
The Effect of Centella asiatica on Brain Malondialdehyde Levels of
Aged Rats
Nathaniel Aditya
1
, Indah Fitriani
1
, Desak Gede Budi Krisnamurti
2,3
, Siti Farida
2,3
, Erni Hernawati
Purwaningsih
2,3
, and Rani Wardani Hakim
2,3
1
Undergraduate Student, Faculty of Medicine, Universitas Indonesia, Jl. Salemba Raya No. 6, Jakarta Pusat, Indonesia
2
Department of Medical Pharmacy, Faculty of Medicine, Universitas Indonesia, Jl. Salemba Raya No. 6, Jakarta Pusat,
Indonesia
3
Drug Development Research Cluster, Indonesian Medical Education and Research Institute (IMERI), Jl. Salemba Raya
No. 6, Jakarta Pusat, Indonesia
Keywords: Aging, Antioxidant, Oxidative Stress, Lipid Peroxidation, Malondialdehyde, MDA, Centella asiatica
Abstract: Background: In 2050, the number of elderly with 65 years of age or more is estimated to reach 1.5 billion.
To better anticipate this problem, a shift of paradigm, from chronological to biological aging, is needed.
Aging is a multifactorial process closely related to oxidative stress, a phenomenon in which the rate can be
indicated through its secondary metabolite level, malondialdehyde (MDA). Objective: This study examines
the effect of a well-known traditional medicinal plant used for its anti-inflammatory properties, Centella
asiatica (CA), to brain MDA levels in aged Sprague-Dawley rats. Methods: The aged male rats were
divided into three groups: negative control, positive control (vitamin E 6 IU), treatment (CA leaves
ethanolic extract 300 mg/kg), with one additional group of untreated young rats. Throughout 28 days, each
rat was given the corresponding treatment. The brains then were collected to be studied using the Lipid
Peroxidation (MDA) Assay Kit. One-way ANOVA is the choice of the statistical analysis method. Results:
We found that the level of MDA in the brain tissues of the treatment group rats had a lower value compared
to that of the control group, although statistically insignificant (P = 0.5683). Unquestionably, the MDA
concentration in the vitamin E-treated rats is the lowest of all.Conclusion: These results implied that CA
may exhibit an antioxidative effect on aged rats which could hinder an aging process, if not prevent it.
1 INTRODUCTION
Today, the world is in a state of demographical shift.
In 2050, the number of elderly with 65 years of age
or more is estimated to be 1.5 billion; four times the
number of that in 2010 (WHO, 2011). Being the
fastest among all age groups, this increasing rate of
older population will be felt mostly in developing
countries such as Indonesia (Jones, 2010). As the
elderly number grows, its financial weight on
national health service sector will also continue to
rise because older people are more susceptible to
external and internal stress; a result of declining
physiological function (Cesari, Prince et al., 2016,
WHO, 2015). Besides, one should also consider
accompanying diseases, e.g. depressive disorders
and anxiety, as the main cause of quality of life
deterioration. To better anticipate this imminent
problem, a shift of paradigm, from chronological to
biological aging, is urgently needed (Cesari, Prince
et al., 2016). One way to address this challenge is by
changing the focus of therapy; from just lengthening
lifespans into increasing health span (Ho, So et al.,
2010, WHO, 2015).
Aging is a multifactorial process closely related
to oxidative stress. The theory of free radical aging
stated that aging process is caused by an imbalance
of an oxidative and antioxidative process (Finkel and
Holbrook, 2000, López-Otín, Blasco et al., 2013).
One biological consequences of the cellular
oxidative stress is lipid peroxidation, a phenomenon
in which the rate can be indicated through its
secondary metabolite, malondialdehyde (MDA)
(Lieberman, Marks et al., 2013). One of the most
important organs which is vulnerable to the harmful
effects of lipid peroxidation is brain, for it is
Aditya, N., Fitriani, I., Krisnamurti, D., Farida, S., Purwaningsih, E. and Hakim, R.
The Effect of Centella asiatica on Brain Malondialdehyde Levels of Aged Rats.
DOI: 10.5220/0008360102090213
In Proceedings of BROMO Conference (BROMO 2018), pages 209-213
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
209

composed of high concentrations of polyunsaturated
fatty acids (PUFAs). In a study done by Dei, Takeda
et al. (2002), an increase of MDA levels with age
had been demonstrated in the cytoplasm of neurons
and astrocytes. To fulfill a high demand of energy,
brain also consumes a large amount of oxygen.
However, compared to other organ, it relatively
lacks antioxidant defenses, such as a lower activity
in glutathione peroxidase and catalase, making it
more vulnerable to oxidative stress. (Kedar, 2003).
