
Image Segmentation using Gradient-based Histogram Thresholding
for Skin Lesion Delineation
Pedro M. M. Pereira
1,3
, Luis M. N. Tavora
2
, Rui Fonseca-Pinto
2
, Rui Pedro Paiva
3,4
,
Pedro A. A. Assuncao
1,2
and Sergio M. M. de Faria
1,2
1
Instituto de Telecomunicac¸˜oes, Portugal
2
ESTG, Polytechnic Institute of Leiria, Portugal
3
DEI - FCTUC, University of Coimbra, Portugal
4
CISUC, Department of Informatics Engineering, University of Coimbra, Portugal
Keywords:
Segmentation, Skin Lesion Detection, Medical Imaging, Dermoscopy.
Abstract:
Image segmentation is a key stage in medical image processing algorithms and machine learning classifiers
where identification of discriminative features are of utmost importance. In the case of skin lesions, most of the
existing image segmentation approaches aim at minimising some error metric between computed and ground-
truth regions of interest (ROI) defined by medical experts, where ROI delineation is not always considered.
This paper proposes an image segmentation method for skin lesion delineation, which expands traditional
histogram and clustering-based approaches to achieve the best trade-off between both. The proposed method
is capable of providing accurate details of the skin lesion borders, without deviating from the coarser borders
of the available ground-truth.
1 INTRODUCTION
In general, medical image processing systems include
a segmentation stage to identify regions of interest
(ROI) for further processing, which may include tex-
ture and colour analysis, feature extraction, etc. In the
case of pigmented skin lesions, due to the rather lim-
ited human capability to discriminate slight variations
in contrast and blur, precise identification of relevant
ROI boundaries poses a problem to dermatologists
(Claridge and Orun, 2002). Morphological aspects
of skin lesions alongside with the large number of
environment-variables (e.g. location in the body, skin
properties, lighting conditions and angle of view) fur-
ther increase the challenge of accurate segmentation
of the most useful ROI (Celebi et al., 2009a). This re-
sults in significant inter and intra-observer variability
and coarse ROI segmentation. Thus, to reduce the de-
pendence of human factors, different types of compu-
tational methods have been used for image segmenta-
tion, spanning over a quite considerable range of cat-
egories (Pathan et al., 2018).
Recent advances in machine learning approaches
are rapidly changing the landscape of medical image
processing algorithms for detection, recognition and
classification, where data sets with accurate ground-
truth image segmentation are increasingly necessary
both for training and validation of such new com-
putational models (Ker et al., 2018; Oliveira et al.,
2018). In the case of skin lesion segmentation, the dif-
ficulty of achieving accurate delineation of ROI bor-
ders manually, has driven research efforts to increase
the availability of ground-truth ROI through com-
putational methods (Cheng et al., 2015; K´echichian
et al., 2014). Particularly relevant in the applica-
tion scope of this work is the mobile system designed
for early detection of melanoma recently proposed in
(Do et al., 2018), which combines fast segmentation,
feature extraction and classification in resource con-
strained devices.
This paper proposes an accurate segmentation
method for pigmented skin lesions, envisaging delin-
eation of melanoma as the main application. In gen-
eral this kind of medical images produce bi-modal
histograms, and although this characteristic has been
used as the basis of different segmentation methods, it
results in either coarse borders or simply fails to pro-
vide significant ROI in images with low colour con-
trast and smooth texture transitions. Therefore, this
work addresses the problem of accurate identification
of the relevant ROI in such images, which includes the
ability to define the external border of the lesion with
84
Pereira, P., Tavora, L., Fonseca-Pinto, R., Paiva, R., Assuncao, P. and M. de Faria, S.
Image Segmentation using Gradient-based Histogram Thresholding for Skin Lesion Delineation.
DOI: 10.5220/0007354100840091
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 84-91
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

high level of precision. Taking into account the clin-
ical relevance of this aspect, a gradient-based metric
is devised to drive the proposed delineation method
across a refinement histogram-based segmentation al-
gorithm. Two different types of medical images are
targeted, which increases the challenge of achieving
efficient segmentation with accurate border details in
both cases, as pointed out in (Zhou et al., 2008). For
this purpose, the Dermofit dataset of macroscopic im-
ages (Ballerini et al., 2013) and the PH
2
dataset of
dermoscopic images (Mendonc¸a et al., 2013) are both
used in this work.
This work is organised as follows: Section 2
presents the background that is relevant for the pro-
posed method. Section 3 describes the Gradient-
based metric and Section 4 introduces the proposed
method. In Section 5 the obtained experimental re-
sults are presented, alongside with the associated dis-
cussion. Finally, in Section 6 some conclusions and
future work perspectives are presented.
2 SKIN LESION SEGMENTATION
- BACKGROUND
As mentioned above, images of skin lesions exhibit
two distinctive regions which are associated, one to
the lesion itself, and another to the surrounding skin.
