
Automatic Detection and Recognition of Swallowing Sounds
Hajer Khlaifi
1
, Atta Badii
2
, Dan Istrate
1
and Jacques Demongeot
3
1
University of Technology of Compiegne, UTC University, Compiegne, France
2
University of Reading, Department of Computer Science, School of Mathematical,
Physical and Computational Sciences, Reading, U.K.
3
Laboratory AGEIS EA 7407, University Grenoble Alpes, Faculty of Medicine, Grenoble, France
Keywords:
Swallowing Sounds, Automatic Detection, Classification, Non-invasive Dysphagia Clinical Assessment
Support.
Abstract:
This paper proposes a non-invasive, acoustic-based method to i) automatically detect sounds through a neck-
worn microphone providing a stream of acoustic input comprising of a) swallowing-related, b) speech and
c) other ambient sounds (noise); ii) classify and detect swallowing-related sounds, speech or ambient noise
within the acoustic stream. The above three types of acoustic signals were recorded from subjects, without
any clinical symptoms of dysphagia, with a microphone attached to the neck at a pre-studied position midway
between the Laryngeal Prominence and the Jugular Notch. Frequency-based analysis detection algorithms
were developed to distinguish the above three types of acoustic signals with an accuracy of 86.09%. Inte-
grated automatic detection algorithms with classification based on Gaussian Mixture Model (GMM) using the
Expectation Maximisation algorithm (EM), achieved an overall validated recognition rate of 87.60% which
increased to 88.87 recognition accuracy if the validated false alarm classifications were also to be included.
The proposed approach thus enables the recovery from ambient signals, detection and time-stamping of the
acoustic footprints of the swallowing process chain and thus further analytics to characterise the swallowing
process in terms of consistency, normality and possibly risk-assessing and localising the level of any swal-
lowing abnormality i.e. the dysphagia. As such this helps reduce the need for invasive techniques for the
examination and evaluation of patient’s swallowing process and enables diagnostic clinical evaluation based
only on acoustic data analytics and non-invasive clinical observations.
1 INTRODUCTION
The act of swallowing or deglutition constitutes a
complex process in humans. It is a critical enabler
for eating and drinking; ensuring the safe transport
of nourishment from the oral cavity to the stomach
through the pharynx and oesophagus while keeping
the epiglottis in the closed position to protect the tra-
chea and thus the airway security. A mouthful of
food, a bolus, or a gulp of water, once ingested will
then go through the oral, pharyngeal and oesophageal
stages of transport as phases of the swallowing pro-
cess. Any impairment along the above stages can
manifest as dysphagia or difficulty in swallowing
leading to laboured aspiration and/or coughing to
avoid chocking by attempts to expel any elements of
the food that may have been accidentally ingested into
the airway or misplaced or stuck somewhere along
the pharyngeal to stomach pathway.. Thus. Dyspha-
gia can be caused by a wide variety of complications
that can alter the functionality of different parts of the
throat beginning at the upper level, the buccal cav-
ity, through the pharynx and then at the oesophagus.
This can present as a pathology larynx, the oesoph-
agus, and finally at the sphincter or a neuro-motor
impairment causing difficulty in the orchestration of
coordinative structures that support the transport of
food from mouth to the stomach. The prevalence of
dysphagia increases with age, from 9% for subjects
aged 65 to 74, to 28% after age 85 (Singh and Hamdy,
2006). Statistics also show that 40% of people over
75 have experienced swal-lowing disorders and up to
66% of those affected are residents in a social/medical
institution (Kawashima et al., 2004). Dysphagia is
also common in the post-stroke population with up
to 76% of this group being affected. The mortal-
ity rate arising from suffocation due to a swallow-
ing disorder is however relatively low in the general
population; for example in France it stands as 5.99
cases per 100000 in men and 6.1 cases per 100000
Khlaifi, H., Badii, A., Istrate, D. and Demongeot, J.
Automatic Detection and Recognition of Swallowing Sounds.
DOI: 10.5220/0007310802210229
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 221-229
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
221
in women according to the National Institute for De-
mographic Studies (INED) (INED, 2013). However,
the majority of dysphagic cases often remain undiag-
nosed (Kawashima et al., 2004) and are therefore not
treated thus leading to otherwise preventable emer-
gencies and even deaths caused by some form of un-
diagnosed dysphagia. In any event once this disease
is diagnosed then a treatment involving physiotherapy
would be helpful but personal daily care by trained
physiotherapists is impossible to provide given their
lack of availability. However ensuring independent
living capability is crucial due to the demographic
trend. According to a recent report by INSEE, dat-
ing from August 2017 in 2013, 21% of men and 48%
of women aged 75 or over lived alone. It is essential
to be supported the safe ingestion of food by those liv-
ing alone and with swallowing difficulties. Respond-
ing to this challenge, this PhD research has designed,
implemented and evaluation a system for the automa-
tion of dysphagia therapy support in a way that should
be cost-effective, non-intrusive, and scalable for long-
term therapy management. Accordingly, the objective
of this research is to develop and validate a system for
an adaptive Swallowing Events Recogniser and Chok-
ing Risk Assessor Adaptation (SER-CORA) through
a non-invasive means of measuring the acoustic sig-
nature of the coordinative actuation of the muscles in-
volved in safe swallowing.
