
Learning to Predict Autism Spectrum Disorder based on the Visual
Patterns of Eye-tracking Scanpaths
Romuald Carette
1
, Mahmoud Elbattah
1
, Federica Cilia
2
, Gilles Dequen
1
, Jean-Luc Guérin
1
and Jérôme Bosche
1
1
Laboratoire MIS, Université de Picardie Jules Verne, Amiens, France
2
Laboratoire CRP-CPO, Université de Picardie Jules Verne, Amiens, France
Keywords: Autism Spectrum Disorder, Machine Learning, Eye-tracking, Scanpath.
Abstract: Autism spectrum disorder (ASD) is a lifelong condition generally characterized by social and
communication impairments. The early diagnosis of ASD is highly desirable, and there is a need for
developing assistive tools to support the diagnosis process in this regard. This paper presents an approach to
help with the ASD diagnosis with a particular focus on children at early stages of development. Using
Machine Learning, our approach aims to learn the eye-tracking patterns of ASD. The key idea is to
transform eye-tracking scanpaths into a visual representation, and hence the diagnosis can be approached as
an image classification task. Our experimental results evidently demonstrated that such visual
representations could simplify the prediction problem, and attained a high accuracy as well. With simple
neural network models and a relatively limited dataset, our approach could realize a quite promising
accuracy of classification (AUC > 0.9).
1 INTRODUCTION
Autism Spectrum Disorder (ASD) is described as a
pervasive developmental disorder characterized by a
set of impairments including social communication
problems, difficulties with reciprocal social
interactions, and unusual patterns of repetitive
behavior (Wing and Gould, 1979). According to the
U.S. Department of Health and Human Services,
ASD has been considered to affect about 1% of the
world’s population (DOH, 2018). Individuals
diagnosed with ASD typically suffer from deficits in
social communication and interaction across
multiple contexts. Particularly, they could be
incapable of making and maintaining eye contact, or
keeping their focus on specific tasks. Such troubling
symptoms can unfortunately place a considerable
strain on their lives and their families.
The diagnosis of ASD is highly desirable at early
stages in terms of benefits for both child and the
family. The diagnosis process usually involves a set
of cognitive tests that could require hours of clinical
examinations. In addition, the variation of symptoms
makes the identification of ASD more complicated
to decide. In this respect, computer-aided
technologies have come into prominence in order to
provide guidance through the course of examination
and assessment. Examples include Magnetic
Resonance Imaging (MRI), Electroencephalography,
and eye-tracking that is the focus of this study.
Eye-tracking is the process of capturing, tracking
and measuring eye movements or the absolute point
of gaze (POG), which refers to the point where the
eye gaze is focused in the visual scene (Majaranta
and Bulling, 2014). The eye-tracking technology
received particular attention in the ASD context
since abnormalities of gaze have been consistently
recognized as the hallmark of autism in general. The
Psychology literature is replete with studies that
analyzed eye movements in response to verbal or
visual cues as signs of ASD (e.g. Coonrod and
Stone, 2004; Jones et al., 2014; Sepeta et al., 2012;
Wallace et al., 2012).
Furthermore, the coupling of eye-tracking
instruments with modern AI techniques is leveraging
further capabilities for advancing the diagnosis and
its applications. Data-driven techniques, such as
Machine Learning (ML), are increasingly embraced
to provide a second opinion that is considered to be
less biased and reproducible. This study follows on
the path of integrating the eye-tracking technology
Carette, R., Elbattah, M., Cilia, F., Dequen, G., Guérin, J. and Bosche, J.
Learning to Predict Autism Spectrum Disorder based on the Visual Patterns of Eye-tracking Scanpaths.
DOI: 10.5220/0007402601030112
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 103-112
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
103

in tandem with ML to support the diagnosis of ASD.
The study is part of interdisciplinary collaboration
between research units of Psychology and AI.
Our approach is distinctively based on the
premise that visual representations of eye-tracking
scanpaths can discriminate the gaze beahviour of
autism. At its core, the key idea is to compactly
render eye movements into an image-based format
while maintaining the dynamic characteristics of eye
motion (e.g. velocity, acceleration) using color
gradients. In this manner, the prediction problem can
be approached as an image classification task. The
potential of our approach is evidently demonstrated
in terms of promising classification accuracy though
using largely simple ML models.
2 RELATED WORK
Plentiful studies sought to take advantage of eye-
tracking for the study and analysis of ASD. For
instance, Vabalas and Freeth (2016) demonstrated
interesting physiological elements based on eye-
tracking experiments. In face-to-face interactions,
eye movements were different among individuals
who fell on different positions on the spectrum of
autism. Specifically, persons with high autistic traits
were observed to have shorter and less frequent
saccades. In another study, eye-tracking was used to
identify ASD-diagnosed toddlers based on the
duration of fixations and the number of saccades
(Pierce et al., 2011). Their results showed that
participants with ASD spent significantly more time
fixating on dynamic geometric images compared to
other groups.
Likewise, a longitudinal study examined the patterns
of eye fixation for infants aged 2 to 6 months (Jones
and Klin, 2013). They notably indicated that ASD-
diagnosed infants exhibited a mean decline in eye
fixation, which was not observed for those who did
not develop ASD afterwards. Moreover, another
cohort study suggested the strong potential of eye-
tracking as an objective tool for quantifying autism
risk and estimating the severity of symptoms
(Frazier et al., 2016).
More recent studies attempted to makes use of
eye-tracking to build predictive ML models. To
name a few, Pusiol et al. (2016) worked on the
analysis of the eye focus on the face during
conversations. This was specifically applied to
developmental disorder (DD) children or Fragile X
Syndrome (FXS) children. A set of classification
models were experimented including Recurrent
Neural Networks (RNN), Support Vector Machines
(SVM), Naive Bayes, and Hidden Markov Model.
