
Towards an “Operational” Educational Model in Healthcare:
Exploiting Computer-Interpretable Guidelines
Alessio Bottrighi
1
, Gianpaolo Molino
2
, Luca Piovesan
1
and Paolo Terenziani
1
1
DISIT, Università del Piemonte Orientale, Viale Teresa Michel 11, Alessandria, Italy
2
Az. Osp. San Giovanni Battista, C.sa Bramante 88, Torino, Italy
Keywords: Clinical Guidelines, Practitioners, Healthcare-agents, Medical Students Education.
Abstract: Clinical guidelines (GLs) encode the best medical practices. GLs have been widely exploited to enhance the
quality of patient care, and to optimize it, and several computer-based approaches to manage computer-
interpretable guidelines (CIGs) have been proposed in the literature. Quite surprisingly, however, the
potentialities of CIG systems in medical education have not been considered yet. In this position paper we
argue that, since CIG systems support the “simulation” of the application of GLs on specific patients, they
can be used to show students how to apply medical knowledge and best practices on specific patients.
Therefore, using CIG systems, students may learn an “operational methodology” that, otherwise, they could
only learn from the medical practice. In this paper, we have taken GLARE (and its extension, META-GLARE)
as an example of CIG system, and we have addressed the roadmap we intend to follow to fully exploit its
potentialities in medical education.
1 INTRODUCTION
Clinical guidelines (GLs) have been defined as
“systematically developed statements to assist
practitioner and patient decisions about appropriate
healthcare under specific clinical circumstances”
(Field and Lohr, 1990). They encode best medical
practices, promoting the adoption of evidence-based
medicine and supporting the quality and the
standardization of healthcare services, and the
optimization of costs. Thousands of GLs have been
devised. For example, the Guideline International
Network (http://www.g-i-n.net) groups 106
organisations in 54 countries, and provides a library
of more than 6500 GLs. In this paper, we argue that
such a valuable body of knowledge may be paired
with software tools supporting its application and
simulation (the so-called Computer-Interpretable
Guideline Systems, see below), and fruitably used to
complement “traditional” education in medicine.
Despite the large number of GLs, and their
diffusion, GLs have not fully achieved all their goals,
in terms of quality and optimization of the healthcare
services. The discussion of the reasons for GL not-
full success is outside the goals of this paper. Here we
just highlight two of such reasons, which have largely
motivated the introduction of ICT support tools to GL
management. Non-computerized GLs are large
bodies of knowledge (even hundreds of pages),
mostly expressed as free text, describing the “best
practice” recommendations for the treatment of a
given disease. Using such large bodies of (textual)
knowledge to diagnose and treat a specific patient is
a difficult issue for physicians, who are left alone
(i) to interpret the textual description (which, as
any natural language text, contains
imprecisions and ambiguities)
(ii) to identify the “mapping” between the general
recommendations in the GL and the specific
patient (and disease or clinical condition) at
hand; indeed, when diagnosing or treating a
patient, physicians should quickly identify,
among pages and pages of free text, the few
parts that are relevant for the specific patient
at hand.
Considering (among the others) issues (i) and (ii)
above, since the 90’s, the medical community has
started to develop many different systems and
projects to support physicians in the management of
GLs. In particular, in Computer-Interpretable
Guidelines (CIG) systems, GLs
(1) are represented in a formal and unambiguous
way, and
402
Bottrighi, A., Molino, G., Piovesan, L. and Terenziani, P.
Towards an “Operational” Educational Model in Healthcare: Exploiting Computer-Interpretable Guidelines.
DOI: 10.5220/0007482604020409
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 402-409
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(2) most CIG systems provide execution tools that
are automatically connected to the patients’
clinical records, so that they are able to
automatically detect the GL recommendations
that are more appropriate for the given patient.