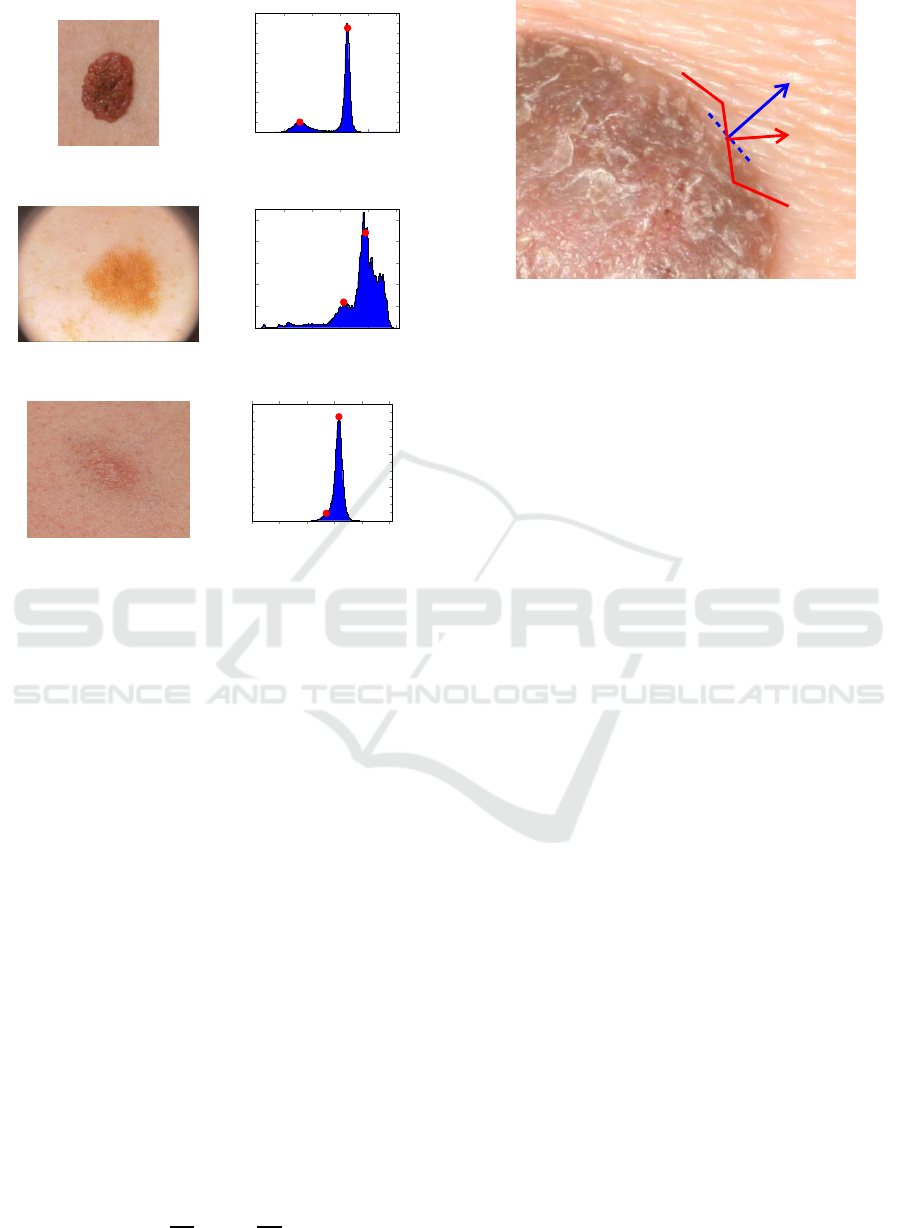
This leads to bi-modal histograms, as shown in Fig. 1,
for images of skin lesions with quite different charac-
teristics. This results not only from the lesion mor-
phology, but also from the use of different image ac-
quisition technologies and lighting conditions. For in-
stance, while accurate segmentation of the skin lesion
shown in Fig. 1a is quite easy to be obtained directly
from its well-defined histogram, in the case of Fig. 1c
it poses a problem due to the blurry borders and low
colour contrast. This can be confirmed by the corre-
sponding histogram shown on the right side of Fig. 1,
where the pixels belonging to the relevant ROI are
quite difficult to identify.
2.1 Histogram Thresholding
Histogram thresholding techniques have long been
used for segmentation of these type of images, where
the region of the lesion can be distinguished by its dif-
ferent tonality (Korotkov and Garcia, 2012). The un-
derlying idea of these methods is to perform a binary
partition of the image based on the luminance level
of each pixel, meaning in this case (e.g. Fig. 1) to
successfully separate the region of the lesion (darker
region ↔ left Y-peak: Y
Pmin
) from the surrounding
skin (right Y-peak: Y
Pmax
). In a simple formulation,
the segmentation challenge to be considered here is to
find an optimum criterion to define a threshold value
(Y
th
) that leads to an accurate ROI extraction, i.e., the
region of the image that contains the lesion. Different
threshold techniques exist for decades (Sahoo et al.,
1988), and this operation can be done either directly
on the Y-histogram or after a transformation T(Y), as
proposed in (Rajab et al., 2004). Nevertheless, the
performance of the method might strongly depend on
the distribution of luminance values, as inferred from
Fig. 1c and even Fig. 1b.
2.2 Clustering
Clustering algorithms have also been used for
skin lesion segmentation based on different ap-
proaches (Melli et al., 2006; Tasoulis et al., 2010;
Iyatomi et al., 2008; Ganster et al., 2001). The al-
gorithms can be fed with image information in differ-
ent formats such as RGB, YUV or YCbCr, but there
are also systems using only the luminance Y channel
since the inherent fusion process of the RGB chan-
nels allows inclusion of the relevant colour informa-
tion (Maglogiannis and Doukas, 2009). A quite ef-
ficient clustering approach is based on the so called
K-Means, or Lloyd’s algorithm (Lloyd, 1982), which
is an iterative data-partitioning algorithm that assigns,
for a predefined number of clusters, every input ob-
servation to only one of the clusters. In skin lesion
images, clustering algorithms may take advantage of
the bi-modal characteristics of the histogram to use
the corresponding peaks as the initial centroids.
