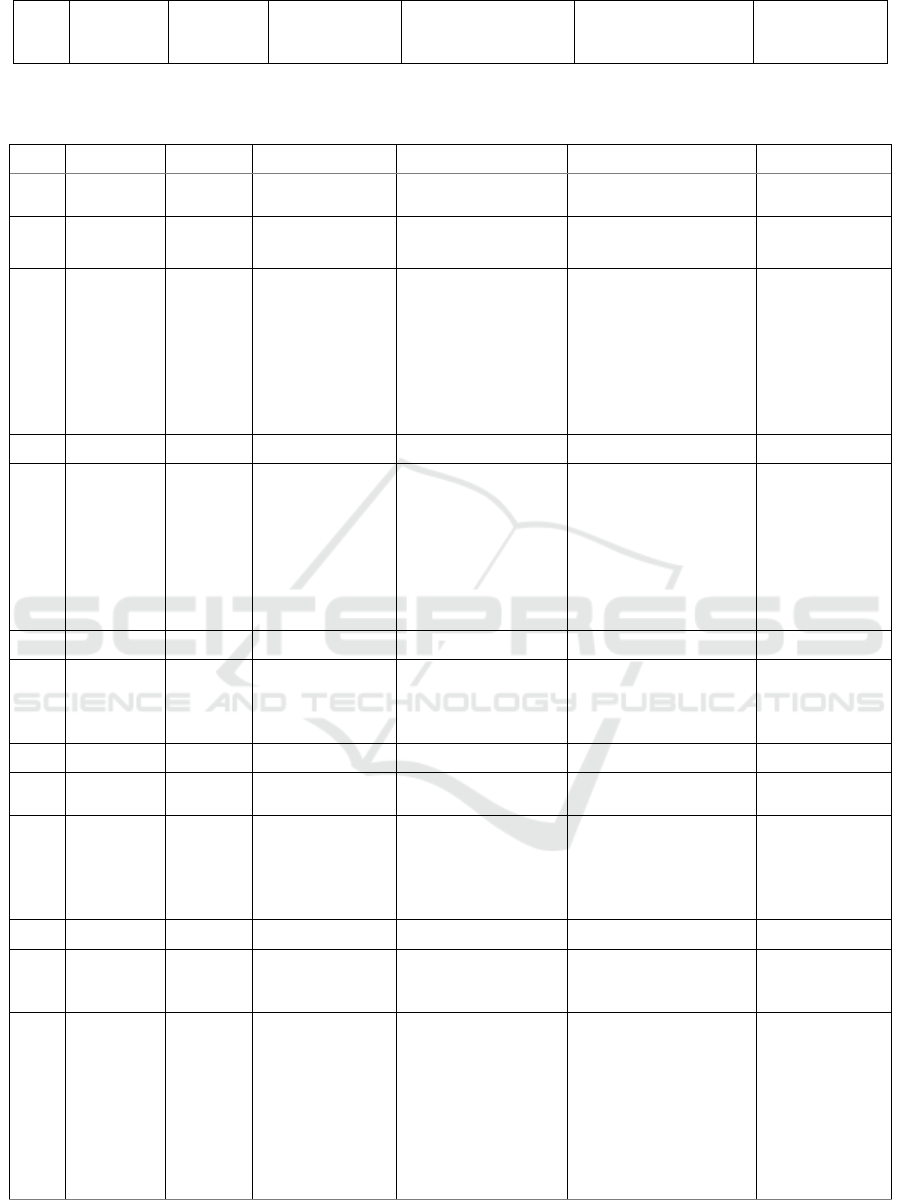
Table 1: Predicted compounds of ethyl acetate extract from M. crenata leaves in DCM solvent
No. RT (min) % Area Measured m/z Molecular Formula Proposed Metabolite Activity
1 1.272 0.3022 150.0280 Unknown Unknown -
2 1.420 0.2059 119.0944 Unknown Unknown -
3 2.118 1.0502 201.1728 C
11
H
23
NO
2
11-Aminoundecanoic
aci
d
-
4 2.598 1.7620 122.0842 C
7
H
10
N
2
2-Pyridylethylamine
Histamine
agonist
(Kunkel and
Dixon, 1984)
5 4.427 0.2680 301.1890 C
15
H
27
NO
5
Megalanthonine
Antifungal
(Reina et al.,
1998
)
6 4.828 0.0245 378.1862 C
21
H
30
O
4
S Tixocortol
Corticosteroid,
antiinflammato
ry (Friedman
and Metcalfe,
1991),
decongestant
(Cuenant et al.,
1986)
7 4.930 0.0063 299.1944 C
12
H
29
NO
7
Unknown -
8 5.193 0.0799 315.1134 Unknown Unknown -
9 5.342 0.1373 149.1203 Unknown Unknown -
10 5.479 0.0713 431.2729 Unknown Unknown -
11 5.662 0.0830 210.1255 Unknown Unknown -
12 5.959 0.0335
519.3245
C
27
H
45
N
5
O
3
S
3,5-
Isothiazoledicarboxa
mide, 4-amino-
N
3
,N
5
-dicyclohexyl-
N
5
-[1-[[(3-
methylbutyl) amino]
carbon
y
l]but
y
l]-
-
13 6.211 0.0193 545.3508 Unknown Unknown -
14 6.623 0.0089 462.2615 C
13
H
39
N
10
O
4
PS Unknown -
15 7.206 0.4010 196.1099 C
11
H
16
O
3
1-carboxy-3-
hydroxyadamantane
-
16 7.972 0.1522 271.1930 C
12
H
26
N
5
P
Pyrrolidine, 1,1',1''-
(hydrazinylidenephos
p
horanylidyne)tris-
-
17 9.733 0.0992 256,1936 C
17
H
24
N
2
1H-Benzimidazole,
1-(2-
cyclohexylethyl)-5,6-
dimethyl-
Antituberculos
is, antibacterial
(Gobis et al.,
2015)
18 10.967 0.4997 191.1309 Unknown Unknown -
19 11.448 1.0321 241.2772 C
16
H
35
N Hexadecylamine
Antibacterial,
adjuvant for
diphtheria,
tetanus toxoid,
and
antiinfluenza
(Attwood and
Florence,
2012)
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
52
20 11.630 0.5779 386.1728 C
22
H
26
O
6
Benzophenone, 2-(1-
ethylacetonyl)-
3',4,4',5-
tetramethox
y
-
-
21 11.882 0.0066 310.1203 C
19
H
18
O
4
Benzylbutylphthalate
Estrogenic
activity (Harris
et al., 1997
)
22 12.111 0.1000 310.1775 C
17
H
26
O
5
Portentol
Antioxidant,
anticancer
(Schröckenede
r
, 2012
)
23 12.842 0.1933 3032925 C
21
H
37
N Pregnan-3-amine -
24 13.345 0.0078 228.1152 C
15
H
16
O
2
Bisphenol A
Estrogenic
activity
(Hewitt and
Korach, 2010)
25 13.940 0.1502 567.4201 C
36
H
58
NO
2
P
Dibenzo[d,f][1,3,2]di