With RNN, they were able to reach a high prediction
accuracy of 86% and 91% for the classification of
female and male FXS patients respectively. Another
recent study applied ML on eye-tracking output in
order to predict ASD (Carette et al., 2017). The ML
model included features related to the saccade eye
movement (e.g. amplitude, duration, acceleration).
Their experiments aimed at detecting ASD among a
set of 17 children aged 8 to 10 years. Despite using a
limited dataset and a relatively simple model, they
demonstrated promising potentials of ML
application in this regard.
Compared to earleir efforts, the main distinction
of the present work is that it is purely reliant on the
visual representation of eye-tracking recordings. We
produce scanpath visualizations that represent the
spatial coordinates of the eye movement along with
its dyanmics. The approach allowed for simplifying
the model training, and attained high accuracy as
well. It is claimed that such approach has not been
applied before in the context of ASD, to the best of
our knowledge.
3 DATA ACQUISTION
3.1 Participants
A group of 59 children took part in this study. It was
highly desirable to have participants at an early stage
of development, as the principal goal was towards
supporting the early detection and diagnosis of ASD.
Specifically, all participants were school-aged
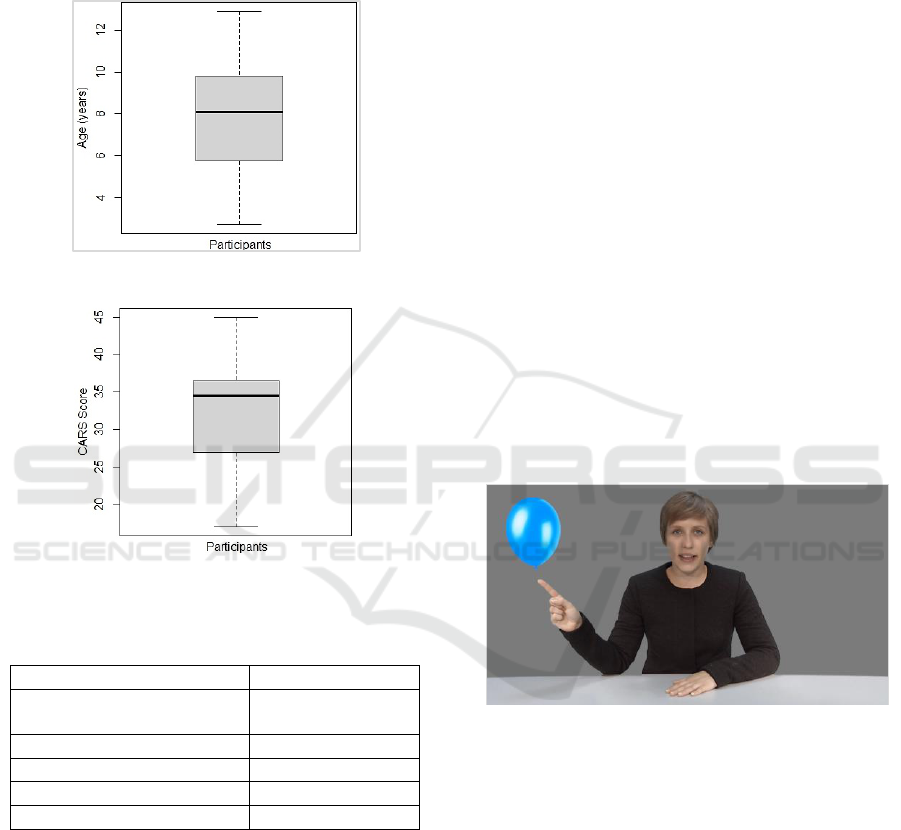
children of average age about 8 years. Figure 1
provides a box plot showing the distribution of age.
A group of typically developing (TD) children
was recruited from a number of French schools in
the region of Hauts-de-France. Parental reports of
any possible concerns were carefully considered.
Both informed consent from parents and assent from
subjects were confirmed for all cases. Moreover, all
procedures involving human participants were
conducted in accordance with the ethical standards
of the institutional and/or national research
committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical
standards.
The participants were broadly organized into two
groups as: i) ASD-Diagnosed, or ii) Non-ASD.
ASD-diagnosed children were examined in
multidisciplinary ASD specialty clinics. The
intensity of autism was estimated by psychologists
HEALTHINF 2019 - 12th International Conference on Health Informatics
104
based on the Childhood Autism Rating Scale
(CARS) (Schopler et al., 1980).
Figure 2 gives the distribution of CARS score
among ASD-diagnosed participants using a box plot.
Further, Table 1 gives summary statistics of the
participants (e.g. gender distribution, age mean).
Figure 1: The age distribution in all participants.
Figure 2: The distribution of CARS in ASD-diagnosed
participants.
Table 1: Summary statistics of participants.
Number of Participants
59
Gender Distribution (M / F)
38 (≈ 64%)
/ 21 (≈ 36%)
Number of Non-ASD
30
Number of ASD-Diagnosed
29
Age (Mean / Median) years
7.88 / 8.1
CARS (Mean / Median)
32.97 / 34.50
3.2 Experimental Protocol
The participants were invited to watch a set of
videos, which included scenarios tailored
specifically to stimulate the eye movement across
the screen area. Participants could be seated on their
own or on their parents’ lap at approximately 60-cm
distance from the display screen. The experiments
were conducted in a quiet room at the University
premises. Physical white barriers were also used to
reduce visual distractions.
The scenarios varied in content and length in
order to allow for analyzing the ocular activity of
participants from different perspectives. In general,
videos were designed to include visual elements that
can be especially attractive to children (e.g. colorful
balloons, cartoons). The position of elements can
also change throughout the course of experiment. In
addition, some videos could include a human
presenter who speaks and attempts to turn the child’s
attention to visible or invisible elements around. All
conversations were performed in French as the
native language of participants. Figure 3 presents a
screenshot captured from one of the videos used in
eye-tracking experiments.