This paper is focused on the automatic recovery of
swallowing sounds from the captured ambient acous-
tic environment mix of sounds (speech plus noise) i.e.
detecting and recognising the swallowing sounds to
pave the way for the subsequent diagnostic analysis
of such acoustic footprints of the swallowing process
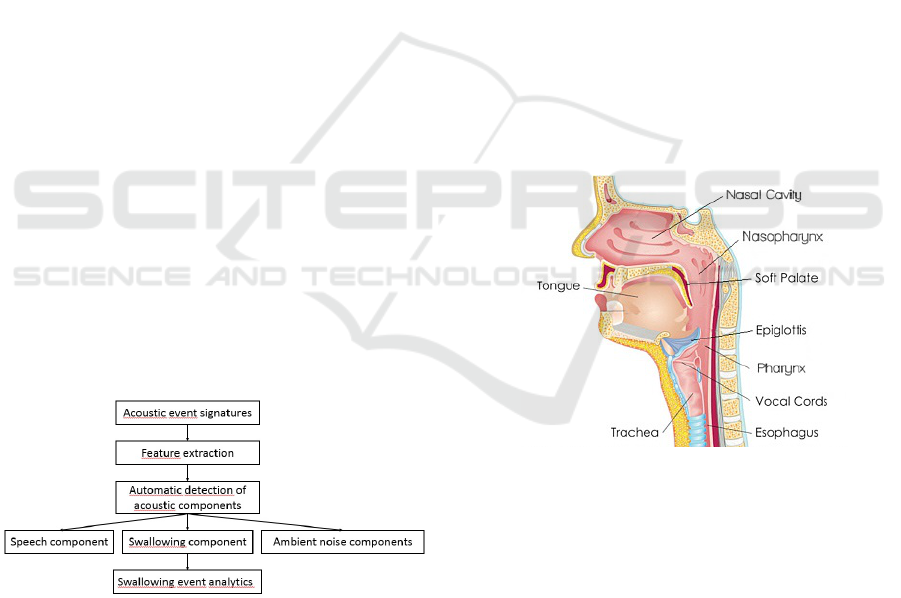
chain as illustrated in Figure 1 below
Figure 1: Analysis procedure of the proposed system.
2 ETHICAL DESIGN AND
DEPLOYMENT
In routine use the system will detect and recognise
swallowing events and can support the characterisa-
tion of the level of risk, sensing any choking precur-
sors and alerting of actual chocking. Such alerts can
be securely transmitted direct to the patient electronic
record via Bluetooth so as to be available for periodic
inspection and analysis by their clinician and phys-
iotherapist to inform diagnostic and remedial proto-
cols to be prescribed for the patient to support ongo-
ing therapy management.
3 PHYSIOLOGY OF THE
NORMAL SWALLOWING
PROCESS
As illustrated in Figure 2 below, the swallowing pro-
cess chain comprises of a set of coordinated muscle
movements controlled by the cranial nerves, trans-
ports food from the mouth to the stomach passing by
the pharynx while ensuring the safety of the respi-
ratory tract as the paths of air and food cross in the
pharynx. The physiology of normal swallowing was
originally described in three sequential phases which
are i) oral phase, ii) pharyngeal phase, and, iii) oe-
sophageal phase for drinking and swallowing foods
(solid/ liquid).
Figure 2: The anatomy of the human swallowing related
organs.
An initial phase can be added to this model which
is the preparatory phase for eating and swallowing
solid foods. Once the bolus is formed and is ready
to be expelled to the pharynx, the tip of the tongue
is raised up and applied against the alveolar ridge of
the upper incisors and the tongue takes the form of a
spoon where the bolus slips and forms a single mass.
The bolus is then moved backwards by the action of
the tongue as gradually applied to the palate from
front to back. At this stage, the soft palate ensures
the closure of the oropharynx and prevents the pen-
etration of the bolus into the pharynx; however, the
larynx is still open. Simultaneously, the velum rises
upwards to close the nasal fossae. By the time the
HEALTHINF 2019 - 12th International Conference on Health Informatics
222

bolus has reached the throat isthmus, the oral phase
(the voluntary phase of the swallowing process) is
over. The back of the tongue then moves forward and
forms an inclination allowing the bolus to move to-
wards the oropharyngeal cavity. In terms of control,
the pharyngeal and oesophageal phases which consti-
tute the swallowing reflex, without voluntary control,
called the swallowing reflex, has thus been triggered
as the food leaves the oral cavity and is moved on to
the pharyngeal stage. Precisely at this mo-ment, the
passage of food into the trachea is also pre-vented.
The larynx opens during chewing to allow breath-
ing and is closed as soon as the bolus arrives at the
base of the tongue. Simultaneously, the vocal cords
close ensuring airway closure, the moving cartilages
of the larynx (arytenoids) swing forward in the laryn-
geal vestibule, covered by the rocking movement of
the epiglottis. The larynx is pulled up and down by
the hyoid muscles, which places it under the protec-
tion of the base of the tongue. Thus, the pharyngeal
phase is triggered by the contact of the bolus with the
sensory receptors of the throat isthmus and of the oro-
pharynx. At this stage, breathing is interrupted and at
the same time the last stage of swallowing begins with
the bolus entering the oesophagus and being moved
on through the oesophageal peristaltic waves (muscle
contractions) to-wards the stomach. The pharyngeal
process is a continuous phenomenon in time, consid-
ered as a reflex accompanied simultaneously by the
velo-pharyngeal closure by the velum, by the laryn-
geal occlusion assured by the elevation of the larynx,
and, by the retreat of the tongue base, the movement
at the bottom of the epiglottis, the pharyngeal peri-
stalsis and finally the opening of the upper sphincter
of the oesophagus allowing the passage of the food
bolus into the oesophagus. This phase lasts less than
one second. The opening of the upper sphincter of the
oesophagus is initiated by the onset of the pharyngeal
peristalsis and food passage through the oesophagus
is ensured by the ongoing coordinated peristalsis of
pharyngeal and oesophageal stages.