oxaphosphepin-6-
amine, N,N-dibutyl-
2,4,8,10-tetrakis(1,1-
dimethylethyl)-
-
26 14.077 0.1513 531.3416 C
28
H
45
N
5
O
5
Glycine, N-[[(E)-2-
(4-
methoxyphenyl)diaze
nyl]carbonyl]leucyl-,
compd. with N-
cyclohexylcyclohexa
namine
(
1:1
)
-
27 15.038 3.7928 627.1884 C
33
H
30
N
5
O
6
Cl
1H,5H-Pyrrolo[3,4-
g][1,2,4]triazolo[1,2-
a]cinnoline-
1,3,8,10(2H,7H,9H)-
tetrone, 7-(3-chloro-
4-hydroxy-5-
methoxyphenyl)-
7a,10a,11,11a-
tetrahydro-2-methyl-
9-[(4-
methylphenyl)amino]
-7a-
p
hen
y
l-
-
28 16.970 36.4625 775.2261 C
38
H
38
N
5
O
11
Cl
(1R,13S,16S,17R,28
R)-28-Amino-20-
chloro-17,25-
dihydroxy-5,8,10,24-
tetramethoxy-N-
methyl-15,29,31-
trioxo-22-oxa-
14,30,32triazahexacy
clo
[14.14.2.2
18,21
.1
2,6
.1
23,
27
.0
7,12
]hexatriaconta-
2(36),3,5
,7,9,11,18,20,23(33),
24,26,34-dodecaene-
13-carboxamide
-
29 17.633 34.5167 592.2692 C
30
H
33
N
12
P Unknown -
30 17.885 10.8884 849.2460 C
52
H
41
N
5
OPSCl Unknown -
31 18.330 6.4550 701.2070 Unknown Unknown -
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata Presl. Using UPLC-QToF-MS/MS
53
32 21.658 0.0608 156.9950 C
4
H
3
N
3
O
2
S
1H-Pyrazolo[3,4-
d]thiazole-
3,5(2H,6H)-dione
-
Table 2: Predicted compounds of ethyl acetate extract from M. crenata leaves in methanol solvent
No. RT (min) % Area Measured m/z Molecular Formula Proposed Metabolite Activity
1 0.581 0.0068 124.9797 C
3
H
5
NCl
2
3,3-Dichloro-2-
p
ropen-1-amine
-
2 1.500 1.0634 235.1421 C
10
H
22
NO
5
Nitromethanetrispropa
nol
-
3 2.266 0.1459 122.0478 C
6
H
6
N
2
O Nicotinamide
Activity of
diphosphate
(ADP) -
ribosyltransfera
se (Maurer et
al., 2012), anti-
SIRT2 (Cui et
al., 2014
)
.
4 4.016 0.0642 124.9789 Unknown Unknown
5 5.045 0.1590 149.1201 C
10
H
15
N
(S)-(+)-
Methamphetamine
Increase activit
y of
neurotransmite
r norepinefrin
and dopamine,
reduce appetite
(Ward et al.,
2016).
6 5.228 0.1070 431.2733 C
18
H
41
NO
10
Unknown
7 5.445 0.0977 466.2989 C
33
H
37
N
3
(1E)-1-(2,2'',4,4'',6,6''-
Hexamethyl1,1':3',1''-
terphenyl-2'-yl)-3-
mesit
y
l-1-triazene
-
8 5.662 0.0169 519.3256 H
34
N
31
OCl Unknown
9 7.206 4.6301 196.1102 C
11
H
16
O
3
1-carboxy-3-
hydroxyadamantane
-
10 8.006 0.2579 125.1882 C
12
H
25
NO
2
12-Aminododecanoic
acid
Hamper
expression of
CD
40
(Albertshofer
et al.,2005
)
11 8.886 0.0908 119.0941 Unknown Unknown