Further stimuli were presented from the SMI
Experiment Center Software. Stimuli represented
multiple distinct types used in the eye gaze
literature. Examples included static and dynamic
naturalistic scenes with and without receptive
language, joint attention stimuli, static face or
objects and cartoons stimuli. The average duration
of eye-tracking experiments was about 5 minutes.
Participants were mainly examined for the
quality of eye contact with the presenter, and the
level of focus on other elements. A five-point
calibration scheme was used. The calibration routine
was followed by a set of verification procedures.
Figure 3: Screenshot captured from one of the videos.
3.3 Eye-tracking Records
The SMI remote eye-tracker (Red-m 250Hz) was the
main instrument used to perform the eye-tracking
function. The device belongs to the category of
screen-based eye-trackers. It can be conveniently
placed at the bottom of the screen of a desktop PC or
laptop. In our case, a 17-inch monitor of 1280x1024
resolution was used.
Three basic categories of eye movements are
aimed to be captured by eye-trackers including: i)
Fixation, ii) Saccade, and iii) Blink. A fixation is the
brief moment that happens while pausing the gaze
on an object in order that the brain can perform
Learning to Predict Autism Spectrum Disorder based on the Visual Patterns of Eye-tracking Scanpaths
105
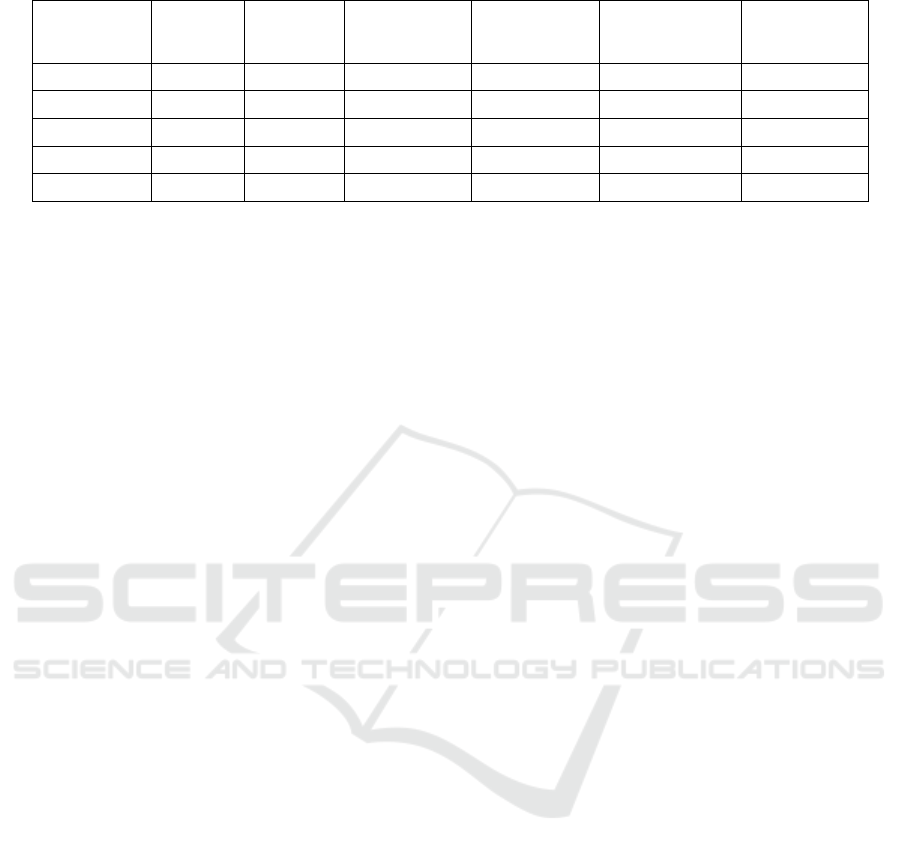
Table 2: A snapshot of eye-tracking records.
Recording
Timestamp
[ms]
Category
Right
Category
Left
Point of
Regard Right
X [px]
Point of
Regard Right
Y [px]
Point of Regard
Right Y [px]
Point of
Regard Left Y
[px]
8005654.069
Fixation
Fixation
1033.9115
834.0902
1033.9115
834.0902
8005673.953
Fixation
Fixation
1030.3754
826.0894
1030.3754
826.0894
8005693.85
Saccade
Saccade
1027.337
826.3127
1027.337
826.3127
8005713.7
Saccade
Saccade
1015.0085
849.2188
1015.0085
849.2188
8005733.589
Saccade
Saccade
613.7673
418.1735
613.7673
418.1735
the perception process. The average duration of
fixation was estimated to be 330 milliseconds
(Henderson, 2003). Further, the accurate perception
requires constant scanning of the object with rapid
eye movements, which are so-called saccades.
Saccades include quick, ballistic jumps of 2
o
or
longer that take about 30–120 milliseconds each
(Jacob, 1995). On the other hand, a blink would
often be a sign that the system has lost track of the
eye gaze.
Likewise, the initial records of our eye-tracking
experiments essentially included the features
described above. In addition, the eye-tracker
provided the (x,y) coordinates that represented the
participant's gaze direction into the screen. The
coordinates were of special significance to
implement our approach for drawing the virtual path
of the viewer’s POG and the dynamics of movement
as well (e.g. velocity, acceleration).
Table 2 provides a simplified snapshot of the
eye-tracking records. The records describe the
category of movement and the POG for the left and
right eyes over time. The table lists five records of
eye movements including two fixations and three
saccades. Due to limited space, many variables are
not included in the table (e.g. pupil size, pupil
diameter, pupil position).