4 DYSPHAGIA
Swallowing disorders or dysphagia can result from a
wide variety of structural and/or functional deficits of
the oral cavity, pharynx or oesophagus. Several ab-
normalities can cause a poorly-functioning oral cav-
ity. These include: i) Cleft lip and palate and (or-
tho) dental pathologies which can inhibit mastication
and impair the swallowing process. ii) Tumours af-
fecting the head or the neck have the potential to
cause oropharyngeal disorders by damaging the cra-
nial nerves. iii) Xerostomia or “dry mouth” can
disrupt the insalivation of the bolus and thus cause
the accidental passage of granular, or inadequately
chewed food, into the respiratory tract. iv) Infections
can cause inflammation and ulceration thus reduc-
ing the masticatory performance. v) Chronic Gastro-
esophageal reflux disease (GERD) can weaken the
oesophagus wall, affecting in some the normal peri-
stalsis contractions for propulsion of food, and the
functioning of the oesophagus sphincter. vi) Zenker’s
diverticulum or pharyngo-oesophageal diverticulum
can occur in the pharynx or oesophagus and cause
regurgitated into the pharynx, which may result in
coughing or aspiration. vii) Dysphagia can also arise
from iatrogenic dysfunction due to medication side-
effects or surgical complications.
5 TEXTURE OF FOOD
Depending on the state of the “swallowing mecha-
nism”, some foods may be hard to swallow and a
physiotherapist can suggest softer foods and bever-
ages to meet nutritional needs. In this context, foods
of varying texture, on a hard to easy-to-swallow
spectrum are considered including solid foods rang-
ing from the hardest-to-the-softest and smoothest to
roughest/most-granular and liquids of varying sticki-
ness and viscosity. Penman and Thomson’s review of
dysphagia diets (Penman and Thomson, 1998), main-
tains that thickened fluid can be classified, graduating
from thin fluid such as water and all juices thinner
than pineapple juice, to thick fluids such as milk and
most fruit juices, and liquids thickened with starch to
pureed consistency like yoghurt and compote. Solid
foods can range from soft to hardest-to-swallow such
as: fruits like melon and tomato, vegetables like car-
rots, potatoes and dry bread which is considered dif-
ficult to chew.
6 STATE OF THE ART IN
SWALLOWING ANALYSIS
Neurological disorders associated with sensory and
neuro-motor impairments arising from degenera-
tive diseases and/or cardio-vascular incidents such
a stroke may lead to disruption of the neuro-motor
orchestration required to accomplish the swallowing
process chain i.e. causing dysphagia. The clinical
evaluation of swallowing disorders involves a num-
ber of tools. The Video-Fluoroscopic Swallowing
Study (VFSS) which enables the real-time visuali-
Automatic Detection and Recognition of Swallowing Sounds
223

sation of bolus flow versus structural motion in the
upper aero-digestive tract, is considered as the pre-
ferred instrument for dysphagia assessment by the
clinicians. The VFSS helps identify the symptoms of
swallowing disorders and localise the physiological
causes triggering the typical repeated attempts at as-
piration associated with dysphagia conditions (Dodds
et al., 1990; Logemann, 1999). This enables the clin-
icians to make an accurate judgment about the pa-
tient’s level of sensory or neuro-motor impairment
and thus evaluate the extent of swallowing deficiency
to be treated (Martin-Harris and Jones, 2008). Fur-
thermore, ultrasound imaging has proven to be an ex-
cellent non-invasive means of studying the dynam-
ics of the oropharyngeal system and the function of
the muscles and other soft tissues of the orophar-
ynx during swallowing (Jones, 2012). This tool is
used to examine movements of the lateral pharyn-
geal wall (Miller and Watkin, 1997), and to visu-
alise and examine tongue movement during swallow-
ing (Shawker et al., 1983; Stone and Shawker, 1986).
It has also been used to track the motion of the hy-
oid bone and thereby evaluate the extent of normal
or abnormal swallowing (Sonies et al., 1996). Ultra-
sound imaging has also been used to assess the laryn-
geal phonation function by identifying the morphol-
ogy of vocal folds and quantifying the horizontal dis-
placement velocity of the tissue in the vibrating por-
tion of vocal folds (Hsiao et al., 2001). Based on the
assumption that it is advisable to lean forward whilst
eating, a system comprising of an Inertial Measure-
ment Unit (IMU) and an Electro-myographic sensor
(EMG), has been presented (Imtiaz et al., 2014) to
measure the head-neck posture and submental mus-
cle activity during swallowing. Another study (Huck-
abee et al., 2005) has concerned the comparative eval-
uation of the influence of two swallowing manoeu-
vres (effortful versus normal swallowing) on an ante-
rior supra-hyoid surface through EMG measurement
and pharyngeal manometric pressure. They found
significant change in both supra-hyoid surface EMG
and pharyngeal pressures during effortful swallowing
compared with normal swallowing. The same lab-
oratory has conducted further research to prove that
the tongue-to-palate emphasis during execution of ef-
fortful swallowing increases the amplitudes of sub-
mental surface EMG, the oro-lingual pressure, and
the upper pharyngeal pressure to a greater degree
than a strategy of inhibiting tongue-to-palate empha-
sis. The assessment of the functioning of the swal-
lowing process is also supported by acoustic mea-
surement and analysis. In 1990, Vice et al. (Vice
et al., 1990) described the detailed pattern of throat
sounds in newly born infants during suckle feeding.