12 10.533 1.4306 180.1148 C
11
H
16
O
2
2-tert-butyl-4-
methoxyphenol
Antioxidant
(Lee et al.,
2006
)
13 11.013 0.6199 224.1886 C
13
H
24
N
2
O
Ethyl (4S)-5-
cyclohexyl-2,2-
difluoro-4-{[(2S)-2-
{[N-(4-
morpholinylsulfonyl)-
L-
phenylalanyl]amino}-
4-pentenoyl]amino}-3-
oxopentanoate
-
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
54
14 11.379 0.2271 340.1314 C
20
H
20
O
5
Morachalcone A
Tyrosinase
Inhibitors
(Nguyen et al.,
2012),
inhibition of
nitric oxide
(Joo et al.,
2014),
pancreatic
lipase
inhibitory
(Jeong et
al.,2015)
15 11.562 3.0017 310.1200 C
14
H
19
N
4
O
2
Cl Lintopride
Treatment of
gastrointestinal
reflux, nausea
and vomiting
(Delvaux et al.,
1995)
16 11.996 0.1281 332.1961 C
16
H
24
N
6
O
2
8-(4-Ethyl-1-
piperazinyl)-3-methyl-
7-(2-methyl-2-propen-
1-yl)-3,7-dihydro-1H-
p
urine-2,6-dione
-
17 12.431 4.2855 503.3096 C
25
H
45
NO
9
Pederin
Antioxidant,
anticancer
(Ghoneim,
2013)
18 12.614 0.6065 693.3941 C
33
H
59
NO
14
Methyl {[(9Z)-17-
{[(2R,3R,4S,5S,6R)-
4,5-dihydroxy-6-
(hydroxymethyl)-3-
{[(2S,3R,4S,5S,6R)-
3,4,5-trihydroxy-6-
(hydroxymethyl)tetrah
ydro-2H-pyran-2-
yl]oxy}tetrahydro-2H-
pyran-2-yl]oxy}-9-
octadecenoyl]ami
no
}
acetate
-
19 12.911 0.2985 363.3127 C
18
H
42
N
5
Cl Unknown
20 13.208 0.7061 276.2087 C
18
H
28
O
2
Phenyl laurate
Antimicroba,
antihipertensio
n (Edwin and
Edmun
d
,1940)
21 13.460 0.3641 495.3566 C
24
H
46
N
9
Cl Unknown
22 13.791 2.6389 531.3408 C
2
H
37
N
29
O
2
S Unknown
23 14.306 0.8403 698.5889 C
30
H
75
N
14
O
2
Cl Unknown
24 15.541 21.6948 698.5885 C
8
NO
15
S
6
Br
2
Unknown
25 16.718 11.5201 698.5896 C
43
H
86
S
3
Unknown
26 17.153 11.2271 592.2689 C
35
H
36
N
4
O
5
Pheophorbide A
Antiinflamatio
n, antioxidant
(Vencl et al.,
2009), anti-
HIV (Zhang et
al., 2003)
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata Presl. Using UPLC-QToF-MS/MS
55

27 17.370 0.6928 592.2694 C
36
H
40
N
4
S
2
1,1'-(1,4-
Butanediyl)bis{2,6-
dimethyl-4-[(3-
methyl-1,3-
benzothiazol-2(3H)-
ylidene)methyl]pyridin
ium}
-
28 18.330 33.0776 698.5885 C
8
NO
15
S
6
Br
2
Unknown -
Table 1 and Table 2 summarise all the
compounds characterized in ethyl acetate extract of
M. crenata, including retention times, % area,
measured m/z, molecular formula, putative
compounds, and its activity based on references.