3.4 Visualization of Eye-tracking
Scanpaths
A scanpath represents the sequence of consecutive
fixations and saccades as a trace through time and
space that may overlap itself (Goldberg and
Helfman, 2010). The premise of the study is based
on learning the visual patterns of scanpaths.
Specifically, the core idea was to compactly render
the eye movements into a visual representation that
can simplify and describe the path and dynamics of
eye movement.
To achieve this, we availed of the coordinates
included in eye-tracking records, which represented
the change in participant's gaze direction into the
screen with respect to time. Based on the change in
position along associated time, we were able to
calculate the velocity of gaze movement. The
acceleration of movement could be computed based
on the change in velocity, and the jerk of movement
could be accordingly computed based on the change
in acceleration. As such, the variation of velocity,
acceleration and jerk could describe properly the
dynamics of eye motion.
Subsequently, the path of eye motion along with
computed dynamics were transformed into images.
For every participant, a set of images could describe
the visual patterns of gaze behavior. Specifically, an
image is constructed as below:
A line is drawn for each transition from
position (x
t
, y
t
) to (x
t+1
, y
t+1
), where t is a
defined point of time during the experiment.
The change in color across the line
represented the movement dynamics. The
values of RGB components were tuned based
on velocity, acceleration and jerk with respect
to time. For instance, the values of velocity
range from black (i.e. low) to red (i.e. high).
In this manner, higher values of velocity shift
gradually towards deeper red values.
Likewise, the acceleration and jerk were set
using color gradients of green and blue
respectively.
The images produced were vertically
mirrored since the origin was located at the
bottom of the screen.
All color values were capped to one-quarter of
the diagonal length of the display screen. With this
cap, images represented the eye movements
including saccades (yellow or white, white
representing very fast movements, exceeding the
cap), and fixations as cyan.
HEALTHINF 2019 - 12th International Conference on Health Informatics
106

Figure 4: Visualization of eye-tracking scanpaths. The left-sided image represents an ASD-diagnosed participant, while the
other is for non-ASD.
In addition, images were constrained to contain
the same level of information approximately.
Specifically, a threshold was applied to limit the
number of points to be drawn. The threshold was
aimed to be high enough to sufficiently describe the
pattern of gaze behavior. However, overly high
values could increase the possibility of producing
cluttered visualizations. Therefore, several tests were
conducted to choose an appropriate value for the
threshold. With a threshold ranging from 100 to 150,
images seemed to include fewer lines, which turned
out to poorly discriminate the two classes of
participants. Eventually, it was decided to set the
threshold at 200, which largely struck an adequate
balance, and could capture the key features of eye
motion. The visualizations were produced using
Python along with the popular Matplotlib library
(Hunter, 2007).
Figure 4 presents examples of scanpaths
visualizations corresponding to ASD and non-ASD
participants. As it appears, the center of both images
includes areas of high density, which probably
represent one of the main points of focus in the
video scenario. The visualizations may also
highlight other points of focus x at the right side of
the screen. These focus points are drawn as cyan
(i.e. low velocity, but high acceleration and jerk),
while other widely diffused lines seem yellow (i.e.
high velocity, medium acceleration and jerk).
The figures can also describe the distinction of
the gaze movement in both cases. For example, it
can be noticed that the ASD-diagnosed participant
had a tendency to look at the bottom of the screen,
where the eye-tracking device was placed.
The visualizations resulted in an image dataset
containing 547 images. Specifically, 328 images for
the non-ASD participants, and 219 images for others
(i.e. ASD-diagnosed). The default image dimension
was set as 640x480. A more comprehensive
presentation of the process of data acquisition and
transformation was elaborated in an earlier
publication (Carette et al., 2018). Further, the dataset
along with metadata files were recently published
and made publicly available on the Figshare data
repository (Figshare, 2018). It is conceived that the
dataset itself could be useful for developing further
applications or discovering interesting insights using
data mining or other AI techniques.
4 DATA AUGMENTATION AND
PRE-PROCESSING
4.1 Image Augmentation
Image augmentation is a common technique to
enlarge datasets, and help models generalize better
and reduce the possibility of overfitting. The basic
idea of augmentation is to produce synthetic samples
using a random set of image transformations (e.g.
rotation, shearing). Augmentation was recognized to
improve the prediction accuracy in image
classification applications (e.g. Xu et al., 2016;
Perez and Wang, 2017).
Similarly, we applied augmentation to produce
variations of the eye- scanpath visualizations. The
dataset was augmented with additional 2,735
samples, where five synthetic images were generated
for each visualization. The data augmentation
process was greatly simplified thanks to the Keras
library (Chollet, 2015), which includes an easy-to-
use API for that purpose.
Learning to Predict Autism Spectrum Disorder based on the Visual Patterns of Eye-tracking Scanpaths
107
Figure 5: The procedures of dimensionality reduction.
4.2 Dimensionality Reduction
The reduction of problem dimensionality is a vital
part in the development of ML models. The
dimensionality refers to the number of variables (i.e.
features) under consideration. In our case, the initial
dimensionality was 640*480*3 (i.e. image size*
RGB components). This translates into more than
900K features to be considered, which can
complicate the model training and largely increases
the possibility of overfitting as well.
To simplify the learning process, a set of image
processing techniques was applied in sequence as
follows. First, all images were consistently scaled
down to 100x100 dimensions. It was expected that
such resizing would not lead to much loss of
information since most images contained a large
blank space of unused pixels. The impact of new
dimensions was also examined in the initial ML
experiments.
Second, the images were converted into a
grayscale format for further simplification.
The grayscale transformation reduces the image
representation by eliminating the hue and saturation
information while retaining the luminance.