In the same way as the normal swallowing process
can be divided into three stages (oral, pharyngeal
and oesophageal phases), the associated swallowing
sounds can be divided into three different segments
(Vice et al., 1990): Initial Discrete Sound (IDS), Bo-
lus Transit Sound (BTS) and Final Discrete Sound
(FDS). The opening of the cricoi-pharynx in the pha-
ryngeal phase contributes to the IDS and the bolus
transition into the oesophagus in pharyngeal phase
contributes to the BTS. The oesophageal phase then
contributes to the FDS. By applying a Hidden Markov
Model (HMM, researchers have also confirmed that
sounds arising from the swallowing process chain
comprise of three stages (Moussavi, 2005). An auto-
mated method has been set up to separate swallowing
sounds from breathing sounds using multilayer feed-
forward neural networks (Aboofazeli and Moussavi,
2004). The algorithm was able to detect the acous-
tic footprints of 91.7% of swallowing events cor-
rectly. Lazareck and Moussavi (Lazareck and Mous-
savi, 2004a; Lazareck and Moussavi, 2004b) pro-
posed a non-invasive, acoustic-based method to dif-
ferentiate between individuals with and without dys-
phagia. A wearable swallowing monitor system was
developed by Dond and Biswas, (Dong and Biswas,
2012) in order to assess the overall food and drink
intake habits as well as other swallowing-related dis-
orders. A piezo-respiratory belt able to be used for
identifying non-intake swallows, solid intake swal-
lows, and drinking swallows is reported to be un-
der development. A study of dietary eating activity
has been conducted using various sensors (Amft and
Troster, 2006; Amft and Tr
¨
oster, 2008). To moni-
tor arm movements, they used an inertial sensor con-
taining an accelerometer, gyro-scope and compass
sensor. For chewing cycle recognition, they used
an ear microphone and finally for swallowing pro-
cess recognition, they used both the stethoscope and
EMG electrodes attached at the infra-hyoid throat po-
sition. They found higher recognition accuracy for
body movements and chewing sounds than for swal-
lowing sounds which needed more investigation. Typ-
ical recognition rates achieved were around 79% for
movements, 86% for chewing and 70% for swallow-
ing. The recognition rate for swallowing was 68%
using the fusion approach of EMG and sound ver-
sus 75% if using EMG alone and 66% using sound
alone. Another study deployed a non-invasive wear-
able sensing system using a bone conduction mi-
crophone for capturing swallowing process sounds,
counting the number of mastications and classifying
the food swallowed according to its texture by means
of frequency spectrum analysis (Shuzo et al., 2009).
The large number of dysphagic patients has continued
HEALTHINF 2019 - 12th International Conference on Health Informatics
224
to inspire research on automated methods of moni-
toring and evaluation and classification of dysphagia
to support functional rehabilitation of patients’ swal-
lowing disorders. In the context of the e-Swallhome
project as part of the ANR Research Programme, our
study involved the monitoring of the patients suffer-
ing from swallowing disorders. Accordingly our ap-
proach was motivated to support the automated moni-
toring of the swallowing process chain through a non-
invasive and minimalist intervention strategy using
only one wearable neck-attached microphone. Thus
we have developed an acoustic-based system to detect
and classify swallowing events to be used to identify
cases of distress during food intake such as laboured
aspiration responsive to swallowing distress, chock-
ing which may or may not lead to asphyxiation and/or
falling.
7 METHODOLOGY
Based on the observations as presented above, our
analysis distinguished the sources of the factors af-
fecting the swallowing process as being of either in-
trinsic (e.g. neuro-physiologically mediated) or ex-
trinsic (e.g. food-texture-related) influence. Con-
sistent with the above etiological analysis of causal
and co-existing conditions, it was decided to recruit
a structured sample of participants for swallowing
event data acquisition including a representative num-
ber of participants to cover the above types of factors.
This was to help enhance the generalisability of the
adaptive algorithms to be designed to support Swal-
lowing Events Recognition and Choking Risk As-
sessment (SERCORA). Accordingly, 27 participants
were identified and a number of increasingly hard-
to-swallow food types were selected for swallowing
experiments to monitor the swallowing process. An
ethical consent confirming process was completed as
a pre-requisite to registration of the participants who
were also to complete a questionnaire to declare any
dysphagia condition or food allergies. Subsequently,
the first phase of the research study was implemented
as follows: In the initial stage of the study, 27 par-
ticipants took part to enable their swallowing event
data to be captured using a throat-mounted micro-
phone while swallowing the following substances to
establish a baseline profile for their swallowing as fol-
lows: i) Saliva; ii) Water; iii) Compote. Additionally,
data was also captured from each participant when
just breathing in order to establish the acoustic sig-
nature baseline associated with only the diaphragm
movement. This protocol was carried out with two
groups of volunteers: a control population of healthy
persons with no dysphagia history and persons with
swallowing disorders.
7.1 The Experimental Environment
In this project, non-invasive equipment was used to si-
multaneously record sounds associated with the swal-
lowing process and also respiratory habits during
food intake and other ambient sounds. These were
recorded using a discrete module of miniature omni-
directional microphone capsule (Sennheiser-ME 102)
with IMG Stageline MPR1 microphone pre-amplifier
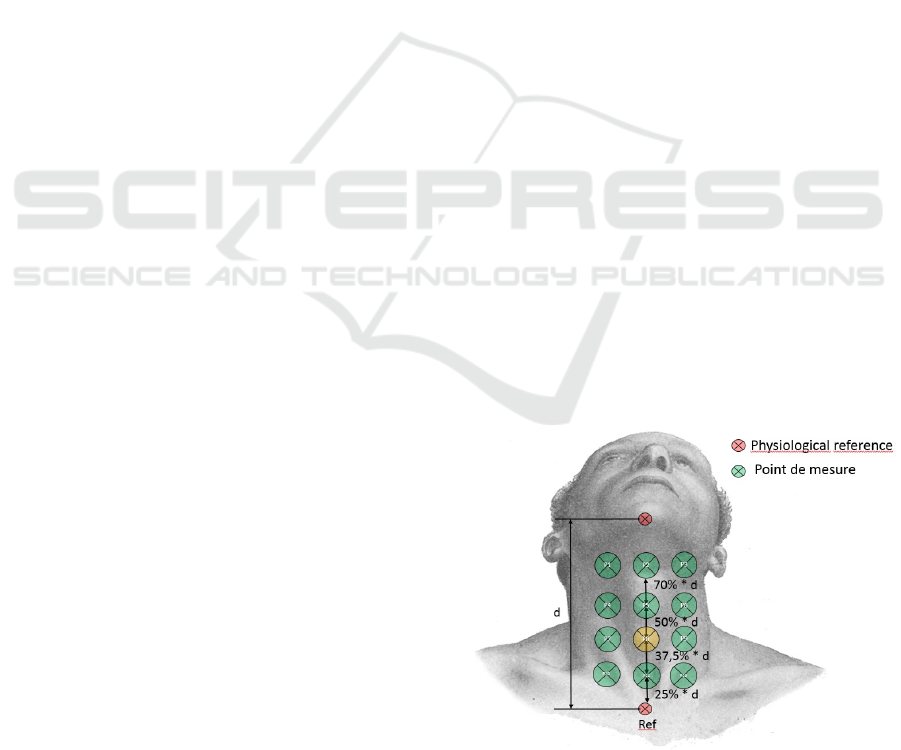
placed on the neck at a pre-studied position midway
between the Jugular Notch and the Laryngeal Promi-
nence optimised for the most effective acquisition of
the swallowing-elated signals, marked as P8 in Figure
3 presented below. Accordingly swallowing-related
sounds were recorded at a 44.1 KHz sampling rate.