2.3 Other Approaches
Several other segmentation approaches are worth-
while to be mentioned. For instance, the concept of
Fuzzy Differential Evolution Entropy (FT) was used
in (Sarkar et al., 2014), through an algorithm that cre-
ates fuzzy partitions from the image histogram. Then
the entropy is optimised to obtain the thresholds us-
ing a differential evolution meta-heuristic. Another
algorithm based on a similar approach is the Fuzzy
Clustering LevelSet (FL) (Li et al., 2011), which
uses a hybrid model that alternates between global
and local region competitions using spatial informa-
tion in the fuzzy clustering technique. For Quantiza-
tion approaches one can find early methods based on
PCT (Principal Components Transform) Median Cut
(PM) (Umbaugh et al., 1993), while Active Contours
were proposed in (Chan et al., 2000) and (Lankton
and Tannenbaum, 2008), referred as Chan-Vese (CV)
and Lankton Mean Separation (LM). In the former a
Mumford-Shah function is minimised over the length
Image Segmentation using Gradient-based Histogram Thresholding for Skin Lesion Delineation
85
0
50
100
150
200
250
0
0.5
1
1.5
·10
4
(a) A121a from (Ballerini et al., 2013)
0
50
100
150
200
250
0
0.2
0.4
0.6
0.8
1
·10
4
(b) IMD390 from (Mendonc¸a et al., 2013)
0
50
100
150
200
250
0
1
2
3
·10
4
(c) A67 from (Ballerini et al., 2013)
Figure 1: Skin lesion images (left) and the corresponding
luminance (Y) histograms (right), were red dots represent
peaks that correspond to the lesion and skin, respectively.
of the contour, while the latter uses local image statis-
tics and evolving contours based on local information.
Additionally, traditional methods like Otsu Threshold
(OT) (Otsu, 1979) and K-Means Colour (KC) (Melli
et al., 2006) also serve as baseline literature.
3 GRADIENT-BASED METRIC
The figure of merit that is herein proposed to assess
the accuracy of a given outside border line is based
on the rationale that the segmentation contour sep-
arates regions with substantially different tonalities.
Accordingly, the magnitude of the image gradient on
contour pixels is expected to yield higher values than
in other regions (i.e., either inside the lesion or in the
remaining skin). Following this argument, segmenta-
tion masks whose border lines exhibit higher gradient
magnitudes should more faithfully separate the two
regions of the image.
For a given image I, the gradient magnitude in
each pixel can be determined by Eq.(1),
~
G
j
=
"
∂I
j
∂x
2
+
∂I
j
∂y
2
#
1/2
, (1)
~
G
l
ˆn
l
Figure 2: Segmentation border line (red) and gradient: the
higher the projection of the image gradient
~
G onto ˆn
l
, the
more accurate is the border line (Image P348a from (Bal-
lerini et al., 2013)).
where j denotes the colour (or luminance) channel
under consideration (e.g., j = R,G,B,Y). For any
point of a border line defined by segmentation, the
projection of the gradient vector
~
G onto the orthogo-
nal direction of the line (ˆn
l
), defines an accuracy met-
ric for the border line. Such projection is given by (2).
G
⊥
l, j
=
~
G
l, j
· ˆn
l
(2)
This concept is depicted in Fig. 2, where one can
observe that the orthogonal direction of the segmen-
tation border line (red) is not aligned with the gradi-
ent at the same point. The higher the projection G
⊥
l, j
computed by (2) the better (i.e. more accurate) is the
lesion contour segment.
Therefore, following the above discussion, the av-
erage value of G
⊥
l, j
over all points of a contour line
l is used as the gradient-based metric to evaluate how
accurately a given segmentation contour represents
the outside border on a skin lesion.
4 PROPOSED METHOD
The image segmentation method proposed for skin le-
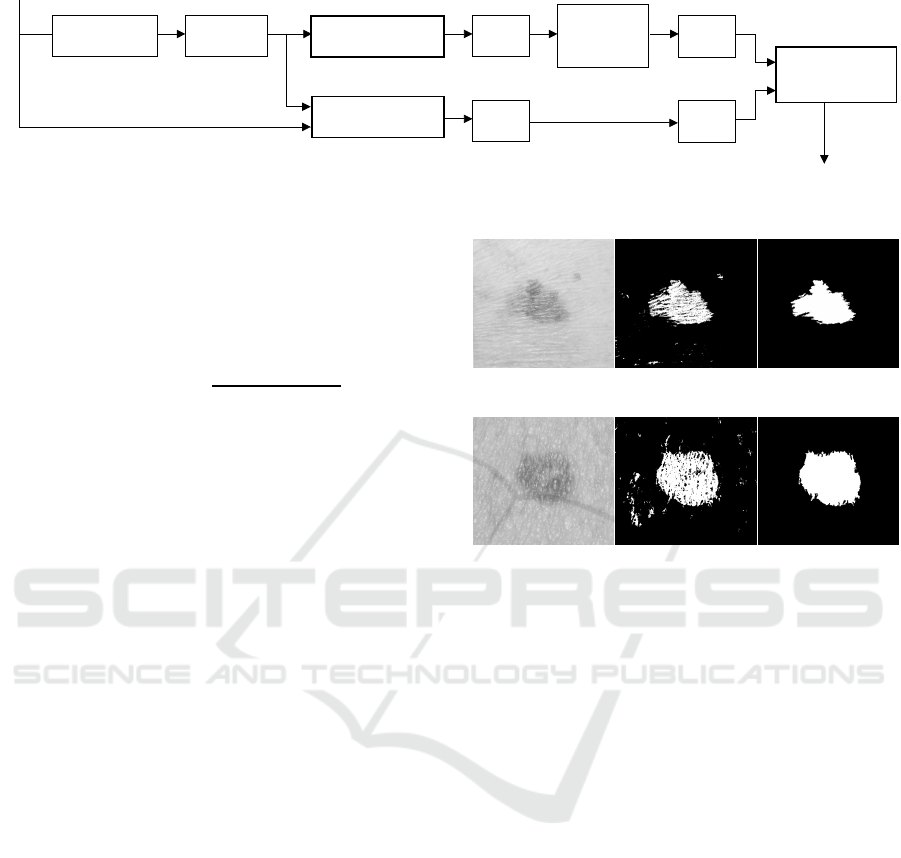
sion delineation follows the processing chain depicted
in Fig. 3. The underlying idea is to find an optimal
ROI delineation based on a trade-off between an ROI
with the highest gradient magnitude in the orthogo-
nal direction of its border line, and another ROI with
larger area but lower gradient. While the former iden-
tifies the sharpest boundary of the skin lesion, the lat-
ter contains more boundary information which is also
useful for medical analysis and monitoring of tempo-
ral evolution. As described next, gradient-based his-
togram thresholding and clustering are used to gener-
ate the two ROIs for final optimisation and delineation
of skin lesions.