Specifically, the grayscale values were computed by
forming a weighted sum of the R, G, and B
components as in the equation below. This
contributed to reducing the visual representation
from 100x100x3 to 100x100x1. It turned out that the
grayscale spectrum was mostly sufficient to
discriminate the eye-tracking patterns in terms of
velocity, acceleration, and jerk.
Luminance=0.299*R + 0.587*G + 0.114*B
Where R, G and B represent the values of the Red,
Green and Blue components, and the coefficients are
used to calculate luminance (ITU, 2017).
Eventually, Principal Component Analysis
(PCA) was implemented to transform grayscale
images into a more compressed format. Using
orthogonal transformations, PCA attempts to convert
a possibly correlated set of data (e.g. signals or
images) into a linearly uncorrelated set of reduced
dimensions. PCA is one of the most popular
techniques for dimensionality reduction that has
been widely applied in problems dealing with data
of high dimensionality such as image compression
(Du and Fowler, 2007), and face recognition (Draper
et al., 2003). In our case, the 10K feature set was
transformed into 50 components. This significantly
reduced the dimensionally into less than 1% of the
original dataset. The number of components was
empirically decided based on the model accuracy.
Figure 5 summarizes the pre-processing procedures
along with the dimensions output from each step.
5 EXPERIMENTAL RESULTS
The experimental results are divided into three
sections as follows. Initially, we aimed to develop a
binary classifier that can basically predict the two
categories of participants. Subsequently, the
accuracy of a multi-label classification model was
experimented. Further, a simple web-based tool is
presented as a practical demo that can be used
during the diagnosis process.
5.1 Binary Classifier
We conducted our experiments using several ML
models. Initially, non-neural network approaches
were tested including: Naive Bayes, Logistic
Regression, SVM, and Random Forests. Those
models were implemented using the Scikit-Learn
library (Pedregosa et al., 2011). Generally, the
accuracy realized by that category of models was
relatively fair (AUC ≈ 0.7).
Subsequently, the model was experimented using
various Artificial Neural Network (ANN) structures
as follows. Initially, the simplest model structure
included a single hidden layer of 50 neurons. The
complexity of the model was gradually increased by
adding more neurons (e.g. 200, 500).
HEALTHINF 2019 - 12th International Conference on Health Informatics
108
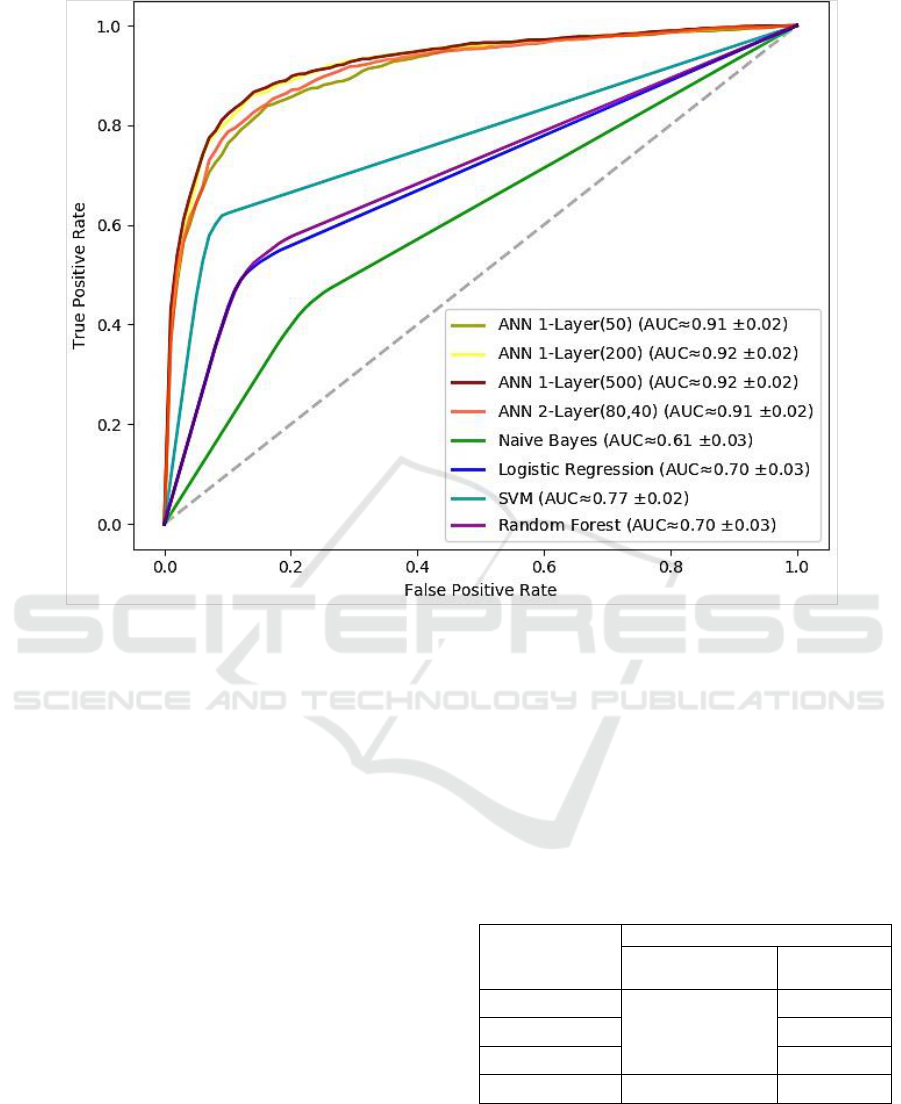
Figure 6: ROC curves of the binary classification models.
Subsequently, another hidden layer was included
in the model. The two layers consisted of 80 and 40
neurons respectively. It turned out the there was no
need to introduce further complexity in the model
based on the prediction accuracy as given in the next
section. Table 3 lists the ANN architectures included
in our experiments. The ML experiments were
implemented using the Keras library (Chollet, 2015)
with Python. The models were trained based on 10
rounds of cross-validation including 100 epochs and
20% dropout.