The Signal was re-sampled at 16 KHz for the pro-
cessing as required. Participants were seated comfort-
ably on a chair. They were told that the equipment
would record swallowing-related sound. The base-
line function of each participant’s swallowing process
for the most fluid-like foods was established by cap-
turing the data from participants during the swallow-
ing of water and saliva as was also the case when
fed with compote in a teaspoon. Additionally, they
were asked to read aloud phonologically balanced
sentences and paragraphs. The sounds of their cough-
ing and yawning were also recorded. There were 39
recordings acquired from 27 people over about 11
hours. The sounds were manually annotated and a
corpus of approximately 45 minutes of swallowing-
related sounds, 90 minutes of speech and 35 minutes
of other ambient sounds were extracted and archived.
The table below shows the composition of the sounds.
Figure 3: Best microphone position.
Automatic Detection and Recognition of Swallowing Sounds
225
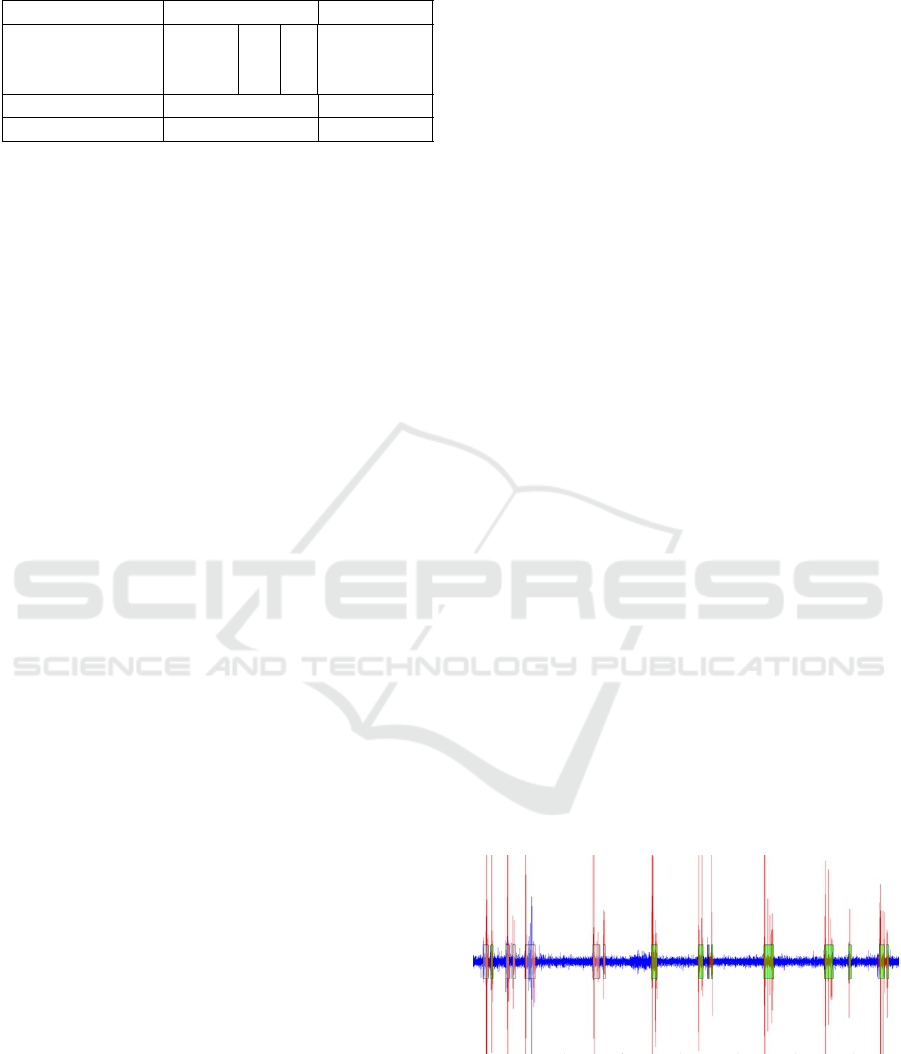
Table 1: Sound database.