BIOIMAGING 2019 - 6th International Conference on Bioimaging
86

4.1 Histogram Thresholding
The bi-modal characteristic of skin lesion image his-
tograms is used to determine the two most impor-
tant peaks, which in turn define the range limits
Y
Pmin
,Y
Pmax
for all possible thresholds, i.e., the best
ROI must be found by cutting the histogram at the
optimum threshold Y
th
∗
∈ {Y
Pmin
,Y
Pmax
} to be found
between the two peaks (e.g. red markers on the his-
tograms of Fig. 1). In the method shown in Fig. 3,
histogram thresholding is performed for all values be-
tween Y
Pmin
and Y
Pmax
, generating an equal number
of images and the corresponding segmentation masks.
After a filtering process to remove small isolated re-
gions and outliers, a clean ROI is determined for each
image and the average gradient G
⊥
is computed, as
defined by (2). Then the ROI whose border yields
the maximum average gradient is selected along with
its histogram threshold. In summary, this process en-
sures that the border line of such ROI is the one with
the highest tonality variations across it. In the remain-
ing sections, the ROI obtained by maximising the gra-
dient border through histogram thresholding is identi-
fied by HT.
4.2 Clustering
In the proposed method, clustering is used to identify
a coarse ROI for the skin lesion where the boundaries
include, in general, the smooth transition regions be-
tween the lesion and the surrounding healthy skin. In
this work the variant K-Means++ was selected, due
to its faster clustering convergence and also good dis-
criminative performance due different heuristics used
for finding centroids (Arthur and Vassilvitskii, 2007).
The iterative clustering process is carried out with a
maximum of 200 iterations seeking for two clusters
with a global minimum of the Euclidean distance to
cluster-centre. For the sake of reproducibility, the
initial centroids are defined as the histogram peaks.
In the remaining sections, the ROI obtained through
clustering is identified by KM.
4.3 Filtering
Due to noise, inherent illumination variations and
other factors, both the histogram thresholding and
clustering methods described above produce ROIs
with binary masks that include not only a large blob
(the skin lesion region) but also other small isolated
regions spread across the whole image. The filter-
ing process devised to remove such unwanted regions
assumes that the lesion region limits are fully lo-
cated within the image, so the first operation is to
remove all isolated regions with any boundary coin-
cident with the image borders. This is done by us-
ing a flood-fill algorithm based on morphological re-
construction (Soille, 2013). This first cleansing op-
eration is especially relevant when processing images
from the PH
2
dataset, as they exhibit a black circu-
lar frame artificially introduced in the dermatoscope
digitisation process. The relevant ROI containing the
lesion is then determined by extracting the blob with
the largest area in the binary mask, using a labelling
procedure (Haralick and Shapiro, 1992, p. 40-48).
4.4 Optimised Segmentation
The optimisation step aims at improving delineation
of skin lesions by selectively expanding the ROI
border that was previously found through histogram
thresholding with gradient maximisation, in order to
further include relevant areas of transition regions.
This is necessary because gradient maximisation of-
ten leads to stringent contour lines which only in-
clude the inner parts of the lesion and leave out rel-
evant transition regions. Taking into account that
clustering-based segmentation usually results in the
inclusion of a larger region around the inner part
of the lesion, the proposed optimisation procedure
achieves the best trade-off between gradient maximi-
sation and increased ROI area to include transition re-
gions. This is done by finding an optimum threshold
(Y
th
∗
) that maximises, simultaneously, both the gradi-
ent of the border line G
⊥
and the ROI area.
For each ROI obtained through histogram thresh-
olding (HT) and clustering (KM), as described above,
let us define the following reference values:
• G
⊥,KM
and G
⊥,HT
: the average gradient of the
border (in (2));
• A
KM
and A
HT
: the area of the ROI, i.e. skin lesion.
The corresponding values associated to an arbitrary
histogram thresholdY
th
are defined as G
⊥,Y
th
and A
Y
th
,
respectively. The ratios R
G
(Y
th
) and R
A
(Y
th
) (expres-
sion (3)) define the relative gradient and relative area
of any ROI obtained with threshold Y
th
, using the HT
and KM ROIs as references.
R
G
(Y
th
) =
G
⊥,Y
th
G
⊥,KM
R
A
(Y
th
) =
A
Y
th
min(A
KM
,A
HT
)
(3)
The optimisation procedure consists in finding the
optimum threshold Y
th
∗
that maximises both R
G
(Y
th
)
and R
A
(Y
th
). This is accomplished by maximising
their product, provided that the selected maximum
does not lead to gradient values below that of the
KM ROI (G
⊥,KM
) and the new ROI area falls between
Image Segmentation using Gradient-based Histogram Thresholding for Skin Lesion Delineation
87
Image
Histogram
Histogram
Histogram
Peak
Detection Thresholding
Segmentation
Filter
Filter
Cut
max(G
⊥
)
G
⊥,HT
A
HT
G
⊥,KM
A
KM
ROI
Clustering
Optimization
Final ROI
Figure 3: Proposed scheme.
those of HT and KM ROIs. This is equivalent to solve
the following constrained maximisation problem:
Y
th
∗
= arg max
Y
th
∈Y
R
G
(Y
th
)R
A
(Y
th
) (4)
subject to:
R
G
≥ 1∧ R
A
≥ 1∧
A
Y
th
max(A
KM
,A
HT
)
≤ 1 (5)
In summary, the optimal histogram threshold Y
th
∗
to be used for delineation of skin lesions is found
through a trade-off between border gradient and
amount of transition area included in the ROI.