The classification accuracy is analyzed based on
the Receiver Operating Characteristics (ROC) curve.
The ROC curve plots the relationship between the
true positive rate and the false positive rate across a
full range of possible thresholds. Figure 6 plots the
ROC curves for the set of ANNs experimented. The
figure also shows the approximate value of the area
under the curve along with its standard deviation
over the 10-fold cross-validation.
At it appears, the neural network models
obviously outperform other approaches. All neural
networks provided a notable prediction accuracy that
went beyond 90%. Specifically, the single-layer
model of 200 could yield the best performance
(≈92.0%).
However, it is noteworthy that there was no
substantial improvement by growing the model
complexity either by increasing the number of
neurons or stacking more hidden layers. Thus, we
can say that a single-layer neural network was
sufficient in our case, which is a promising outcome
using a relatively limited dataset.
Table 3: ANN architectures.
Model Architecture
Hidden Layers
Number of
Neurons
Experiment #1
Single-Layer
50
Experiment #2
200
Experiment #3
500
Experiment #4
Two-Layer
(80, 40)
5.2 Multi-Label Classifier
A finer classification of ASD-diagnosed participants
was applied to allow for training a multi-label
model. We followed the basic grouping that describe
Learning to Predict Autism Spectrum Disorder based on the Visual Patterns of Eye-tracking Scanpaths
109
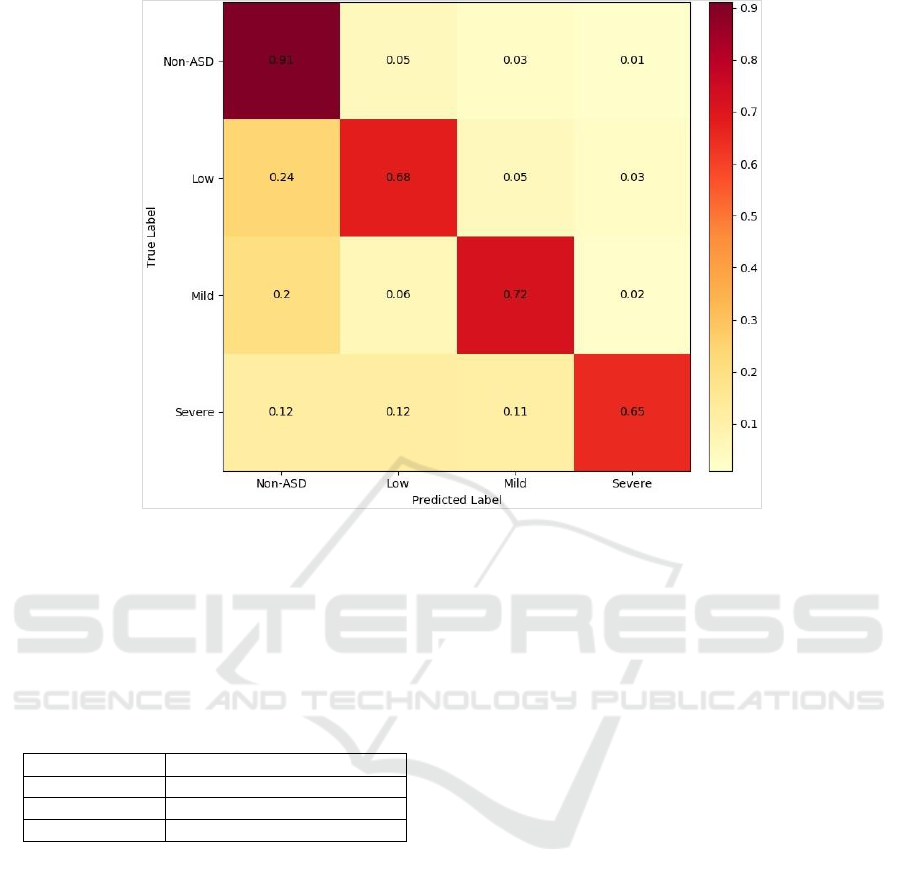
Figure 7: Confusion matrix of the multi-label classifier.
the severity of autism symptoms based on the CARS
score (Schopler et al., 1985). Specifically, ASD
participants were organized into smaller groups as
follows: i) Low, ii) Mild, and iii) Severe. Table 4
gives the specific categoreis of ASD participants
based on the CARS score.
Table 4: Classification of ASD participants.
ASD Category
Range of CARS Score
Low
CARS < 30
Mild
30<= CARS < 36
Severe
CARS >= 36
The multi-label classification model was trained
using neural networks only. We experimented
single-layer model of 200 neurons and two-layer
model as before. The average accuracy of the single-
layer model was still higher (≈83%) compared to
about 81%.
Though the accuracy relatively declined, the
approach still proved very good performance. Figure
7 provides a confusion matrix that visulaizes the
normalized classification accuracy (single-layer
model). The model turned out to discriminate the
non-ASD labels very well compared to others. The
prediction accuracy of ASD labels was 20% lower
(at least), especially for the severe-ASD examples.
5.3 Demo Application
A demo application was developed to serve as a
practical illustration of our approach. The
application links the three components altogether
including eye-tracking, visualization and ML to
support the diagnosis of ASD. The application
includes three layers as: i) Presentation, and ii) Web
services, and iii) Prediction.
The presentation layer performs the basic user
interface functionality and interactivity. The
presentation elements were delivered using
ASP.NET along with JavaScript. The layers of web
services and prediction were fully implemented by
the Azure ML Studio. Specifically, Azure ML is
employed to host the classification model and the
Python implementation used to produce
visualizations. The Azure ML platform provides an
ideal environment for data analytics with the ability
to deploy ML models as web services. In this
manner, ML models can be conveniently used
through web services using request/response calls.
