
A Machine Learning-based Approach for Collaborative Non-Adherence
Detection during Opioid Abuse Surveillance using a Wearable Biosensor
Rohitpal Singh
1
, Brittany Lewis
1
, Brittany Chapman
2
, Stephanie Carreiro
2
and Krishna Venkatasubramanian
1
1
Worcester Polytechnic Institute, Worcester, MA, U.S.A.
2
University of Massachusetts Medical School, Worcester, MA, U.S.A.
Stephanie.Carreiro@umassmed.edu, kven@wpi.edu
Keywords:
Opioid Epidemic, Wearable Technology, Biosensor, Adherence, Machine Learning.
Abstract:
Wearable biosensors can be used to monitor opioid use, a problem of dire societal consequence given the cur-
rent opioid epidemic in the US. Such surveillance can prompt interventions that promote behavioral change.
The effectiveness of biosensor-based monitoring is threatened by the potential of a patient’s collaborative
non-adherence (CNA) to the monitoring. We define CNA as the process of giving one’s biosensor to someone
else when surveillance is ongoing. The principal aim of this paper is to leverage accelerometer and blood vol-
ume pulse (BVP) measurements from a wearable biosensor and use machine-learning for the novel problem
of CNA detection in opioid surveillance. We use accelerometer and BVP data collected from 11 patients who
were brought to a hospital Emergency Department while undergoing naloxone treatment following an opioid
overdose. We then used the data collected to build a personalized classifier for individual patients that capture
the uniqueness of their blood volume pulse and triaxial accelerometer readings. In order to evaluate our detec-
tion approach, we simulate the presence (and absence) of CNA by replacing (or not replacing) snippets of the
biosensor readings of one patient with another. Overall, we achieved an average detection accuracy of 90.96%
when the collaborator was one of the other 10 patients in our dataset, and 86.78% when the collaborator was
from a set of 14 users whose data had never been seen by our classifiers before.
1 INTRODUCTION
The Center for Disease Control (CDC) in the US re-
ports that on average 192 Americans die every day
from an opioid (e.g., heroin, oxycontin, morphine)
overdose (CDC, 2017). This is a multi-faceted prob-
lem of immense societal consequence that needs to
be addressed in many ways, such as improving data
quality and the monitoring of opioid use and relapse,
developing opioid use prevention strategies, provid-
ing support health providers, equipping first respon-
ders with the antidote naloxone to minimize overdose
related deaths, and encouraging the public to take safe
precautions with opioid use (CDC, 2017).
In this work, we focus on the issue of data qual-
ity and monitoring opioid use for people with opioid
use disorders (OUD). Effective monitoring is crucial
to the proper treatment of patients with OUD. Opioid
use monitoring is typically done through surveys and
patient evaluations, and blood/urine tests during office
visits (Weiss, 2004). These approaches are inherently
reactive in nature and can only detect drug use after
the fact. One way of addressing this problem close to
real-time is to use wearable biosensors.
Wearable biosensors are wearable devices that can
measure a variety of physiological (e.g., blood vol-
ume pulse, and electrodermal activity) and move-
ment (e.g., triaxial accelerometer) streams from the
patient they are deployed on. These biosensors are
of tremendous interest in the drug abuse treatment
space for their potential to detect drug use in near
real time (Carreiro et al., 2015)(Carreiro et al., 2016).
They provide a significant advantage for behavioral
interventions aimed at harm reduction or abstinence
as they can alert medical practitioners of changes in
physiological markers, practitioners can then inter-
vene. Biosensors can not only be useful in tracking
long-term reactions to drug withdrawal or therapeutic
measures, but can also detect immediate harm to the
patient such as an overdose.
Success in this type of clinical application for bio-
sensors relies upon patient’s adherence, that is the
patient’s wearing of the biosensor given to them. A
major concern in this context, therefore, is that pa-
tients under surveillance can simply give the biosen-
sor to another person to wear during the period of sub-
310
Singh, R., Lewis, B., Chapman, B., Carreiro, S. and Venkatasubramanian, K.
A Machine Learning-based Approach for Collaborative Non-Adherence Detection during Opioid Abuse Surveillance using a Wearable Biosensor.
DOI: 10.5220/0007382503100318
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 310-318
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

stance use to avoid opioid use (or relapse) detection
1
.
In this paper, we present a machine learning-based
detection approach for collaborative non-adherence
(CNA). We define CNA as the process of giving one’s
biosensor to someone else when surveillance is ongo-
ing. The reason we focus on CNA is because patients
with OUD are usually stigmatized in the society. Con-
sequently, they have considerable incentive to keep
any drug use or relapse from others, especially in the
presence of any systematic surveillance of their opi-
oid use (Olsen and Sharfstein, 2014). It is therefore
imperative that as we work on developing new mon-
itoring tools for the opioid epidemic, we also, at the
same time, investigate ways to deal with deliberate
non-adherence to the monitoring regime (Figure 1).