Therefore, protecting the brain from excessive
oxidative damage might ameliorate the balance
between pro-oxidants and antioxidants, hence
promoting a healthier aging process.
One preventive effort to ensure this healthy
aging is reflected in phytotherapy, known as herbal
medicine, which utilizes therapeutic potential of a
certain plant (Ho, So et al., 2010). Centella asiatica
(CA), a medicinal tropical plant from the family
Apiaceae used commonly in Southeast Asia, had
shown to have neuroprotective and cognitive-
enhancement effect which could play an important
role in aging (Dev, 2009, Mukherjee, Kumar et al.,
2007, Tiwari, Singh et al., 2008, Veerendra Kumar
and Gupta, 2003). However, there were only a
limited number of researches examining the
antioxidative properties possessed by this plant,
especially its role in brain aging and lipid
peroxidation. The animal subjects which were used
was also limited to a single breed of rat; not to
mention the lack of comparison with a proven
exogenous antioxidant.
In the present study, we compared the brain
MDA levels between CA-treated aged Sprague-
Dawley rats and their younger counterparts. The
antioxidative properties of CA on aged rats were
also compared to a well-known antioxidant agent,
vitamin E. We hypothesized that aged rats which
were treated with CA extract would have a lower
level of brain MDA compared to those untreated,
thus raising the potential of CA as an antioxidant
which could promote a healthier aging process.
2 METHODS
2.1 Study Design and Subjects
The subject used in this experiment, the male
Sprague-Dawley rats, is a distinct outbred albino rat
used commonly in nutritional and medical research
settings. These rats were obtained from the National
Institute of Health Research and Development,
Ministry of Health Republic of Indonesia. Sprague-
Dawley rat has an elongated head structure and a tail
longer than its body. These rats are first bred by R.
W. Dawley from the Sprague-Dawley Animal
Company in Wisconsin, United States in 1925. Their
docile characteristics make them easy to handle.
The rats were divided into two groups according
to their age; young rats (8-12 weeks old) and aged
rats group (20-24 months old). The aged rats were
further divided into three final groups according to
the treatment given; negative control (water as
placebo), positive control (vitamin E), and treatment
(Centella asiatica ethanolic leaves extract) group. In
total, there were 4 experimental groups.
To differentiate individual rats in every group, a
color-coding system was used; each rat possessed a
distinct mark on a certain part of its body. The rats
have initial weights ranging from 183 to 308 g for
the young rats, and 333 to 490 g for the aged, all in
healthy state. Using Federer’s formula, a minimum
of 24 subjects was needed to achieve the optimal
sample size. However, to anticipate the possibility of
subject exclusion due to death or other unforeseen
causes, a total of 27 rats were used.
2.2 Extract Preparation
Centella asiatica (CA) leaves were dried under the
sunlight until the water content fully evaporated and
grinded to small fractions. The active substances of
these grinded particles were then extracted by
soaking them to a solvent, ethanol, for 24 to 48
hours repeatedly. To obtain and separate the active
substances from its solvent, a rotary evaporator was
utilized. Subsequently, the percentage of active
substances contained in the viscous solution
produced from this process was measured using
gravimetric analysis.
2.3 Treatments
Prior to the 28-day treatment, all rats underwent a
one-week acclimatization at the experiment room,
adapting to a 24
o
C temperature and a light-dark
cycle of 12:12 with lights on at 9.00 PM.
Throughout the study, all groups were fed daily with
10 g of standard pelleted chow (protein 18.5-20.5 %;
fat ± 4%; fiber ± 6%; calcium ± 0.9%; phosphor ±
0.7%) and provided with water ad libitum.
After the aged rats were randomly distributed
into the three groups, the following treatment was
started at day-1 and ended at day-28 accordingly;
water as placebo (negative control), CA leaves
ethanolic extract with 300 mg/kg bodyweight dosage
(treatment), and 6 IU of vitamin E (positive control).
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
210

All treatments were given twice daily. As for the
young rats group, no additional treatment was given
(water as placebo).
2.4 Termination
In the last day of treatment (day-28), all rats were
sedated under ketamine and xylazine prior to
termination and brain collection procedure. The
brains were weighed, put in a sterile container, and
preserved in a -20
o
C container.
2.5 Outcomes
2.5.1 Tissue Homogenate
One hundred milligrams of tissue from each brain
was dissolved with 1 ml of 0.01 M phosphate-
buffered saline (PBS) with a pH of 7.4 before
homogenized. It was then centrifuged at 3500 rpm
for 10 minutes. Then, the supernatant was obtained
and kept in a -20
o
C container.