Classes Nomber of files Duration
Swallowing
Compote
1943
565 45 minutes
Water 644
Saliva 734
Speech 705 1h 31 minutes
Sounds 839 35 minutes
8 ALGORITHMS AND RESULTS
8.1 Automatic Detection Algorithm
Automatic feature detection algorithms have been ex-
ploited in different domains (Boyer et al., 2016; Bap-
tista et al., 2018; Brune et al., 2017). Recently, the
analysis of swallowing sounds has received particular
attention (Lazareck and Moussavi, 2002; Aboofazeli
and Moussavi, 2004; Shuzo et al., 2009; Amft and
Troster, 2006) whereby swallowing sounds have been
recorded using microphones and accelerometers. The
algorithms developed in our research enabled the au-
tomatic detection of swallowing-related sounds from
a mixed stream of acoustic input as acquired through
the neck-worm microphone positioned as described
above. Frequency domain analysis enabled the de-
termination of the frequency band associated with
swallowing-related process chain; within this band
relative prominence of frequencies varied according
to the texture of the food being ingested. Our analysis
established that swallowing of liquid-like foods such
as compote was associated with a frequency signature
with an upper range of 3617 Hz; whereas swallowing
water was associated with a frequency signature be-
low 2300 Hz and for saliva the corresponding max-
imum frequency component remained below a max-
imum of 200 Hz. Wavelet decomposition was then
achieved using symlet wavelets at level 5. After com-
parison of different combinations of wavelet detail co-
efficients, details 5, 6 and 7 were selected for opti-
mal decomposition and resolution of the swallowing-
related sounds corresponding respectively to the fre-
quency bands 500-1000 Hz, 250-500 Hz and 125-250
Hz; accordingly a new signal was reconstituted as the
linear combination of the swallowing-related sounds
thus recovered. This resulting signal was analysed us-
ing a sliding window running along the signal with an
overlap of 50%. For each position of the current slid-
ing window, simultaneously, an associated energy cri-
terion was initially computed as the average energy of
the ten preceding windows and accordingly a thresh-
old for the start and end points of swallowing-related
sounds was established. This threshold was calcu-
lated as a function of the average energy as follows:
T hreshold = ε ∗ APW
i
+ α (1)
where APW
i
corresponds to an average of the
ten preceding windows of each position (position i).
Thus, following this framework, the starting point of
a swallowing-related signal would be detected where
the value of its associated energy as calculated ac-
cording to the above method, exceeded the thresh-
old and its end-point would be detected when the
associated energy fell below the threshold level of
the energy. However experiments showed the ap-
plication of the above associated energy criterion to
result in significant start-end detection errors as the
extreme peaks of the signal still affected the start-
end points detection disproportionately. Accordingly
it was proposed not to stop detection of swallow-
ing related sounds at the position where the associ-
ated energy falls below the threshold computed as
described above but to continue detecting and vali-
dating through a two-pass process whereby validated
start-end points were ultimately established accord-
ingly to a reference which results in greater than 80%,
recovery of swallowing events. Further-more, partial
detection was characterised when the associated en-
ergy falls below 80% of the above reference and a
false alarm was the detection when the associated en-
ergy did not correspond to that any annotated event
as described above. The overall validated detection
rate was of 86.09%. The validated reference rate was
22.19% and the partial reference rate was 63.90%.
The rate of missed references was 13.91% and the
false alarm rate was 24.97%. Figure 4 below dis-
plays the typical result of the automatic detection of
sounds in our experiment whereby the green panel
corresponds to the validated detections and the light
grey corresponds to partial automatic detections
Figure 4: Example of validated and partial detections of
swallowing-related sounds.
The overall automatic detection algorithm results
are set out in table 2 as presented below.
HEALTHINF 2019 - 12th International Conference on Health Informatics
226
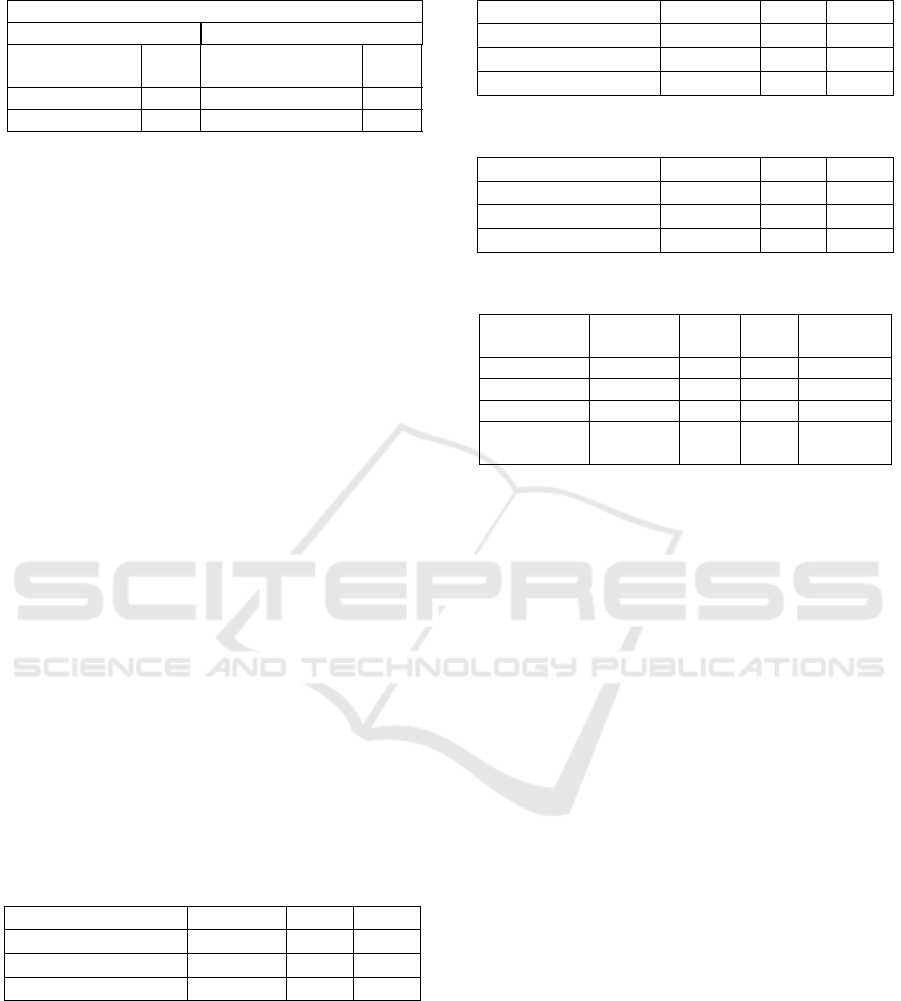
Table 2: Automatic detection results.