Note that currently there is no data on CNA in peo-
ple with OUD to our knowledge. This is because the
field of wearable biosensor-based interventions in this
population itself is in early stages. However, given the
propensity for individuals to try to “cheat” other wear-
able tracking devices such as step counters (Alshurafa
et al., 2014) and the stigma associated with drug use,
we view this as more of an eventuality than a theoreti-
cal concern. Further, since such behaviors would dra-
matically lower the success rates of biosensor-based
interventions, we are proactively seeking solutions
that can be build into interventions from the start.
Figure 1: Collaborative Non-Adherence (CNA).
To develop and evaluate our detection approach,
we rely on the simulation of CNA using biosensor data
collected from overdosing patients in the University
of Massachusetts medical center emergency depart-
ment (ED). We used an Empatica E4 wrist-mounted
biosensor (Empatica, Milan, Italy) for our data col-
lection. Data was collected from 11 patients who pre-
sented to the ED for medical care following an opioid
overdose. The patients were in various states of re-
1
They could also simply take off the device, in which
case their data-stream would flatline and would lead to an
intervention by the caregiver, which we assume the patients
want to avoid.
covery subsequent to an administration of naloxone
2
.
Our patients were real medical patients suffering from
OUD, and all data was gathered in a way that prior-
itized patient care and wellness over research goals
with approval from our Institutional Review Board
(IRB) (ethics board).
In order to detect CNA, we use machine learning
techniques to develop personalized classifiers for in-
dividual patients that capture the uniqueness of their
cardiac physiology (blood volume pulse) and move-
ment (accelerometer). This personalized classifier for
a given patient in our dataset is trained using biosen-
sor data from that patient when they are not intoxi-
cated (also referred to as neutral state) and data be-
longing to the rest of the 10 patients in our dataset.
This data from the other 10 patients can be any of
three physiological dates: neutral, withdrawal, or in-
toxicated/overdosed. This way of building the clas-
sifier simulates the case where a patient gives their
biosensor to someone else (a collaborator) of whom
they have no control. This collaborator can therefore
themselves decide to abuse drugs or abstain. The per-
sonalized classifier is able to detect the presence or
absence of CNA because the patterns in the physio-
logical and movement data obtained from a patient
are unique to that patient (i.e., act as a biometric), ir-
respective of their health state. Note that, our chosen
physiological signals have been used before as bio-
metrics to identify a person uniquely (Sarkar et al.,
2016)(Yang et al., 2015). However, none of these
studies involved participants in various stages of drug
use. To the best of our knowledge, this is the first
work on developing an automated approach for CNA
detection in the context of drug abuse. An analysis
of our detection approach demonstrates its viability.
Overall, the results show the efficacy of our personal-
ized CNA detection approach. We achieved an aver-
age detection accuracy of 90.96% when the collabo-
rator was one of the other 10 patients in our dataset,
and 86.78% when the collaborator was from a set of
14 individuals, whose data has never been seen by our
classifiers during training.
2 SYSTEM MODEL AND
PROBLEM STATEMENT
In this paper we make several assumptions based on
our eventual goal of identifying CNA. First, since the
patient is compromising the source of the biosensor
data, we assume that they have the ability to hand
off the biosensor for any length of time. We also
2
Naloxone is a antidote that is given to someone who is
overdosing on opioids. It immediately reverses the effect of
opioids by competitively binding to the opioid receptors in
the body.
A Machine Learning-based Approach for Collaborative Non-Adherence Detection during Opioid Abuse Surveillance using a Wearable
Biosensor
311

assume that the collaborator has unfettered access to
the biosensor after it has been handed off. Second,
we assume that the patient does not have the abil-
ity to mount other forms of non-adherence against
the surveillance, such as replaying their own histor-
ical biosensor data. Finally, we assume that the user
has no control over the collaborator once the device
is handed off. This means that the collaborator could
choose to use opioids themselves.
In this context, the principal problem we address
in this paper is to detect if the data received from a
wearable biosensor assigned to person X is truly com-
ing from person X (and not from person Y, where X 6=
Y); and that person X is not exhibiting opioid toxicity.
3 RELATED WORK
CNA During Physical Activity Monitoring: In (Al-
shurafa et al., 2014) the authors present an approach
to detect CNA in the use of fitness trackers to fool in-
surance companies who might want to give premium
reductions for people who exercise. Their approach
was specifically focused on the use of accelerometer
data, collected from a wrist-worn fitness tracker and
machine-learning, for detecting CNA. Further, their
paper only focused on providing cross validation re-
sults for their models and did not test their models
using data from previously unseen user data. Their
accuracy in this work for cross validation was around
90%. In this work, we apply CNA detection to the
domain of opioid abuse. Since the intake of opioid
affects the physiology of the patient as much as their
movement, we use both accelerometer and blood vol-
ume pulse from the patients as a way to identify CNA.