2.5.2 MDA Calculation
Four hundred microliters mixture of water, MDA
standard, and supernatant were put into each of two
1.5 mL tubes. Into every tube, 200 µL of
trichloroacetic acid (TCA) 20% was added, then
vortexed and centrifuged at 5000 rpm for 10
minutes. After the supernatants were transferred to 2
mL tubes, 400 µL of thiobarbituric acid (TBA)
0.67% was added before the tubes were incubated
for 10 minutes in a water bath with a temperature of
96-100
o
C. Following the incubation, the tubes were
left out in the air until they reached room
temperature before their wave absorbance at 530 nm
were measured using a spectrophotometer. The final
MDA concentrations were calculated based on the
MDA wave absorbance standard curve.
2.6 Statistical Analysis
The data acquired were processed and analyzed
through GraphPad Prism ver. 7.00 statistical
software. The results are shown as mean ± SEM.
Shapiro-Wilk normality test was performed to see if
the data came from a Gaussian distribution.
Ordinary one-way ANOVA was the chosen
parametric test, followed by Tukey’s multiple
comparisons test with a single pooled variance as the
follow-up. The statistical significance was defined as
a P value of <0.05.
2.7 Ethical Consent
The study protocol and the usage of rats as
experimental subjects was approved by the Health
Research Ethical Committee, Faculty of Medicine,
Universitas Indonesia – Cipto Mangunkusumo
Hospital in December 2016 with the registration
number 1016/UN2.F1/ETIK/2016.
3 RESULTS & DISCUSSION
Of all 27 healthy male rats involved at the beginning
of the study, only 21 rats were alive at the time of
termination. In different periods, each of the 6 rats
appeared sick initially, and then died for unknown
reasons.
From our data, brain MDA concentration was
found to be lowest in those treated with vitamin E
(positive control) with a mean and SEM of 3.12 ±
0.39 nmol/L. In the negative control group
consisting of untreated aged rats, the MDA
concentration measured 3.78 ± 0.44 nmol/L, closely
followed by the young rats at 3.70 ± 0.21 nmol/L as
the second highest. With a mean difference of only
0.29 nmol/L with the vitamin E-treated group, CA-
treated aged rats had brain MDA concentration of
3.41 ± 0.25 nmol/L (Figure 1).
Although the findings were not statistically
significant (P = 0.5683), the decrease of brain MDA
seen in CA-treated aged rats from that of untreated
aged rats correlates with a similar previous finding
(Kumar and Gupta, 2002). That study demonstrated
a significant decrease of brain MDA in male Wistar
rats treated with 200 and 300 mg/kg of CA whole-
plant aqueous extract. However, the research did not
provide information about the age of the rats used;
The Effect of Centella asiatica on Brain Malondialdehyde Levels of Aged Rats
211
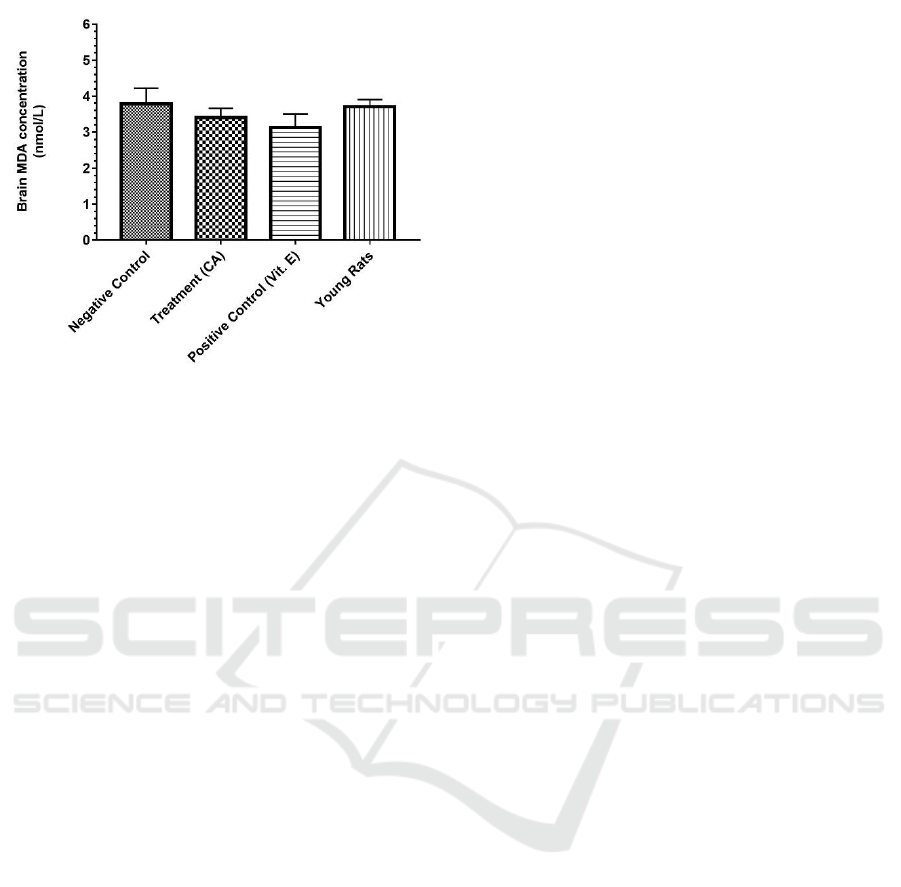
Figure 1: Brain MDA concentration (nmol/L) in different
groups of aged male Sprague-Dawley rats and young
Sprague-Dawley rats. Data are shown in mean ± SEM (P
= 0.5683).