Figure 8 sketches the application architecture.
The application goes through three steps as
follows. First, the user is asked to upload the eye-
tracking data. The data records should describe the
coordinates of the viewer’s gaze into the screen
along with associated time as shown earlier in
Table2. Second, the application produces a
HEALTHINF 2019 - 12th International Conference on Health Informatics
110
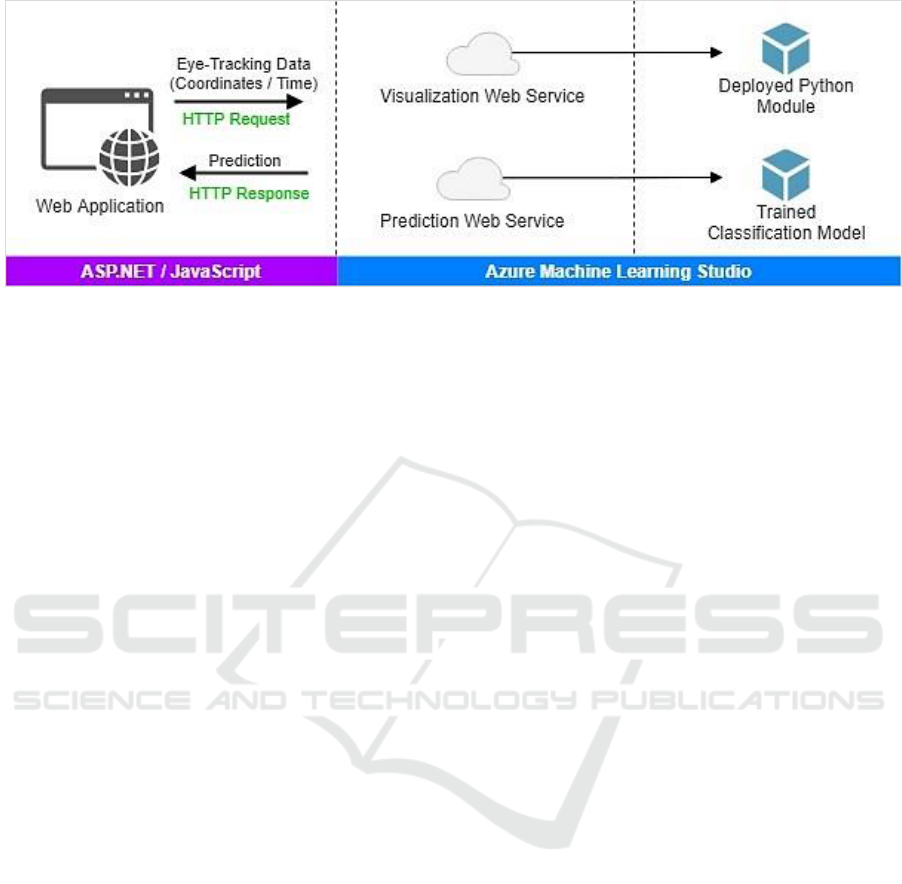
Figure 8: The demo application architecture.
visualization of the eye-tracking records. The
visualization is constructed through calling a web
service hosted on the Azure ML Studio. The web
service executes a Python module deployed to create
visualizations of the eye movement and its
dynamics.
Eventually, the application calls the prediction
web service, which returns the prediction from the
trained classification model. All communication
with the web services is conducted through standard
HTTP requests/response. The application can be
accessed online via <https://goo.gl/i4N7Zj>.
6 LIMITATIONS
Even though the results presented in this study are
promising, a set of limitations need to be highlighted
as follows. The primary limitation could be the
relatively small number of participants.
Another relevant issue of concern is the duration
of video scenarios, which was fairly short. Perhaps
longer scenarios could have allowed for a richer
visual representation of the gaze behavior of ASD.
Despite limitations, the study is still believed to
serve as a kernel for further interesting applications
of the proposed approach.
7 CONCLUSIONS
The coupling of eye-tracking, visualization and ML
can hold a strong potential for the development of an
objective tool to assist the diagnosis of ASD. The
ML experiments confirmed the core idea behind our
approach, which hinges on the visual representation
of eye-tracking scanpaths. The classification
accuracy indicated that visualizations were able to
successfully pack the information of eye motion and
its underlying dynamics.
From a practical standpoint, it is noteworthy that
we could reach that high accuracy with largely
simple ML models. Using simple ANN Classifiers,
the prediction accuracy could go beyond 90%. This
should be compared positively to related efforts that
used different sets of features and more complex
models (e.g. Wan et al., 2018; Carette et al., 2017).
It is conceived that our approach might be
applicable to comparable diagnostic problems. In a
broader sense, the visualization of eye-tracking
scanpaths could possibly be utilized for assisting the
diagnosis of similar psychological disorders.
ACKNOWLEDGMENTS
This work has been generously supported by the
Evolucare Technologies company. Run by a team of
medical information technology professionals,
Evolucare aspires to bring a broad range of products
and services to the whole healthcare community.
www.evolucare.com.
REFERENCES
Carette, R., Cilia, F., Dequen, G., Bosche, J., Guerin, J.-L.
and Vandromme, L. (2017). Automatic autism
spectrum disorder detection thanks to eye-tracking and
neural network-based approach. In Proceedings of the
International Conference on IoT Technologies for
HealthCare (HealthyIoT). Springer.
Carette, R., Elbattah, M., Dequen, G., Guérin J.L. and
Cilia F. (2018). Visualization of eye-tracking patterns
in autism spectrum disorder: method and dataset. In
Proceedings of the 13th International Conference on
Digital Information Management. IEEE.