Further, we evaluate our model using unseen test data,
which shows the efficacy of our detection approach.
Wearable Biosensor Tracking of Opioid Use:
Some work has been done in identifying opi-
oid use using a wearable biosensor (Carreiro
et al., 2016)(Carreiro et al., 2015)(Smelson et al.,
2014)(Chintha et al., 2018). However, these ef-
forts have focused on detecting opioid ingestion us-
ing physiologic measurements made using wearable
biosensors. They have not explored issues with pa-
tient adherence. In our work, we focus around the
novel problem of collaborative non-adherence.
4 CNA DETECTION APPROACH
In this paper we are primarily interested in determin-
ing the feasibility of our non-adherence detection us-
ing the movement and cardiac signals in people who
are drug users. Consequently, we decided to initially
build and test our CNA detection approach by simu-
lating CNA behavior using biosensor data collected
from actual patients admitted to the University of
Massachusetts medical center emergency department
from drug overdose. Before we describe the details of
the simulation (in Sections 5 and 6), we present the
details of our data collection and the CNA detection
approach.
4.1 Data Collection and Cleaning
The data used for building and evaluating our detec-
tion approach was collected from ED patients who
received the opioid antagonist naloxone for known
or suspected diagnosis of opioid toxicity. These pa-
tients were chosen as they are part of our target de-
mographic. Once study staff obtained informed con-
sent, the E4 was placed on the participant’s non-
dominant wrist and continuous biometric data was ob-
tained until a predefined endpoint was reached (either
discharge from or admission to the hospital).
Patients (i.e., study participants) were assessed ap-
proximately every few hours while enrolled in the
study by a research staff member. Based on physi-
cal exam findings, they were noted to be in one of
three states: neutral, opioid intoxication, or opioid
withdrawal. The neutral state is defined as the pa-
tient being sober and awake. In the intoxication state
the patient has exhibits signs and symptoms of in-
toxication or overdose. Finally, in the withdrawal
state, the patient exhibits signs and symptoms of opi-
oid withdrawal due to the administration of the opioid
antagonist naloxone. The data from the patients in
the intoxication and withdrawal states were collected
while they were primarily lying still in a hospital bed.
In the neutral state, the patients were more likely to
stand/walk ad lib. This would probably include brief
bouts of activity (like walking to the bathroom), as
opposed to any prolonged or brisk activity. They also
may be sitting up, having conversations with clini-
cians, or eating and drinking lightly.
We used the Empatica E4 (Empatica, Milan, Italy)
wrist-worn biosensor for our data collection. The E4
is capable of monitoring a variety of physiological
(e.g., blood volume pulse, heart rate variability, skin
conductance) and movement (e.g., triaxial accelerom-
eter) information. For this work we only use the triax-
ial accelerometer (sampled at 32 Hz) and blood vol-
ume pulse (sampled at 64 Hz) data from the E4 de-
vice. We collected accelerometer data because it has
been used before in classification and authentication
tasks similar to ours, and we believed it would be able
to reasonably accurately identify our patients (Zheng
et al., 2014) and (Alshurafa et al., 2014). Further, we
used blood volume pulse (BVP) data because opioid
use directly affects a person’s breathing (Santiago and
Edelman, 1985), which is reflected in the BVP signal.
HEALTHINF 2019 - 12th International Conference on Health Informatics
312

Once the data is collected, the next step is to clean
it and extract features from its data streams which can
be used to identify the source of data. In order to ac-
count for noise in the BVP data, a low pass filter with
finite-duration impulse response was utilized on the
data. The filter utilized a pass-band frequency of 0.6
Hz. This was chosen because it aligns with a heartbeat
of approximately 40 beats per minute (bpm), which is
less than the range of 50-80 bpm expected for a heart
at rest (Spodick, 1993). Similarly, the stop-band fre-
quency was chosen at 3.33 Hz (aligning with approx-
imately 200 bpm) based on estimates by Tanaka et. al
(Tanaka et al., 2001). The decision was made to only
apply the low pass filter to the BVP data and not to
the accelerometer data to avoid accidentally remov-
ing variations in the accelerometer data that might be
the results of physiological reactions such as shaking
from withdrawal. Finally, we re-sampled the triaxial
accelerometer data to 64 Hz to match the sampling
rate of the BVP measurement.
Feature Extraction: Inspired by Alshurafa et al.