5 EXPERIMENTAL RESULTS
AND DISCUSSION
The performance of the segmentation algorithm de-
scribed in Section 4 was evaluated using sets of im-
ages from different databases. A total of 195 images
from the Dermofit dataset (Ballerini et al., 2013) and
32 images from the PH
2
dataset (Mendonc¸a et al.,
2013) were used. This selection followed two main
criteria: i) images without hair strands crossing the
lesion, i.e., as hairless as possible and ii) lesion limits
within the image, i.e., the whole lesion boundary fully
located inside the image.
In the first stage the input grayscale image passes
through two segmentation processes, namely His-
togram Thresholding and Clustering Segmentation.
As pointed out in section 4, the output of both algo-
rithms may exhibit some image artefacts, which can
be observed in Fig. 4b and Fig. 4e. Then the effi-
ciency of the filtering stage, that is used after both
segmentation algorithms (described in Section 4.3),
in removing the small isolated regions, is shown in
Fig. 4c and Fig. 4f. In these images it is possible to
observe that the filtering process is quite effective in
providing an accurate lesion/skin segmentation mask
without harming the border details.
After the segmentation and filtering stages, accu-
rate ROI delineation is performed, following the opti-
misation procedure described in the previous section.
(a) Image B964 (b) Histogram TH (c) Filtered
(d) image B241 (e) Clustering (f) Filtered
Figure 4: Image segmentation using Histogram thresh-
olding (HT) and K-Means (KM) clustering: (a,d) original
grayscale images from (Ballerini et al., 2013), (b,e) seg-
mentation output, (c,f) final binary mask after the filtering
operation.
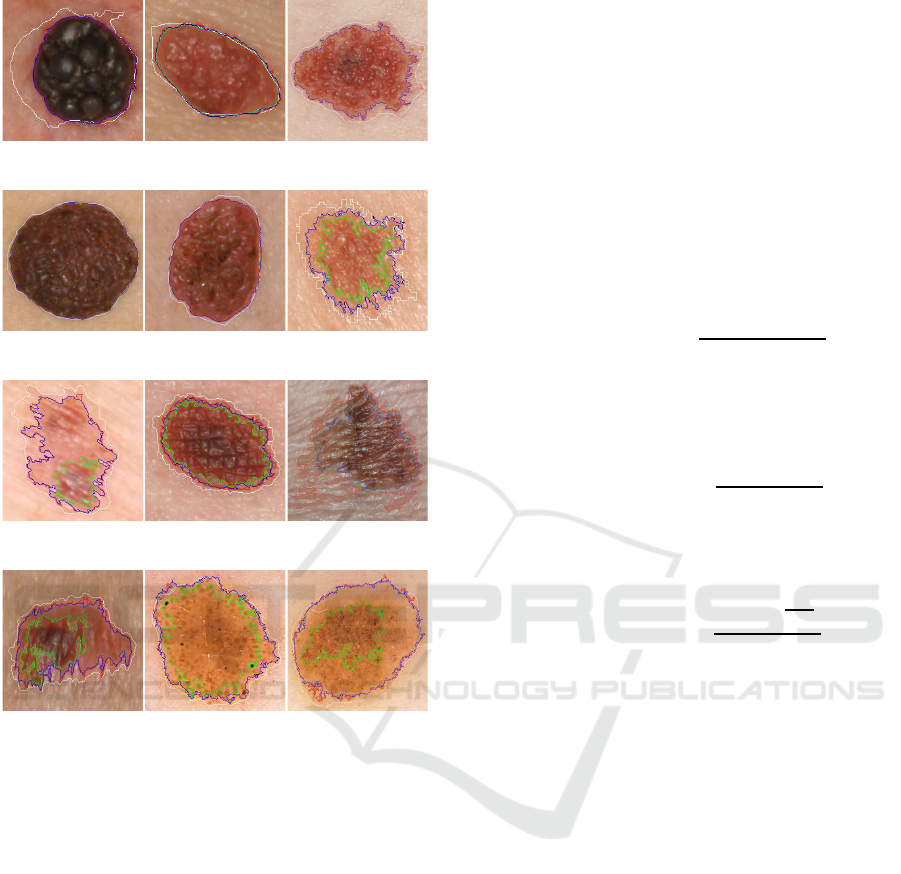
For visual evaluation and discussion, a set of rep-
resentative types of skin lesions have been selected
from the datasets to represent the segmentation re-
sults, which is depicted in Fig. 5.
In Fig. 5 the lesion segmentation using KM is rep-
resented by a red line, the HT by a green line and the
Proposed method by a blue line. The white line rep-
resents the ground-truth (GT) provided by the dataset.
From the representative results presented in
Fig. 5a to Fig. 5e, it can be observed that the algo-
rithms are in general quite effective in the segmenta-
tion of images and delineation of the relevant ROI.
The Histogram Thresholding method (HT) is able
to achieve accurate delineation when there is a sharp
tonality difference between the skin lesion and the
surrounding skin. However, as mentioned before, in
images with smoother lesion-to-skin transitions, the
highest value of G
⊥
, may result in a segmented re-
gion that is smaller than expected. This effect can be
seen in images from Fig. 5f to Fig. 5l. This kind of
output is not the most useful from the clinical point of
view, as it may exclude a relevant part of the lesion.