albeit the weight range was stated to be 200-250 g.
This was almost half of the aged Sprague-Dawley
rats used in current study (333-490 g). This
reduction of MDA as a lipid peroxidation marker
indicate that there was also a decrease in the lipid
peroxidation process itself. This decrease may be
due to the electron and H
+
donating capacity of
flavonoids present in CA (Subathra, Shila et al.,
2005). Furthermore, beside its established role as an
oxidative stress indicator, MDA was also known for
causing yet another secondary oxidative stress to
proteins nearby. In one research which studied the
interaction between MDA and bovine serum
albumin (BSA), it was found that an oxidative
process called protein glycooxidation played the key
role. The research hypothesized that this process was
one of the main cause of molecular aging (Traverso,
Menini et al., 2004). Hence, by decreasing the MDA
levels on brain tissue, not only the lipid peroxidation
of the PUFAs will be reduced, but also the
secondary detrimental damage caused by MDA to
proteins will also be prevented.
In lipid peroxidation process of PUFAs, which
can be found at large amount in brain tissue,
chemical reactions induced by lipid peroxyl radical
(LOO
.
) appear to be responsible for aging and other
age-dependent diseases (Spiteller, 2007). Compared
to other organs in our bodies, brain also has a higher
risk for oxidative damage because (1) it requires
significant amounts of oxygen per weight
(approximately 20% of the total oxygen used in
humans) while (2) not highly equipped with
antioxidant protective mechanisms. In addition, key
ingredients behind the cause of cell membrane lipid
peroxidation, Fe and ascorbate, was found to be at
high concentration in brain tissue (Floyd, 1999).
This means that CA capability to lower lipid
peroxidation process in the aging brain could
translate into a potent antioxidant effect in an organ
inherently faced with a pro-oxidative state. Centella
asiatica positive effects on brain aging have been
attributed to its two major triterpene saponosides;
asiatic and madecassic acides, as well as their
heterosides; asiaticoside and madecassoside (Orhan,
2012).
Our data also demonstrate that vitamin E reduced
MDA brain concentration in aged rats. This finding
was relevant with a proven role of vitamin E as a
peroxyl radical scavenger which terminates chain
reactions and protecting long-chain PUFAs for
important cellular signaling events (Traber and
Atkinson, 2007). Nonetheless, one in vivo study
showed that supplemental vitamin E given to healthy
persons had no effect to the rate of lipid peroxidation
(Meagher, Barry et al., 2001). A difference on the
marker used on that observation (urinary isoprostane
called iPF
2α
-VI and urinary 4-hydroxynonenal) and
the fact that it measured whole-body lipid
peroxidation instead of a single organ might be the
cause of this contradicting finding, among many
others.
Unexpectedly, in this current study, one finding
raised questions; the resemblance between the brain
MDA levels of young rats to that found in the
untreated aged rats. Statistically, the comparison
between these two groups were proven to be the
most insignificant (P = 0.9987). This result was not
analogous with a previous study done by Subathra,
Shila et al. (2005) which displayed a significantly
lower level of MDA in various brain regions of
young rats when contrasted to untreated aged rats.
Some plausible explanation behind these disparities
are the difference in the strain of rat (Wistar vs.
Sprague-Dawley), the age range of young rats (3-4
months vs. 2-3 months) and aged rats (>24 months
vs. 20-24 months), and the type of CA extract used
(whole-plant vs. leaves). A shorter duration of
treatment in present study (28 days) could also play
a key role in these different findings. Likewise, 6
rats which died in the middle of the study, thus
altering the previously optimal number of subjects,
might contribute to the change in mean calculations.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
212

4 CONCLUSION
From our results, we concluded that Centella
asiatica may exhibit an antioxidative effect on aged
rats, comparable to that of vitamin E, which was
demonstrated by its capacity to reduce
malondialdehyde levels in aged brain rats. Despite
the insignificance found, the study suggests a
potential future role of Centella asiatica in hindering
aging process, if not preventing it.