(Alshurafa et al., 2014), our feature extraction pro-
cess relies on statistical features extracted from the
accelerometer and BVP data collected using the wear-
able biosensor. The features taken for accelerometer
are: Mean, Median, Skewness, Variance, Standard
Deviation, Mean Crossing Rate, Maximum Value,
Minimum Value, Mean Derivatives, Inter-Quartile
Range, Zero Crossing Rate, and Kurtosis. Those
taken for BVP data are: number of peaks, mean of
the peaks, standard deviation of the peaks, root mean
square, distance from the maximum to the minimum
of the peaks, mean peak-to-peak distance, standard
deviation of peak-to-peak distance, and power spec-
tral density of the signal. Since there are 12 sta-
tistical features gathered from each of accelerome-
ter axes and 8 statistical features from BVP measure-
ment, there are a total of 44 features that are extracted.
Identifying features to improve the accuracy of CNA
detection is important future work.
4.2 Training and Detection
Once we have the dataset and know which features
to extract, the next step is to build the CNA detec-
tion approach. Our detection approach uses a ma-
chine learning-based classifier to address our princi-
pal question: data received from a wearable biosensor
assigned to patient X are actually coming from patient
X and that patient X is not opioid intoxicated.
Our classifier learns the essential uniqueness of
the accelerometer and BVP data of a patient’s neutral
state. We only consider the neutral state of the patient
for two reasons: (1) it may be impossible or imprac-
tical to gather data from the patient during training
for intoxication or withdrawal state in the real world,
and (2) since the purpose of our system is to help in-
Figure 2: Overview of collaborative non-adherence (CNA)
detection approach.
troduce monitoring of the patient for their physical
well-being, we think that showing that they are in the
neutral state is necessary to meet that goal. As long
as any newly received accelerometer and BVP snippet
match the understanding of a patient’s neutral state,
the classifier can be sure there is no CNA. Our detec-
tion approach has to two phases: the training phase
and the detection phase (see Figure 2).
Training Phase: In the training phase, we build
a classifier to identify the uniqueness of a patient in
their neutral state so that it can later be used to identify
whether the received accelerometer and BVP mea-
surements are also representations of the same pa-
tient’s neutral state. We build a personalized (binary)
classifier for each patient in our dataset, whose two
class points are obtained as follows. (1) Positive class
points: During training, for a given patient, we take
that patient’s neutral state data, and divide it up into
windows of length w time-units. We then extract our
44 features from each of these windows. That is,
we produce a 44-dimensional feature point per win-
dow. We call the feature points as the positive class
points and these signify no CNA. (2) Negative class
points: We then divide the neutral, intoxicated, and
withdrawal state data from all remaining 10 patients
in our dataset into w-size windows, extract the 44 fea-
tures from each of these windows, and call them the
negative class points. Since we have no real instances
of CNA, these negative class points are used to simu-
late CNA in our training set. Once we have the pos-
itive and negative class points, we take a subset of
these feature points to train a classifier which forms
our CNA detection approach and use the rest for vali-
dation purposes.
Detection Phase: Once the classifier is trained for
a patient, it is now able to classify whether an unseen
feature point derived from a w-sized snippet of ac-
celerometer and BVP measurements came from that
patient, or whether it is an instance of CNA. When
A Machine Learning-based Approach for Collaborative Non-Adherence Detection during Opioid Abuse Surveillance using a Wearable
Biosensor
313

Figure 3: Overview of our data curation process.
an unseen data point is evaluated on our classifier, it
returns a confidence value from 0 to 1 with 1 indicat-
ing that the model has full confidence that the unseen
snippet belongs to the patient in the neutral state, and
with 0 indicating full confidence that the point does
not belong to that patient’s neutral state and hence sig-
nifies CNA in our setup. We are then able to decide
whether to accept or reject that data point depend-
ing on whether its confidence value meets a chosen
threshold.
5 EVALUATION
METHODOLOGY
Given that patients in our dataset were examined by
clinicians intermittently (every hour), we do not have
the ground-truth about the patient’s health state at all
times. Consequently, we curate the biosensor data we
collected from the 11 patients, to extract a dataset that
captures the blood volume pulse and the accelerome-
ter readings from the patients when we are reasonably
confident of their health states (i.e., neutral, intoxi-
cated or withdrawal). This curated dataset can then
be used for training our detection classifiers and eval-
uating their efficacy. In this section, we describe our
dataset curation process, and its use for training and
evaluating any CNA detection, followed by a brief de-
scription of our evaluation metrics.
5.1 Dataset Curation
Overall, we have data collected using the wearable
biosensor from 11 patients who were brought to the
ED as a result of opioid overdose for several hours.