In the case of Clustering ROI segmentation (KM),
BIOIMAGING 2019 - 6th International Conference on Bioimaging
88
(a) D427b (b) D155b (c) B447a
(d) D384 (e) A121a (f) B311c
(g) A92 (h) B17a (i) B964
(j) B1075 (k) IMD103 (l) IMD175
Figure 5: Skin lesion segmentation using KM (red), HT
(green) and Proposed (blue). The white line corresponds
to the dataset provided ground-truth (GT). Images (a)
to (j) are from (Ballerini et al., 2013), and (k) and (l)
from (Mendonc¸a et al., 2013).
in general, the segmented region may include smooth
transition regions between the lesion and the sur-
rounding healthy skin. This commonly results in
a larger region than that obtained by the Histogram
Threshold method, as can be seen in images Fig. 5h,
Fig. 5i, Fig. 5j and Fig. 5l. In such cases, this might
not represent the best option as well.
In order to overcome the HT underestimation of
the ROI and the possible KM overestimation, the pro-
posed combined method relies on a trade-off between
the ROI and the border gradient. As can be observed
in all images of Fig. 5, the blue line always represents
a more precise delineation of the ROI.
Some authors compare the segmentation results
with the ground-truth (GT) segmentation masks pro-
vided in the databases. Nevertheless, as can be visu-
ally observed in Fig. 5a, Fig. 5b and Fig. 5f, the GT
borders are not as accurate and spatially detailed as
those obtained with the used algorithms. It can also
be observed that the GT lines often miss areas with
high texture variations.
In numerical terms, other performance indicators
are usually considered as benchmarks (Hance et al.,
1996; Celebi et al., 2009b; Garnavi et al., 2011):
• Border Error (BE), in (6), a measure of the
fraction of non-overlapping (exclusive-OR, ⊕)
segmentation regions (Area) between the pro-
posed segmentation (PS) method and the dataset
ground-truth segmentation (GT);
BE(PS, GT) =
Area(PS⊕ GT)
Area(GT)
(6)
• True Detection Rate (TDR), in (7), an indicator of
the ratio of number of pixels (np) that are correctly
classified as lesion;
TDR(PS, GT) =
np(PS ∩ GT)
np(GT)
(7)
• False Positive Rate (FPR), in (8), which accounts
for the number of pixels that are incorrectly clas-
sified as lesion.
FPR(PS,GT) =
np(PS ∩
GT)
np(GT)
(8)
The results obtained for these indicators are pre-
sented in Table 1, alongside with those from the meth-
ods presented in Section 2.3. It can been seen that the
performance of the proposed algorithm (Prop) is gen-
erally inline with others published in the literature,
though not always consistent for all metrics. How-
ever, it should be kept in mind that these indicators
use the GT as reference, which does not provide seg-
mentation masks with as much spatial details as those
herein obtained. Such difference can be clearly ob-
served in Fig. 5
The gradient metric defined in Section 3 was
also used to assess the performance of the proposed
method. The quotient of gradient between delin-
eations for both datasets was determined for such pur-
pose and the results are presented in Table 2. Observ-
ing its first three lines, it can be seen that HT has on
average the highest G
⊥
values, as the method was op-
timised for such purpose, though in some cases this
also corresponds to inaccurate segmentation. The sec-
ond group of three lines make it clear that the Prop
method outperforms KM while only slightly compro-
mising the G
⊥
value in comparison with the max-
imum of HT. The remaining data on the table also
shows that the Prop method produces segmentations
Image Segmentation using Gradient-based Histogram Thresholding for Skin Lesion Delineation
89
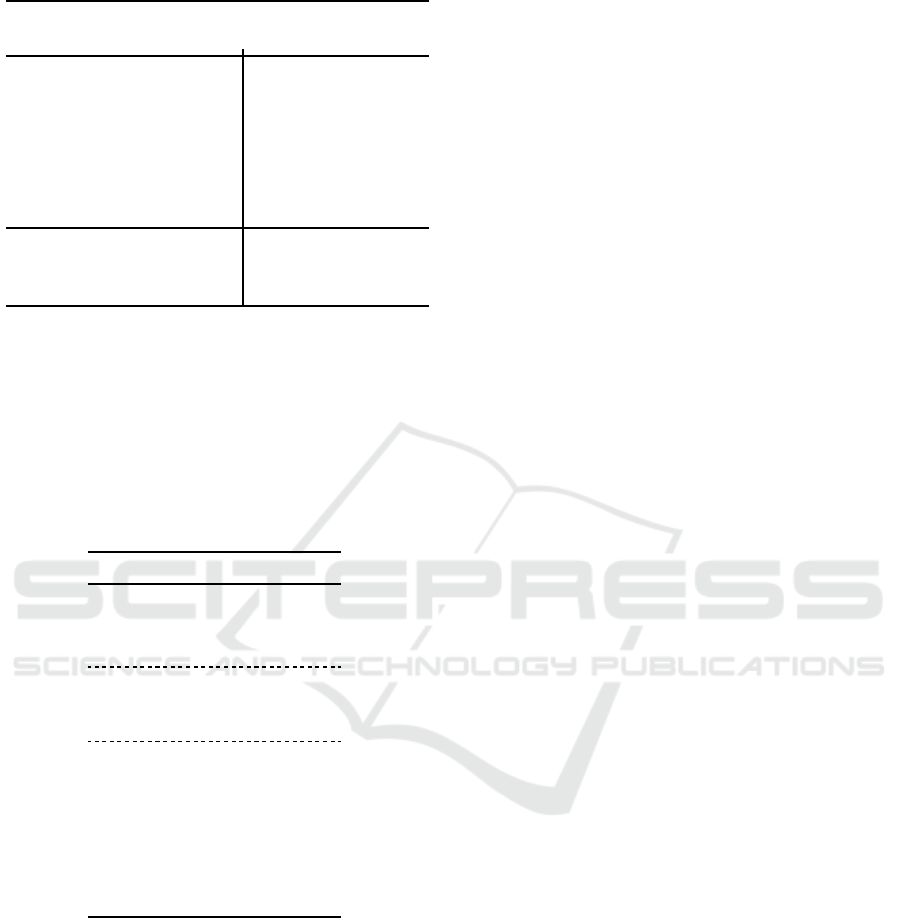
Table 1: Ground Truth (GT)-based indicators (%).