Number of references to be detected = 3501
Events References
Validated
events
2365 Validated references22.19%
Partial events 1052 Partial references 63.90%
False alarms 24.97% Missed references 13.91%
8.2 Classification
The Gaussian Mixture Model (GMM) have been es-
tablished as one of the most statistically mature meth-
ods for clustering (Reynolds and Rose, 1995). GMM
have been deployed widely in acoustic signal pro-
cessing such as speech recognition and music clas-
sification. Accordingly GMM are used to classify
signals without a-priori information about the gener-
ation process; this was conducted in two steps as re-
quired: a training step and a recognition step. The
training step with the Expectation Maximisation al-
gorithm was used for classification of the three types
of sounds in the input stream mix as set out previ-
ously: the swallowing-related, the speech and the am-
bient noise signal components. Parameters computed
on sounds were the Cepstre at the outlet of a Mel
scale filter bank, the Mel Frequency Cepstral Coeffi-
cients (MFCC) and in the linear scale, the Linear Fre-
quency Cepstral Coefficients (LFCC), and their delta
and delta-delta, differences between coefficients ob-
tained from one analysis window to another, to in-
clude the signal temporal variations. In order to take
into consideration the swallowing-related frequency
band, only 14 coefficients were chosen through 24 fil-
ters. The parameters yielding the best results were
the 14 LFCCs coefficients with delta-delta. Results
with manually annotated segments showed an overall
recognition rate of 95.49% as shown in table 3:
Table 3: Confusion matrix of manually annotated refer-
ences.
ORR = 95.49% Swallowing Speech Sounds
Swallowing 100% 0 0
Speech 0 91.49% 8.51%
Sounds 0 5.02% 94.98%
Next, a test was applied to automated detections.
For validated detections, a satisfactory recognition ac-
curacy of 93.32% for swallowing-related events was
achieved and an overall recognition rate of 87.60%.
For partial detections, an overall recognition rate of
71.59% was realised and a higher recognition rate of
80.42 was achieved for swallowing-related events us-
ing three classes as shown in the tables 4, 5 and 6
below.
Table 4: Confusion matrix of validated detections.
ORR = 87.60% Swallowing Speech Sounds
Swallowing 93.32% 0.65% 6.03%
Speech 3.83% 91.87% 4.30%
Sounds 20.43% 1.96% 77.60%
Table 5: Confusion matrix of partial detections.
ORR = 71.59% Swallowing Speech Sounds
Swallowing 80.42% 18.11% 1.47%
Speech 14.19% 75.31% 10.49%
Sounds 39.52% 1.43% 59.05%
Table 6: Confusion matrix of partial detections.
ORR =
88.87%
Swallowing Speech SoundsFalse alarm
Swallowing 87.10% 12.58% 0.32 0%
Speech 3.46% 95.60% 0.94 0%
Sounds 25.44% 1.78% 72.78 0%
False
alarm
0 0 0 100%
9 CONCLUSIONS AND
OUTLOOK
In this paper, the rationale for a cost-effective non-
intrusive automated care therapy management sup-
port system for dysphagia patients has been pre-
sented. This has enabled non-invasive adaptive Swal-
lowing Event Recognition and Choking Risk Assess-
ment “SER-CORA” using a minimalist intervention
methods enabling the recovery and detection of the
acoustic foot-prints of the swallowing-related events
based on the acoustic stream captured through a sin-
gle neck-worn microphone. With routine usage this
method can con-tribute valuable data intelligence to
establish refer-ence baselines to help characterise nor-
mal versus deviant swallowing event analytics to sup-
port the assessment of dysphagia conditions by clin-
icians, and the ongoing monitoring of the level of
improvement responsive to any interventions or the
emergence of any new pathology as determined and
managed by the clinicians. Accordingly such capa-
bility for non-invasive Swallowing Events Recogni-
tion and Choking Risk Assessment Adaptation (SER-
CORA) for each particular patient supports the detect
of changes and therefore informs the initial screen-
ing and diagnosis of potential pathologies affecting
the swallowing process chain and the localisation of
the causes of dysphagia thus informing clinical inves-
tigation and treatment. This paper presented the initial
phase of the SERCORA research study and the work
Automatic Detection and Recognition of Swallowing Sounds
227

ongoing in implementation of the final phase includ-
ing a large number of participants and a wider variety
of food types to realise the objective of providing scal-
able cost effective remote therapy management and
early warning support dysphagia conditions.
ACKNOWLEDGEMENTS
This work was conducted within the eSwallHome
(ANR research Programme) and was also funded
within the framework of eBioMed chair of BMBI lab-
oratory. This work is also a part of the STIC AMSUD-
EMONITOR project. We would also like to thank
Open Health Institute for their support for our re-
search study.
REFERENCES
Aboofazeli, M. and Moussavi, Z. (2004). Automated clas-
sification of swallowing and breath sounds. IEEE, Vol
3:7803–8439.
Amft, O. and Troster, G. (2006). Methods for detection
and classification of normal swallowing from muscle
activation and sound. In 2006 Pervasive Health Con-
ference and Workshops, pages 1–10.
Amft, O. and Tr
¨
oster, G. (2008). Recognition of dietary
activity events using on-body sensors. Artificial In-
telligence in Medicine, 42(2):121 – 136. Wearable
Computing and Artificial Intelligence for Healthcare
Applications.