Patient data was gathered in accordance with hospi-
tal policy and with approval through our institution’s
Institutional Review Board (IRB) approval process
(ethics committee review). During this time, patients
were experiencing different health states (i.e., neu-
tral, intoxicated or withdrawal) depending upon when
they were administered naloxone. We extracted wear-
able biosensor data for each patient from the various
health states. This was done by extracting 20 min-
utes of biosensor data immediately surrounding a pa-
tient evaluation by the research/clinical staff, which
resulted in the recording of their health state at the
time. This translates to 10 minutes before and 10 min-
utes after the evaluation. Since the patient is in a hos-
pital setting, we can have reasonable assurance that
during these 20-minutes there would not be a signifi-
cant change in this health state (see Figure 3).
Note that, depending upon when they were
brought into the ED after their overdose, when nalox-
one was administered, and when evaluations were
conducted, some patients may only have data col-
lected in their neutral state, others may have intoxi-
cation and neutral state data, or withdrawal and neu-
tral state data. All patients in our dataset have some
neutral health state data. Once we have extracted the
pertinent data from the patients, we have the neces-
sary dataset for answering our principal question of
whether CNA can be detected.
Data Used for Training: To be able to train the
classifier to detect CNA, we have to compensate for
the idiosyncrasies in our curated dataset that origi-
nated from our data collection protocol. For instance,
during the data collection, we were not able to care-
fully control the patients’ actions (especially in the
neutral state). Further, given the way we extracted
data for different health states for a patient, we do
not have enough contiguous data to train our classi-
fier in any one health state. Therefore, in order to
train our classifier, we first generate the positive and
negative class points (as described in Section 4.2) and
then shuffle them. We then use the first 8 minutes’
worth of feature points for training. Since a feature
point is produced from w-sized windows, 8 minutes
of data will have 8/ w number of feature points. The
rest of the shuffled feature points are used as testing
(i.e., CNA detection). We chose the value of 8 min-
utes because every patient had at least 10 minutes of
neutral state data. This allowed us to train our clas-
sifier for every patient and still have some data left
over to test for non-CNA scenarios. After the data is
shuffled and training data is chosen, all of the result-
ing negative and positive samples are fed into a clas-
sifier (more details on the classifier is given in Sec-
tion 6) to train it. The bottom part of Figure 3 shows
the data curation for training. As mentioned before,
this way of building the classifier simulates scenarios
where a patient gives their biosensor to someone else
(a collaborator) of whom they have no control. This
collaborator can themselves decide to abuse drugs or
abstain.
Data Used for Evaluating CNA Detection:
Once our classifier is chosen and trained, the next step
is to see how well it performs in detecting the pres-
ence and absence of CNA. We do this by feeding the
patient-specific classifiers in our CNA detection ap-
HEALTHINF 2019 - 12th International Conference on Health Informatics
314

proach: w-second snippets of completely unseen tri-
axial accelerometer and BVP data. These simulate
the CNA detection approach determining whether the
patient data collected every w-seconds (3 seconds) is
coming from the patient in their neutral state or not.
Each previously unseen w-second snippet produces
a feature point which is evaluated by the classifier.
These unseen test feature points come from: (1) the
validation set, portions of the data of the 11 patients
in our dataset that were not used in training, and (2)
the external set of 14 healthy/non-opioid using indi-
viduals, whose data has never been seen before in any
form by any of our classifiers. Out of those 14 indi-
viduals, 9 were at rest, and 5 were moving around in
a busy conference setting (see Table 1). The bottom
right of Figure 3 shows the data curation for perform-
ing evaluation.
Table 1: Demographics from both data sets used for our
evaluation.
Set Count Avg. Age (std.)
♂
♀
Validation 11 37.09 ± 10 9 2
External 14 32.8 ± 5.3 10 4
5.2 Metrics
In order to evaluate the efficacy of our system, we use
the following five core metrics. (1) True Reject Rate
(TRR): the rate at which true negative feature points
(data points from patients other than the one that the
system is trained for) is rejected by the detection ap-
proach. This indicates the presence of CNA. (2) False
Accept Rate (FAR): the rate at which true negative fea-
ture points are accepted by the detection approach.
This indicates a false alarm. (3) True Accept Rate
(TAR): the rate at which positive feature points (the
patient’s own unseen data) is accepted by the detec-
tion approach. This indicates the absence of CNA.
(4) False Reject Rate (FRR): the rate at which an un-
seen positive feature points (the patient’s own data) is
rejected from the detection approach. This indicates
when CNA was missed. (5) Equal Error Rate (EER):
The rate at which the FAR and the FRR are equal.
This is the point at which our detection approach bal-
ances the accuracy of its detection with usability.
6 CLASSIFIER TRAINING
In this section, we describe how we identify the clas-
sifier of choice for our detection approach along with
how we choose the window size (w), given the classi-
fier of choice.