In total there were 32 peak of compounds
identified in the DCM solvent, and 28 peak in the
methanol solvent. The use of two types of solvent
aimed to elute the ethyl acetate extract optimally.
From all the peaks, only 36 peaks can be identified,
while the rest are unknown compounds.
Unknown compounds may be identified as
impure compounds which are still detected by the
instrument, or they may be a new compounds, which
is undetectable in chemspider database, especially
unknown compounds with high concentrations.
Based on the results of this study, it is not yet
known which compounds are likely to have activity
as phytoestrogens, but when viewed from the
activity data in Table 1 and Table 2, it is known
some compounds have activity as antioxidants.
Where antioxidants is one form of phytoestrogens
activity, the ER-independent pathway.
Phytoestrogens can work through two pathways,
both ER-dependent and ER-independent pathway.
Although most biological actions of phytoestrogens
are mediated through ERs in cells (ER-dependent),
its can exert antioxidant effects and suppress
oxidative stress through an ER-independent
pathway. Phytoestrogens effectively prevent pro-
oxidant stress by limiting ROS release from
damaged mitochondria, and provides antioxidant
activity in cells (Cui et al., 2013).
4 CONCLUSIONS
From UPLC-QToF-MS/MS analysis, it is concluded
that ethyl acetate extract of M. crenata leaves
contain various types of compounds, either detected
compounds (36 compounds), or unknown
compounds. The unknown compounds still need to
be investigated further, especially those with high
concentrations.
REFERENCES
Adityara, R. A. 2017. Uji aktivitas antiosteoporosis fraksi
etil asetat daun Marsilea crenata Presl. dalam
meningkatkan kepadatan tulang trabekula femur
mencit betina. Skripsi : Universitas Airlangga.
Aemi, N. Y. 2012. Uji aktivitas antiosteoporosis fraksi n-
heksana daun Marsilea crenata Presl. dalam
meningkatkan kepadatan tulang trabekular vertebra
mencit betina. Skripsi : Universitas Airlangga.
Albertshofer, K., Siwkowski, A. M., Wancewicz, E. V,
Esau, C. C., Watanabe, T., Nishihara, K. C., Maier, M.
A. 2005. Structure - Activity Relationship Study on a
Simple Cationic Peptide Motif for Cellular Delivery of
Antisense Peptide Nucleic Acid. J Med Chem. Vol.