Method
Dermofit PH2
BE TDR FPR BE TDR FPR
OT 32.569 76.386 7.483 20.907 83.999 7.061
KC 53.475 78.439 8.581 18.174 86.041 6.313
FT 1.017 37.819 25.731 50.499 50.734 15.319
FL 2.439 49.500 50.084 20.352 82.846 6.872
PM 1.504 97.456 23.096 1.107 93.442 24.524
CV 2.383 69.381 35.618 48.521 68.161 14.119
LM 2.164 0.0003 52.467 1.604 94.543 36.550
HT 36.253 64.920 9.409 34.763 65.586 11.165
KM 21.592 79.336 5.554 17.732 85.113 6.085
Prop 23.034 78.283 6.008 20.336 81.676 6.950
with G
⊥
values higher than any of the other algo-
rithms previously introduced. This means that skin
lesion delineation obtained by the proposed method
is more accurate than the others because the border
line is found where the gradient is higher, i.e., a bet-
ter discrimination between lesion and normal skin is
obtained.
Table 2: Average Border Gradient indicators.
Indicators Dermofit PH2
G
⊥,HT
/ G
⊥,KM
1.173 1.271
G
⊥,Prop
/ G
⊥,HT
0.933 0.917
G
⊥,Prop
/ G
⊥,KM
1.082 1.086
G
⊥,HT
/ G
⊥,GT
3.908 4.742
G
⊥,KM
/ G
⊥,GT
3.364 3.997
G
⊥,Prop
/ G
⊥,GT
3.688 4.301
G
⊥,Prop
/ G
⊥,OT
1,831 1,916
G
⊥,Prop
/ G
⊥,KC
1,831 1,921
G
⊥,Prop
/ G
⊥,FT
2,268 1,787
G
⊥,Prop
/ G
⊥,FL
6,429 1,829
G
⊥,Prop
/ G
⊥,PM
2,894 2,701
G
⊥,Prop
/ G
⊥,CV
1,175 1,086
G
⊥,Prop
/ G
⊥,LM
3,376 3,191
6 CONCLUSIONS
This paper addressed the segmentation of skin lesion
images using both histogram thresholding and clus-
tering algorithms to overcome the limitations of each
one on its own. A gradient-based method was devised
for optimised thresholding and ROI border quality pa-
rameter. The segmentation masks obtained for the fi-
nal ROIs indicate that this method is quite accurate in
delineation of the relevant lesion regions containing
for a wide range of images. The experimental vali-
dation, using two publicly available images datasets,
show that the proposed approach is effective in delin-
eating skin lesions with detailed geometry in regions
with quite diverse tonality variations. The accurate
delineation of skin lesions is a relevant achievement
to provide discriminative features for machine learn-
ing algorithms and also to investigate patterns of tem-
poral evolution of the borders.
ACKNOWLEDGEMENTS
This work was supported by the Fundac¸˜ao para a
Ciˆencia e Tecnologia, Portugal, under PhD Grant
SFRH/BD/128669/2017 and project PlenoISLA
PTDC/EEI-TEL/28325/2017, in the scope of R&D
Unit 50008, through national funds and where
applicable co-funded by FEDER – PT2020.
REFERENCES
Arthur, D. and Vassilvitskii, S. (2007). k-means++: The
advantages of careful seeding. In Proceedings of the
eighteenth annual ACM-SIAM symposium on Discrete
algorithms, pages 1027–1035. Society for Industrial
and Applied Mathematics.
Ballerini, L., Fisher, R. B., Aldridge, B., and Rees, J.
(2013). A color and texture based hierarchical k-nn
approach to the classification of non-melanoma
skin lesions. In Color Medical Image Analysis,
pages 63–86. Springer. https://licensing.edinburgh-
innovations.ed.ac.uk/i/software/dermofit-image-
library.html.
Celebi, M. E., Iyatomi, H., Schaefer, G., and Stoecker, W. V.
(2009a). Lesion border detection in dermoscopy im-
ages. Computerized medical imaging and graphics,
33(2):148–153.
Celebi, M. E., Schaefer, G., Iyatomi, H., Stoecker, W. V.,
Malters, J. M., and Grichnik, J. M. (2009b). An im-
proved objective evaluation measure for border detec-
tion in dermoscopy images. Skin Research and Tech-
nology, 15(4):444–450.
Chan, T. F., Sandberg, B. Y., and Vese, L. A. (2000). Ac-
tive contours without edges for vector-valued images.
Journal of Visual Communication and Image Repre-
sentation, 11(2):130–141.
Cheng, I., Sun, X., Alsufyani, N., Xiong, Z., Major, P.,
and Basu, A. (2015). Ground truth delineation for
medical image segmentation based on local consis-
tency and distribution map analysis. In 37th Annual
International Conference of the IEEE Engineering in
Medicine and Biology Society (EMBC), pages 3073–
3076.
Claridge, E. and Orun, A. (2002). Modelling of edge pro-
files in pigmented skin lesions. In Proceedings of med-
ical image understanding and analysis, pages 53–56.
BIOIMAGING 2019 - 6th International Conference on Bioimaging
90

Do, T. T., Hoang, T., Pomponiu, V., Zhou, Y., Zhao, C.,
Cheung, N. M., Koh, D., Tan, A., and Hoon, T.