Choosing the Classifier: In order to choose a ma-
chine learning classifier that would be effective, we
Figure 4: ROC curves for 5-fold cross validation on various
machine learning algorithms. Here, we set the window size
(w) to 5 seconds, provisionally.
used 5-fold stratified cross validation on our training
data. Since there are far more negative class points
than positive class points, we use weighted classi-
fiers in order to avoid the model favoring the negative
state. The weighted classifiers consider the correct
minority (positive) class decision much more valuable
than a correct majority (negative) class decision. We
used a value of 1:10 for the ratio of the weighting as
there are 11 patients in our data set, and so for ev-
ery 1 positive point from a particular patient, there are
approximately 10 points belonging to other patients.
The weighted algorithms used were weighted Ran-
dom Forest, weighted Adaboost, weighted Support
Vector Machine (SVM) with a radial-bias function
(rbf) kernel, and weighted logistic regression. Our
cross validation was performed with a 5-second win-
dow, which was initially chosen arbitrarily, as we tune
our window size after the algorithm is chosen.
The summary of our evaluation in the form of re-
ceiver operating characteristic (ROC) curves is seen in
Figure 4. ROC curves plot TAR against FAR in order
to form a curve. The closer the area under the curve
(AUC) is to 1, the better the algorithm performed in
cross validation. These curves are averaged overall
11 patients. In addition, for evaluation we looked
at the equal error rate (EER) which is the point at
which FAR and FRR are equal (in other words the
place with the lowest combined error). Algorithms
with a lower EER were considered preferable to those
with a higher EER if the AUC was the same. As can
be seen in Figure 4, the best performing algorithm is
weighted Random Forest, which we chose as our ma-
chine learning classifier.
Choosing Window Size (w): Once the machine
learning algorithm is chosen, the next step is to find
the appropriate window size. Once again, we run with
5-fold cross validation on our training data where the
A Machine Learning-based Approach for Collaborative Non-Adherence Detection during Opioid Abuse Surveillance using a Wearable
Biosensor
315

Figure 5: ROC curves for 5-fold cross validation on penal-
ized Random Forest with various window sizes. A window
size (w) of 3 seconds was chosen based on these results.
data was split by windows of varying size. As can
be seen in Figure 5, which shows the average ROC
curves for various window sizes, w=3 seconds had the
highest average AUC of 0.94 and the lowest EER at
12.0%. Therefore, we chose 3 seconds as the window
size. Contrary to expectation, here, a smaller win-
dow size gives a better result. We hypothesize that
the smaller window is more reliable at capturing the
sudden variability in the blood volume pulse that ap-
pear from opioid ingestion and naloxone use. We plan
to investigate this further in the future.
7 CNA DETECTION
EVALUATION
In this section we present the results of our classifiers
using the validation set and external set to show the
efficacy of our CNA detection approach.
Validation Set Results: For the validation set, we
achieved promising performance from our CNA de-
tection approach. Figure 6 shows the ROC curve of
the 11 patient-specific models. The average AUC on
the validation set comes to 0.94. The EER is of course
different for each patient-specific detection approach.
The average EER for all patients is 9.04% (i.e. aver-
age accuracy of 90.96%). The performance variabil-
ity for different patients can be largely accounted for
by the difference in level of activity between different
patients. The ROC results show how the classifier’s
performance varies for individual patients, but overall
is quite accurate for the validation set.
External Set Results: In addition to testing the
performance of our classifiers on our validation set,
we performed additional testing on an external set.
The data in the external set has never been seen by
Figure 6: Graph of ROC curves for validation set test fea-
tures using weighted random forest with a window of 3 sec-
onds.
our detection approach classifiers in any form before.
These results show the generalizability of our data-
driven CNA detection approach. This data in the
external set was collected from healthy individuals
while they were attending a conference. This simu-
lates the case, where a patient gives their watch to
another person (i.e., engages in CNA) who is healthy,
does not abuse opioids, and is completely unknown to
the surveillance system. Since all the data in the ex-
ternal set comes from someone unknown, ideally, all
the feature points generated from this data should be
rejected (i.e., classified as negative). Figure 7 shows
the ROC curve for the external set. Here, we use the
patient’s own test data to form the TAR for our ap-
proach. As expected, the average AUC is slightly
lower than the validation set at 0.92, with a slightly
higher average EER at 13.22% (i.e. average accuracy
of 86.78%). These results show that we are overfitting
our detection models.
8 LIMITATIONS
In this preliminary study, we showed the promise of
our system for detecting CNA in an opioid surveil-
lance program that relies on a wearable biosensor.