Baptista, M., Silva, R., Jardim, H., and Quintal, A. (2018).
Perioperative electronic system - a new approach for
perioperative nursing performance. pages 505–509.
Boyer, C., Dolamic, L., Ruch, P., and Falquet, G. (2016).
Effect of the named entity recognition and sliding
window on the honcode automated detection of hon-
code criteria for mass health online content. In In
Proceedings of the 9th International Joint Conference
on Biomedical Engineering Systems and Technologies
(BIOSTEC 2016), volume 5, pages 151–158.
Brune, T., Brune, B., Eschborn, S., and Brinker, K. (2017).
Automatic visual detection of incorrect endoscope
adaptions in chemical disinfection devices. In In Pro-
ceedings of the 10th International Joint Conference
on Biomedical Engineering Systems and Technologies
(BIOSTEC 2017), pages 305–312.
Dodds, W. J., Logemann, J. A., and Stewart, E. T. (1990).
Radiologic assessment of abnormal oral and pharyn-
geal phases of swallowing. American Roentgen Ray
Society, Vol 154:965–974.
Dong, B. and Biswas, S. (2012). Swallow monitoring
through apnea detection in breathing signal. In 2012
Annual International Conference of the IEEE Engi-
neering in Medicine and Biology Society, pages 6341–
6344.
Hsiao, T. Y., Wang, C. L., Chen, C. N., Hsieh, F. J., and
Shau, Y. W. (2001). Noninvasive assessment of la-
ryngeal phonation function using colour doppler ul-
trasound imaging. Ultrasound in Med. & Bioll, Vol
27:1035–1040.
Huckabee, M.-L., Butler, S. G., Barclay, M., and Jit, S.
(2005). Submental surface electromyographic mea-
surement and pharyngeal pressures during normal and
effortful swallowing. Arch Phys Med Rehabil, Vol
86:2144–2149.
Imtiaz, U., Yamamura, K., Kong, W., Sessa, S., Lin, Z.,
Bartolomeo, L., Ishii, H., Zecca, M., Yamada, Y., and
Takanishi, A. (2014). Application of wireless iner-
tial measurement units and emg sensors for studying
deglutition preliminary results. IEEE, Vol 978:4244–
7929.
INED (February 12, 2013). ”journ
´
ees d’information sur
les troubles de la d
´
eglutition”. https://etablissements.
fhf.fr/annuaire/hopital-actualite.php?id=998.
Jones, B. (2012). Normal and abnormal swallowing: Imag-
ing In Diagnosis And Therapyl. Springer, 2nd edition.
Kawashima, K., Motohashi, P. Y., and Fujishima, P. I.
(2004). Prevalence of dysphagia among community-
dwelling elderly individuals as estimated using a
questionnaire for dysphagia screening. Dysphagia,
19:266–271.
Lazareck, L. J. and Moussavi, Z. (2002). Automated algo-
rithm for swallowing sound detection. In Canadian
Med. and Biol. Eng. Conf.
Lazareck, L. J. and Moussavi, Z. (2004a). Classification of
normal and dysphagic swallows by acoustical means.
IEEE Transactions on Biomedical Engineering, Vol
51:0018–9294.
Lazareck, L. J. and Moussavi, Z. (2004b). Swallowing
sound characteristics in healthy and dysphagic indi-
viduals. IEEE, Vol 3:7803–8439.
Logemann, J. A. (1999). Behavioral management for
oropharyngeal dysphagia. Folia Phoniatr Logop, Vol
51:199–212.
Martin-Harris, B. and Jones, B. (2008). The videofluoro-
graphic swallowing study. Phys Med Rehabil Clin N
Am, Vol 19:769–785.
Miller, J. L. and Watkin, K. L. (1997). Lateral pharyngeal
wall motion during swallowing using real time ultra-
sound. Dysphagia, Vol 12:125–132.
Moussavi, Z. (2005). Assessment of swallowing sounds
stages with hidden markov model. IEEE, Vol 6:7803–
8740.
Penman, J. and Thomson, M. (1998). A review of the tex-
tured diets developed for the management of dyspha-
gia. J Hum Nutr Diet, Vol 11:51–60.
Reynolds, D. and Rose, R. (1995). Robust text-independent
speaker identification using gaussian mixture speaker
models. Speech Commun, 17:121 – 136. Wearable
Computing and Artificial Intelligence for Healthcare
Applications.
Shawker, T. H., Sonies, B., Stone, M., and Baum,
B. J. (1983). Realtime ultrasound visualization of
tongue movement during swallowing. Dysphagia, Vol
11:485–490.
HEALTHINF 2019 - 12th International Conference on Health Informatics
228

Shuzo, M., Lopez, G., Takashima, T., Komori, S., Delau-
nay, J., Yamada, I., Tatsuta, S., and Yanagimoto, S.
(2009). Discrimination of eating habits with a wear-
able bone conduction sound recorder system. In SEN-
SORS, 2009 IEEE, pages 1666–1669.
Singh, S. and Hamdy, S. (2006). Dysphagia in stroke pa-
tients. Postgraduate medical journal, Vol 82:383–391.
Sonies, B. C., Wang, C., and Sapper, D. J. (1996). Eval-
uation of normal and abnormal hyoid bone move-
ment during swallowing by use of ultrasound duplex-
doppler imaging. Ultrasound in Med. & Bml, Vol
22:1169–1175.
Stone, M. and Shawker, T. H. (1986). An ultrasound exam-
ination of tongue movement during swallowing. Dys-
phagia, Vol 1:78–83.
Vice, F. L., Heinz, J. M., Giuriati, G., Hood, M., and Bosma,
J. F. (1990). Cervical auscultation of suckle feeding in
newborn infants. Developmental Medicine and Child
Neurolog, Vol 32:760–768.
Automatic Detection and Recognition of Swallowing Sounds
229