ACKNOWLEDGEMENTS
This research was funded and supported by the
PITTA (Publikasi Terindeks Internasional Untuk
Tugas Akhir Mahasiswa UI) grant provided by
DRPM (Direktorat Riset dan Pengabdian Kepada
Masyarakat) Universitas Indonesia.
REFERENCES
Cesari, M. et al. 2016. Frailty: an emerging public
health priority. Journal of the American Medical
Directors Association 17(3) 188-192.
Dei, R. et al. 2002. Lipid peroxidation and advanced
glycation end products in the brain in normal
aging and in Alzheimer's disease. Acta
neuropathologica 104(2) 113-122.
Dev, R.D.O. 2009. Comparison on cognitive effects
of Centella asiatica in healthy middle age female
and male volunteers. Annals of Nutrition and
Metabolism 55 709.
Finkel, T. and Holbrook, N.J. 2000. Oxidants,
oxidative stress and the biology of ageing.
Nature 408(6809) 239-247.
Floyd, R.A. 1999. Antioxidants, oxidative stress,
and degenerative neurological disorders (44448).
Proceedings of the Society for Experimental
Biology and Medicine 222(3) 236-245.
Ho, Y.-S., So, K.-F. and Chang, R.C.-C. 2010. Anti-
aging herbal medicine—How and why can they
be used in aging-associated neurodegenerative
diseases? Ageing research reviews 9(3) 354-362.
Jones, G.W. 2010. The 2010 – 2035 Indonesian
population projection. UNFPA Indonesia.
Kedar, N. 2003. Can we prevent Parkinson’s and
Alzheimer’s disease? Journal of postgraduate
medicine 49(3) 236.
Kumar, M.V. and Gupta, Y. 2002. Effect of different
extracts of Centella asiatica on cognition and
markers of oxidative stress in rats. Journal of
ethnopharmacology 79(2) 253-260.
Lieberman, M., Marks, A.D. and Peet, A. 2013.
Marks basic medical biochemistry: Wolters
Kluwer Health/Lippincott Williams & Wilkins.
López-Otín, C. et al. 2013. The hallmarks of aging.
Cell 153(6) 1194-1217.
Meagher, E.A. et al. 2001. Effects of vitamin E on
lipid peroxidation in healthy persons. Jama
285(9) 1178-1182.
Mukherjee, P.K., Kumar, V. and Houghton, P.J.
2007. Screening of Indian medicinal plants for
acetylcholinesterase inhibitory activity.
Phytotherapy research 21(12) 1142-1145.
Orhan, I.E. 2012. Centella asiatica (L.) Urban: from
traditional medicine to modern medicine with
neuroprotective potential. Evidence-based
complementary and alternative medicine 2012.
Spiteller, G. 2007. The important role of lipid
peroxidation processes in aging and age
dependent diseases. Molecular biotechnology
37(1) 5-12.
Subathra, M., Shila, S., Devi, M.A. and
Panneerselvam, C. 2005. Emerging role of
Centella asiatica in improving age-related
neurological antioxidant status. Experimental
gerontology 40(8) 707-715.
Tiwari, S. et al. 2008. Effect of Centella asiatica on
mild cognitive impairment (MCI) and other
common age-related clinical problems. Digest
Journal of Nanomaterials and Biostructures 3(4)
215-220.
Traber, M.G. and Atkinson, J. 2007. Vitamin E,
antioxidant and nothing more. Free Radic Biol
Med 43(1) 4-15.
Traverso, N. et al. 2004. Malondialdehyde, a
lipoperoxidation-derived aldehyde, can bring
about secondary oxidative damage to proteins.
The Journals of Gerontology Series A: Biological
Sciences and Medical Sciences 59(9) B890-
B895.
Veerendra Kumar, M. and Gupta, Y. 2003. Effect of
Centella asiatica on cognition and oxidative
stress in an intracerebroventricular streptozotocin
model of Alzheimer's disease in rats. Clinical
and Experimental Pharmacology and Physiology
30(5‐ 6) 336-342.
WHO. 2011. Global health and aging. Geneva:
World Health Organization.
WHO. 2015. World report on ageing and health.
World Health Organization.
The Effect of Centella asiatica on Brain Malondialdehyde Levels of Aged Rats
213