Even though our system focuses on collaborative non-
adherence during opioid abuse surveillance, it can
also detect accidental non-adherence as well. This
means that if someone else puts on a patient’s biosen-
sor by mistake or if the biosensor fell off or was
recording incorrect data, it would be detected as well
because the received data would not match the data
expected by our classifier for the patient.
HEALTHINF 2019 - 12th International Conference on Health Informatics
316
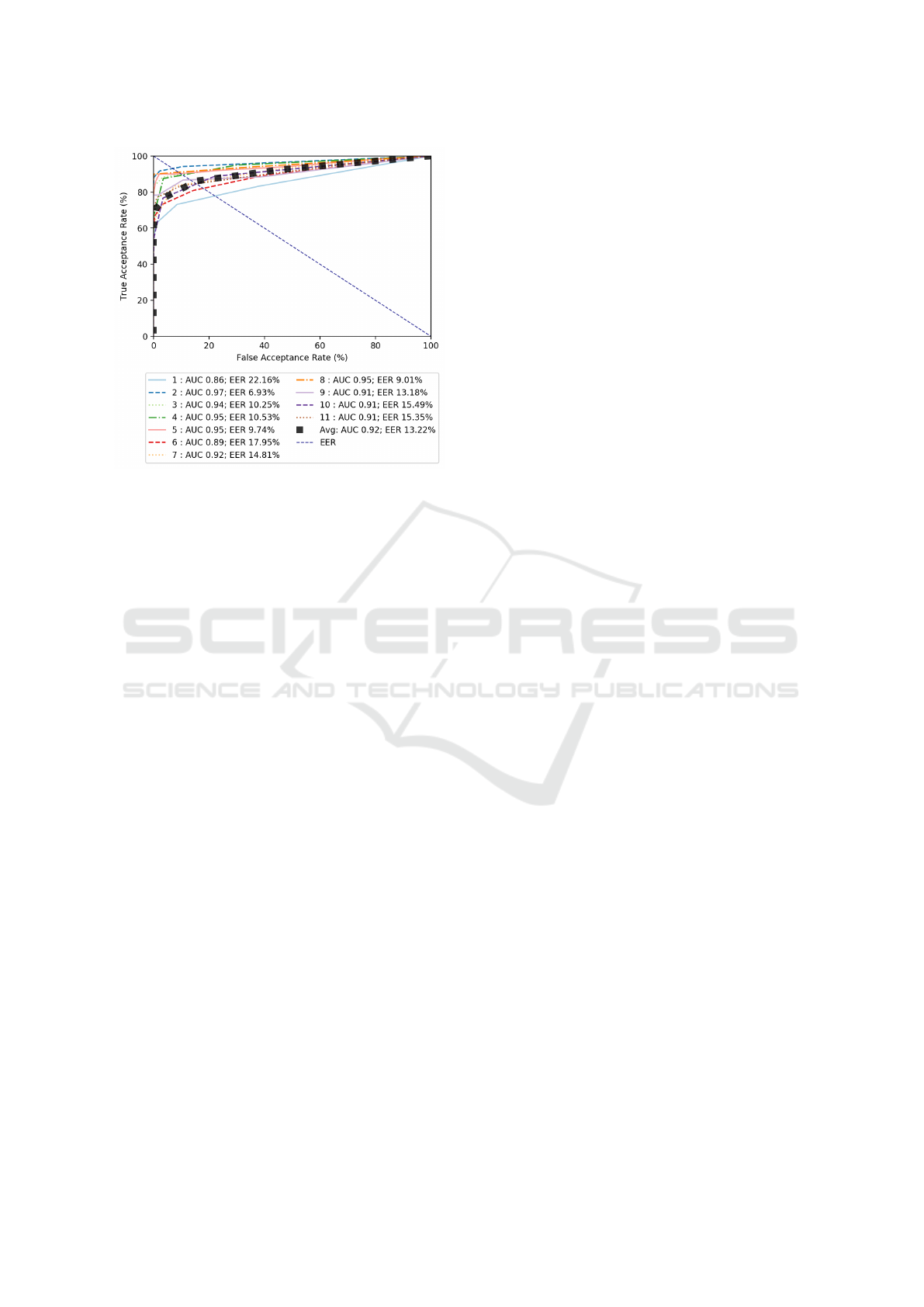
Figure 7: Graph of ROC curves for validation set test fea-
tures using weighted random forest with a window of 3 sec-
onds.
However, there are two major limitations to our
current work that we plan to address in the future.
First, in this work we view the neutral state as a mono-
lithic state. We were able to do it largely because the
data was collected in the ED, where the patient was
not liable to be doing many different things in the
neutral state. However, that is not true outside the
ED as the patient may be performing a variety of ac-
tions when they are in the neutral state. Our classifiers
therefore by necessity have to be aware of the various
activities the patient is engaged in and differentiate
these activities from one patient to another. This will
result in detection models that are much more com-
plicated. Further, this will reduce false alarms.