48(21): 6741–6749.
Attwood D., and Florence, A.T. 1983. Surfactant Systems:
Their chemistry, pharmacy and biology. Chapman and
Hall Ltd. London:.
Cos, P., Bruyne, T. D., Apers, S., Berghe, D. V., Pieters,
L., Vlietinck, A. J. 2003. Planta Med. Vol. 69: 589-
599.
Cuenant, G., Stipon, J. P., Plante-Longchamp, G.,
Baudoin, C., Guerrier, Y. 1986. Efficacy of Endonasal
Neomycin-Tixocortol Pivalate Irrigation in the
Treatment of Chronic Allergic and Bacterial Sinusitis.
J Otorhinolaryngol Relat Spec. 48(4):226-32.
Cui, H., Kamal, Z., Ai, T., Xu, Y., More, S. S., Wilson, D.
J., & Chen, L. 2014. Discovery of Potent and Selective
Sirtuin 2 (SIRT2) Inhibitors Using a Fragment-Based
Approach, 2. J Med Chem. Vol. 57(20):8340-8357.
Cui, J., Shen,Y., Li R. 2013. A Review: Estrogen
synthesis and signaling pathways during aging: from
periphery to brain. Trends in Molecular Medicine.
Vol. 19, No. 3.
Delvaux, M., Maisin, J. M., Arany, Y., Atlan, P., Prieto-
Cabanis, M. J., Canal, M., Frexinos, J. 1995. The
effects of lintopride, a 5HT-4 antagonist, on
oesophageal motility. Aliment Pharmacol Ther. Vol.
9(5):563-9.
Edwin, H. B., and Edmund, J. D. 1940. The Fries
rearrangement of phenyl laurate and phenyl stearate.
Journal of chemical society.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
56

Friedman, B.S., and Metcalfe, D.D. 1991. Effects of
tixocortol pivalate on gastrointestinal disease in
systemic mastocytosis: a preliminary study. Clinical
and Experimental Allergy. Vol. 21:183-188.
Ghoneim, K.S. 2013. Human dermatosis caused by
vesicating beetle products (insecta), cantharidin and
pederin: an overview: world journal of medicine and
medical science. 1(1).
Gobis K., Foks H., Serocki M. 2014. Synthesis evaluation
of in vitro antimycobacterial activity of novel 1H-
benzo[d]imidazole derivatives and analogues.
European Journal of Medicinal Chemistry.
Harris A. C., Henttu P., Parker G. M., and Sumpter J. P.
1997. The Estrogenic Activity of Phtalate Esters In
Vitro. Environmental Health Perspectives. Vol. 105,
No. 8.
Hewitt C. S., and Korach S. K. 2011. Estrogenic Activity
of Bisphenol A and 2,2-bis(p-Hydroxyphenyl)-1,1,1-
trichloroethane(HPTE) Demonstrated in Mouse
Uterine Gene Profiles. Environmental Health
Perspectives. Vol 119, No. 1.
Jeong, J. Y., Jo, Y. H., Kim, S. B., Liu, Q., Lee, J. W., Jin,
E., Lee, M. K. 2015. Pancreatic lipase inhibitory
constituents from Morus alba leaves and optimization
for extraction conditions. Bioorganic & Medicinal
Chemistry Letters. Bioorg Med Chem Lett. Vol. 1;25
(11):2269-74
Joo, T., Sowndhararajan, K., Hong, S., Lee, J., Park, S. Y.,
Kim, S., Jhoo, J. W. 2014. Inhibition of nitric oxide
production in LPS-stimulated RAW 264.7 cells by
stem bark of Ulmus pumila L. Saudi J Biol Sci. Vol.
21(5):427-35.
Kunkel, H. G., and Dixon, F. J. 1984. Advances in
Immunology. Academic Press Inc. London. Vol. 35
Laswati, H. 2011. Green Clover Potentiates Delaying the
Increment of Imbalance Bone Remodeling Process in
Postmenopausal Women. Folia Medica Indonesiana.
Vol. 47. No. 2. Page 112-117.
Ma’arif B., Agil, M., Laswati H. 2016. Phytochemical
assessment on n-hexane extract and fractions of
Marsilea crenata Presl. leaves through GC-MS. Trad.