Chollet, F. (2015). Keras. https://github.com/fchollet/keras
Learning to Predict Autism Spectrum Disorder based on the Visual Patterns of Eye-tracking Scanpaths
111

Coonrod, E. E. and Stone, W. L. (2004). Early concerns of
parents of children with autistic and nonautistic
disorders. Infants & Young Children, 17(3), 258–268.
DOH-U.S. (2018). Data and statistics | Autism Spectrum
Disorder (ASD). URL:
https://www.cdc.gov/ncbddd/autism/data.html
Draper, B. A., Baek, K., Bartlett, M. S. and Beveridge, J.
R. (2003). Recognizing faces with PCA and ICA.
Computer Vision and Image Understanding, 91(1-2),
115-137.
Du, Q. and Fowler, J. E. (2007). Hyperspectral image
compression using JPEG2000 and principal
component analysis. IEEE Geoscience and Remote
Sensing Letters, 4(2), 201-205.
Figshare (2018). URL:
https://figshare.com/s/5d4f93395cc49d01e2bd
Frazier, T. W., Klingemier, E. W., Beukemann, M., Speer,
L., Markowitz, L., Parikh, S., … and Strauss, M. S.
(2016). Development of an objective autism risk index
using remote eye tracking. Journal of the American
Academy of Child and Adolescent Psychiatry, 55(4),
301–309.
Goldberg, J.H. and Helfman, J.I., (2010). Visual scanpath
representation. In Proceedings of the 2010 Symposium
on Eye-Tracking Research & Applications (203-210).
ACM.
Henderson, J. M. (2003). Human gaze control during real-
world scene perception. Trends in Cognitive
Sciences, 7(11), 498-504.
Hunter, J.D., (2007). Matplotlib: A 2D graphics
environment. Computing in Science & Engineering,
9(3), 90-95.
ITU. (2017). Rec.ITU-R BT.601-7 Studio encoding
parameters of digital television for standard 4:3 and
wide screen 16:9 aspect ratios. International
Telecommunication Union-Radiocommunication
Sector, Geneva.
Jacob, R. (1995). Eye tracking in advanced interface
design. In W. Barfield W and T.A. Furness (eds),
Virtual Environments and Advanced Interface Design
(pp. 258–288). New York: Oxford University Press.
Jones, E., Gliga, T., Bedford, R., Charman, T. and
Johnson, M.H. (2014). Developmental pathways to
autism: a review of prospective studies of infants at
risk. Neuroscience & Biobehavioral Reviews, 39, 1-
33.
Jones, W. and Klin, A. (2013). Attention to eyes is present
but in decline in 2–6-month-old infants later diagnosed
with autism. Nature, 504(7480), 427-431.
Majaranta, P. and Bulling, A. (2014). Eye tracking and
eye-based human–computer interaction. In P.
Majaranta, H. Aoki, M. Donegan, D. W. Hansen, J.P.
Hansen, A. Hyrskykari and K.J. Räihä (Eds.), Gaze
Interaction and Applications of Eye Tracking:
Advances in Assistive Technologies (pp. 39-65).
Hershey: IGI-Gloal.
Pedregosa, F., Varoquaux, G., Gramfort, A., Michel, V.,
Thirion, B., Grisel, O., Blondel, M., Prettenhofer, P.,
Weiss, R., Dubourg, V. and Vanderplas, J. (2011).
Scikit-learn: Machine learning in Python. Journal of
Machine Learning Research, 12(Oct), 2825-2830.
Perez, L. and Wang, J. (2017). The effectiveness of data
augmentation in image classification using deep
learning. arXiv:1712.04621.
Pierce, K., Conant, D., Hazin, R., Stoner, R. and
Desmond, J. (2011). Preference for geometric patterns
early in life as a risk factor for autism. Archives of
General Psychiatry, 68(1), 101–109.
Pusiol, G., Esteva, A., Hall, S., Frank, M., Milstein, A. and
Fei-fei, L. (2016). Vision-based classification of
developmental disorders using eye-movements. In
Proceedings of the International Conference on
Medical Image Computing and Computer-Assisted
Intervention (pp. 317–325). Springer.
Sepeta, L., Tsuchiya, N., Davies, M. S., Sigman, M.,
Bookheimer, S. Y. and Dapretto, M. (2012). Abnormal
social reward processing in autism as indexed by
pupillary responses to happy faces. Journal of
Neurodevelopmental Disorders, 4(17), 1–9.
Schopler, E., Reichler, R.J., DeVellis, R.F. and Daly, K.,
(1980). Toward objective classification of childhood
autism: Childhood Autism Rating Scale (CARS).
Journal of Autism and Developmental Disorders,
10(1), 91-103.
Vabalas, A. and Freeth, M. (2016). Brief report: patterns
of eye movements in face to face conversation are
associated with autistic traits: evidence from a student
sample. Journal of Autism and Developmental
Disorders, 46(1), 305–314.
Wallace, S., Coutanche, M. N., Leppa, J. M., Cusack, J.,
Bailey, A. J. and Hietanen, J. K. (2012). Affective –
motivational brain responses to direct gaze in Children
with autism spectrum disorder. Journal of Child
Psychology and Psychiatry, 7(53), 790–797.
Wan, G., Kong, X., Sun, B., Yu, S., Tu, Y., Park, J., ... and
Lin, Y. (2018). Applying eye tracking to identify
autism spectrum disorder in children. Journal of
Autism and Developmental Disorders, 1-7.
Wing, L. and Gould, J. (1979). Severe impairments of
social interactin and associated abnormalities in
children: epidemiology and classification. Journal of
Autism and Developmental Disorders, 9(1), 11-29.
Xu, Y., Jia, R., Mou, L., Li, G., Chen, Y., Lu, Y. and Jin,
Z. (2016). Improved relation classification by deep
recurrent neural networks with data
augmentation. arXiv:1601.03651.
HEALTHINF 2019 - 12th International Conference on Health Informatics
112