(2018). Accessible melanoma detection using smart-
phones and mobile image analysis. IEEE Transactions
on Multimedia, pages 1–1.
Ganster, H., Pinz, P., Rohrer, R., Wildling, E., Binder,
M., and Kittler, H. (2001). Automated melanoma
recognition. IEEE transactions on medical imaging,
20(3):233–239.
Garnavi, R., Aldeen, M., and Celebi, M. (2011). Weighted
performance index for objective evaluation of border
detection methods in dermoscopy images. Skin Re-
search and Technology, 17(1):35–44.
Hance, G. A., Umbaugh, S. E., Moss, R. H., and Stoecker,
W. V. (1996). Unsupervised color image segmenta-
tion: with application to skin tumor borders. IEEE
Engineering in Medicine and Biology Magazine,
15(1):104–111.
Haralick, R. M. and Shapiro, L. G. (1992). Computer and
robot vision. Addison-wesley.
Iyatomi, H., Oka, H., Celebi, M. E., Hashimoto, M., Hagi-
wara, M., Tanaka, M., and Ogawa, K. (2008). An
improved internet-based melanoma screening system
with dermatologist-like tumor area extraction algo-
rithm. Computerized Medical Imaging and Graphics,
32(7):566–579.
K´echichian, R., Gong, H., Revenu, M., Lezoray, O., and
Desvignes, M. (2014). New data model for graph-
cut segmentation: Application to automatic melanoma
delineation. In 2014 IEEE International Conference
on Image Processing (ICIP), pages 892–896.
Ker, J., Wang, L., Rao, J., and Lim, T. (2018). Deep learning
applications in medical image analysis. IEEE Access,
6:9375–9389.
Korotkov, K. and Garcia, R. (2012). Computerized anal-
ysis of pigmented skin lesions: A review. Artificial
intelligence in medicine, 56(2):69–90.
Lankton, S. and Tannenbaum, A. (2008). Localizing region-
based active contours. IEEE transactions on image
processing, 17(11):2029–2039.
Li, B. N., Chui, C. K., Chang, S., and Ong, S. H. (2011). In-
tegrating spatial fuzzy clustering with level set meth-
ods for automated medical image segmentation. Com-
puters in biology and medicine, 41(1):1–10.
Lloyd, S. (1982). Least squares quantization in pcm. IEEE
transactions on information theory, 28(2):129–137.
Maglogiannis, I. and Doukas, C. N. (2009). Overview of ad-
vanced computer vision systems for skin lesions char-
acterization. IEEE transactions on information tech-
nology in biomedicine, 13(5):721–733.
Melli, R., Grana, C., and Cucchiara, R. (2006). Comparison
of color clustering algorithms for segmentation of der-
matological images. In Medical Imaging 2006: Image
Processing, volume 6144, page 61443S. International
Society for Optics and Photonics.
Mendonc¸a, T., Ferreira, P. M., Marques, J. S., Mar-
cal, A. R., and Rozeira, J. (2013). Ph2-a der-
moscopic image database for research and bench-
marking. In Engineering in Medicine and Biol-
ogy Society (EMBC), 2013 35th Annual International
Conference of the IEEE, pages 5437–5440. IEEE.
http://www.fc.up.pt/addi/ph2%20database.html.
Oliveira, R. B., Papa, J. P., Pereira, A. S., and Tavares,
J. M. R. (2018). Computational methods for pig-
mented skin lesion classification in images: review
and future trends. Neural Computing and Applica-
tions, 29(3):613–636.
Otsu, N. (1979). A threshold selection method from gray-
level histograms. IEEE transactions on systems, man,
and cybernetics, 9(1):62–66.
Pathan, S., Prabhu, K. G., and Siddalingaswamy, P. (2018).
Techniques and algorithms for computer aided diag-
nosis of pigmented skin lesions – a review. Biomedi-
cal Signal Processing and Control, 39:237–262.
Rajab, M., Woolfson, M., and Morgan, S. (2004). Applica-
tion of region-based segmentation and neural network
edge detection to skin lesions. Computerized Medical
Imaging and Graphics, 28(1):61–68.
Sahoo, P. K., Soltani, S., and Wong, A. K. (1988). A survey
of thresholding techniques. Computer vision, graph-
ics, and image processing, 41(2):233–260.
Sarkar, S., Paul, S., Burman, R., Das, S., and Chaudhuri,
S. S. (2014). A fuzzy entropy based multi-level im-
age thresholding using differential evolution. In In-
ternational Conference on Swarm, Evolutionary, and
Memetic Computing, pages 386–395. Springer.
Soille, P. (2013). Morphological image analysis: princi-
ples and applications. Springer Science & Business
Media.
Tasoulis, S., Doukas, C., Maglogiannis, I., and Plagianakos,
V. (2010). Classification of dermatological images us-
ing advanced clustering techniques. In Engineering in
Medicine and Biology Society (EMBC), 2010 Annual
International Conference of the IEEE, pages 6721–
6724. IEEE.
Umbaugh, S. E., Moss, R. H., Stoecker, W. V., and
Hance, G. A. (1993). Automatic color segmenta-
tion algorithms-with application to skin tumor feature
identification. IEEE Engineering in Medicine and Bi-
ology Magazine, 12(3):75–82.
Zhou, H., Chen, M., Zou, L., Gass, R., Ferris, L., Dro-
gowski, L., and Rehg, J. M. (2008). Spatially con-
strained segmentation of dermoscopy images. In
Biomedical Imaging: From Nano to Macro, 2008.
ISBI 2008. 5th IEEE International Symposium on,
pages 800–803. IEEE.
Image Segmentation using Gradient-based Histogram Thresholding for Skin Lesion Delineation
91