Med. J, 2016; 21(2):77-85.
Ma'arif, B., Agil, M., Laswati, H. 2018. Alkaline
Phosphatase Activity of Marsilea crenata Presl.
Extract and Fractions as Marker of MC3T3-E1
Osteoblast Cell Differentiation. Journal of Applied
Pharmaceutical Science Vol. 8(03), pp. 55-59.
Maurer, B., Sirt, D., Rumpf, T., Scharfe, M., Stolfa, D. A.,
Schmitt, M. L., Jung, M. 2012. Inhibitors of the NAD
+‑Dependent Protein Desuccinylase and
Demalonylase Sirt5. ACS Med Chem Lett. Vol. 3(12):
1050–1053.
Nguyen, N. T., Nguyen, M. H., Nguyen, H. X., Bui, N. K.,
Nguyen, M. T. 2012. Tyrosinase inhibitors from the
wood of Artocarpus heterophyllus. J Nat Prod. Vol.
75(11):1951-5.
Nurjanah, A. A., and Abdullah, A. 2012. Aktivitas
Antioksidan dan Komponen Bioaktif Semanggi Air
(Marsilea crenata). Jurnal Inovasi dan Kewirausahaan.
Vol. 1. No. 3. Page 152-158.
Ososki, A. L., Kennelly, E. J. 2003. Phytoestrogens: a
Review of the Present State of Research. Phytotherapy
Research. Vol. 17. Page 845-869.
Patil, J. S., Suresh, S., Sureshbabu, A. R., Rajesh, M. S.
2011. Development and Validation of Liquid
Chromatography-Mass Spectrometry Method for the
Estimation of Rifampicin in Plasma. Indian J Pharm
Sci. 2011 Sep-Oct; 73(5): 558–563.
Reina, M., Gonzalez-Coloma, A., Gutierrez, C., Cabrera,
R., Henriquez, J., Villarroel, L. 1998. Pyrrolizidine
Alkaloids from Heliotropium megalanthum. Journal of
Natural Products. Vol. 61, No. 11.
Schröckeneder, A. 2012. Towards the Total Synthesis of
Portentol A Formal Synthesis of Dimethylglutamine
The Crystal Structure of the Dess-Martin Periodinane
[Disertasi]. München: Ludwig Maximilians
Universität München.
Vencl, F. V, Gómez, N. E., Ploss, K., & Boland, W. 2009.
The Chlorophyll Catabolite , Pheophorbide a , Confers
Predation Resistance in a Larval Tortoise Beetle
Shield Defense. J Chem Ecol. Vol. 35(3), page 281–
288.
Villiers, T. J. 2009. Bone health and osteoporosis in
postmenopausal women. Elsevier : Best Practice &
Research Clinical Obstetrics and Gynaecology. Vol.
23. Page 73-85.
Ward, L. F., Enders, J. R., Bell, D. S., Cramer, H. M.,
Wallace, F. N., Mcintire, G. L., Supelco, S. 2016.
Improved Chiral Separation of Methamphetamine
Enantiomers Using CSP-LC – MS-MS, (2), 1–9.
Widiasari, F. A. 2017. Uji aktivitas antiosteoporosis fraksi
etil asetat daun Marsilea crenata Presl. dalam
meningkatkan kepadatan tulang trabekula vertebra
mencit betina. Skripsi : Universitas Airlangga.
Yang, T-S., Wang, S-Y., Yang, Y-C., Su, C-H., Lee, F-K.,
Chen, S-C., Tseng, C-Y., Jou, H-J., Huang, J-P.,
Huang, K-E. 2012. Effects of standardized
phytoestrogen on Taiwanese menopausal women.
Elsevier : Taiwanese Journal of Obstetrics &
Gynecology. Vol. 51. Page 229-235.
Zhang, H., Tan, G. T., Hoang, V. D., Hung, N. Van,
Cuong, N. M., Soejarto, D. D., Fong, H. H. S. 2003.
Natural Anti-HIV Agents. Part IV. Anti-HIV
Metabolite Profiling of Ethyl Acetate Extract from Marsilea crenata Presl. Using UPLC-QToF-MS/MS
57