Second, we have only simulation of CNA in this
work. However, to be truly effective these classifiers
have to detect CNA where patients actively try put
the sensor on a different person. To be able to achieve
this we need to create one or more clinical studies that
allow patients to intermittently put the sensor on an-
other person and then record (e.g., using a survey app)
the fact that there was collaborative non-adherence.
This will allow us to train classifiers and check to see
if they are able to detect CNA in the real world. Fur-
ther, this non-adherence study has to include a larger
participant pool than what we used in this paper which
is somewhat limited.
9 CONCLUSION
In this paper we presented an approach for CNA
detection for a patient who is being surveilled, us-
ing a wrist-worn wearable medical device, for opioid
abuse. In the future, we plan to immediately expand
on this work in several directions: (1) undertake clin-
ical studies that create actual CNA scenarios to fine-
tune our models including activities outside of a hos-
pital setting, (2) identify improved features and ma-
chine learning classifiers to increase the detection ac-
curacy, and (3) build a cloud-based application that
can detect the presence of CNA at scale. In addi-
tion, we have the long-term goal of exploring appli-
cations of our CNA detection approach to other non-
adherence scenarios such as pre-exposure HIV pro-
phylaxis medications, using wearable biosensors.
REFERENCES
Alshurafa, N., Eastwood, J.-A., Pourhomayoun, M., Nya-
mathi, S., Bao, L., Mortazavi, B., and Sarrafzadeh,
M. (2014). Anti-cheating: Detecting self-inflicted and
impersonator cheaters for remote health monitoring
systems with wearable sensors. In BSN, volume 14,
pages 1–6.
Carreiro, S., Fang, H., Zhang, J., Wittbold, K., Weng, S.,
Mullins, R., Smelson, D., and Boyer, E. W. (2015).
imstrong: deployment of a biosensor system to detect
cocaine use. Journal of medical systems, 39(12):186.
Carreiro, S., Wittbold, K., Indic, P., Fang, H., Zhang, J.,
and Boyer, E. W. (2016). Wearable biosensors to de-
tect physiologic change during opioid use. Journal of
medical toxicology, 12(3):255–262.
CDC (2017). Understanding the epidemic. CDC website.
Chintha, K. K., Indic, P., Chapman, B., Boyer, E. W., and
Carreiro, S. (2018). Wearable biosensors to evalu-
ate recurrent opioid toxicity after naloxone adminis-
tration: a hilbert transform approach.
Olsen, Y. and Sharfstein, J. M. (2014). Confronting the
stigma of opioid use disorder—and its treatment.
Jama, 311(14):1393–1394.
Santiago, T. V. and Edelman, N. (1985). Opioids
and breathing. Journal of Applied Physiology,
59(6):1675–1685.
Sarkar, A., Abbott, A. L., and Doerzaph, Z. (2016). Biomet-
ric authentication using photoplethysmography sig-
nals. In Biometrics Theory, Applications and Systems
(BTAS), 2016 IEEE 8th International Conference on,
pages 1–7. IEEE.
Smelson, D. A., Ranney, M. L., Boudreaux, E. D., and
Boyer, E. W. (2014). Real-time mobile detection of
drug use with wearable biosensors: A pilot study.
Spodick, D. H. (1993). Survey of selected cardiologists for
an operational definition of normal sinus heart rate.
The American journal of cardiology, 72(5):487–488.
Tanaka, H., Monahan, K. D., and Seals, D. R. (2001). Age-
predicted maximal heart rate revisited. Journal of the
American College of Cardiology, 37(1):153–156.
Weiss, R. D. (2004). Adherence to pharmacotherapy in pa-
tients with alcohol and opioid dependence. Addiction,
99(11):1382–1392.
Yang, J., Li, Y., and Xie, M. (2015). Motionauth: Motion-
based authentication for wrist worn smart devices. In
A Machine Learning-based Approach for Collaborative Non-Adherence Detection during Opioid Abuse Surveillance using a Wearable
Biosensor
317

Pervasive Computing and Communication Workshops
(PerCom Workshops), 2015 IEEE International Con-
ference on, pages 550–555. IEEE.
Zheng, Y.-L., Ding, X.-R., Poon, C. C. Y., Lo, B. P. L.,
Zhang, H., Zhou, X.-L., Yang, G.-Z., Zhao, N., and
Zhang, Y.-T. (2014). Unobtrusive sensing and wear-
able devices for health informatics. IEEE Transac-
tions on Biomedical Engineering, 61(5):1538–1554.
HEALTHINF 2019 - 12th International Conference on Health Informatics
318