Besides disease-based support tools (e.g., software
tools considering only a specific disease), several
disease-independent tools for CIGs have been
proposed in the literature (see e.g. (Fridsma, 2001;
Gordon and Christensen, 1995; Peleg, 2013)). Such
systems usually provide facilities to acquire,
represent and/or execute GLs in different (medical)
domains. Such tools have been mainly developed to
support physicians in patient care, while, quite
surprisingly, their impact in medical education has
been quite neglected until now.
2 CIG SYSTEMS AND
EDUCATION
Since GLs encode “best medical practices”, their role
in the medical practice has been widely investigated.
Indeed, several studies have shown that the quality of
patient treatments is higher in case physicians have
been educated to adopt GLs (see, e.g., (Corriere et al.,
2014) as regards the case of diabetes).
CIG systems have been quite widely used in order
to acquire GLs into a computer format, and to support
practitioners in the diagnosis and treatment of patients.
However, to the best of our knowledge, the
possible impact of the adoption of CIG systems in
education has not been investigated yet. Indeed, the
main claim of this position paper is that although CIG
systems until now have been conceived as a support
to practitioners to deal with patients affected by
specific diseases, they can evolve to support also
medical education. Indeed, in this paper we argue that
CIGs have the potentiality of drastically improving
the education of healthcare agents (medical
students/ practitioners/ nurses), proposing them an
“operational” methodology showing how to (reason
in order to) apply best practice recommendations to
specific patients.
First of all, it is important to point out that the
approach we propose may support the education of
both practitioners (e.g., their continuous education)
and medical students. Indeed, though the former have
responsibilities that the latter do not have, both of
them have to learn how to apply best medical
practices to patients. Indeed, for the same reason, also
other types of healthcare agents (e.g., nurses) can take
advantage of our approach.
Second, we want to highlight that the approach we
propose is not intended to substitute “traditional”
education in medicine (erogated through courses and
textbooks), but to complement it.
As a matter of facts, “traditional” education
provided by medical texts and courses covers a wide
range of knowledge, ranging from human anatomy to
the description of diseases, and of their treatments.
While such aspects are certainly very important (and
necessarily needed), another aspect should also
deserve a specific attention, the “operational” (our
terminology) aspect: how to “operate” on a specific
patient? How to proceed to diagnose and treat
her\him through the best medical practices? Such a
kind of “operational” knowledge is usually not
considered in textbooks, so that it can be learned by
medical students (and healthcare agents in general)
only “by practicing”.
On the other hand, CIG systems have the potentiality
of enriching education through an operational
methodology, showing how to proceed to diagnose
and to treat specific patients. Indeed, as discussed in
the introduction, any CIG supports healthcare agents
in the treatment of a specific patient, by automatically
“focusing” on the part of the GL which is relevant to
the (status of) the patient at hand (see problem (ii) and
the solution (2) in the introduction). This is, indeed,
the process that students have to learn to be able to
cope with real patients: how to focus on the more
appropriate parts of their general medical knowledge
and of the best practices and to apply them when
considering the patient.
Until now, CIG system have been developed in
order to support practitioners in the diagnose and
treatment of patients. To do so, most CIG systems
provide execution modules, which support
physicians in the application of a general GL to a
specific patient. Execution modules take in input the
patient’s clinical record and, on the basis of the
patient’s data, suggest the proper actions and, above
all, they help physicians to take the decision
appropriate to the patient. Intuitively speaking, one
can say that CIG execution tools support the focusing
on the specific parts of the general GL that are
appropriate for the patient at hand, and help to take
the best decisions on the basis of the patient’s data. In
other words, they make best medical practices
“operational”, by supporting the application of
general GLs to the patients.
In this paper, we propose a new use of CIG
systems. Instead of being used to support physicians
in the execution (application) of a GL to a real patient,
they could also be used in education, to simulate such
an execution (application). The learning healthcare
Towards an “Operational” Educational Model in Healthcare: Exploiting Computer-Interpretable Guidelines
403
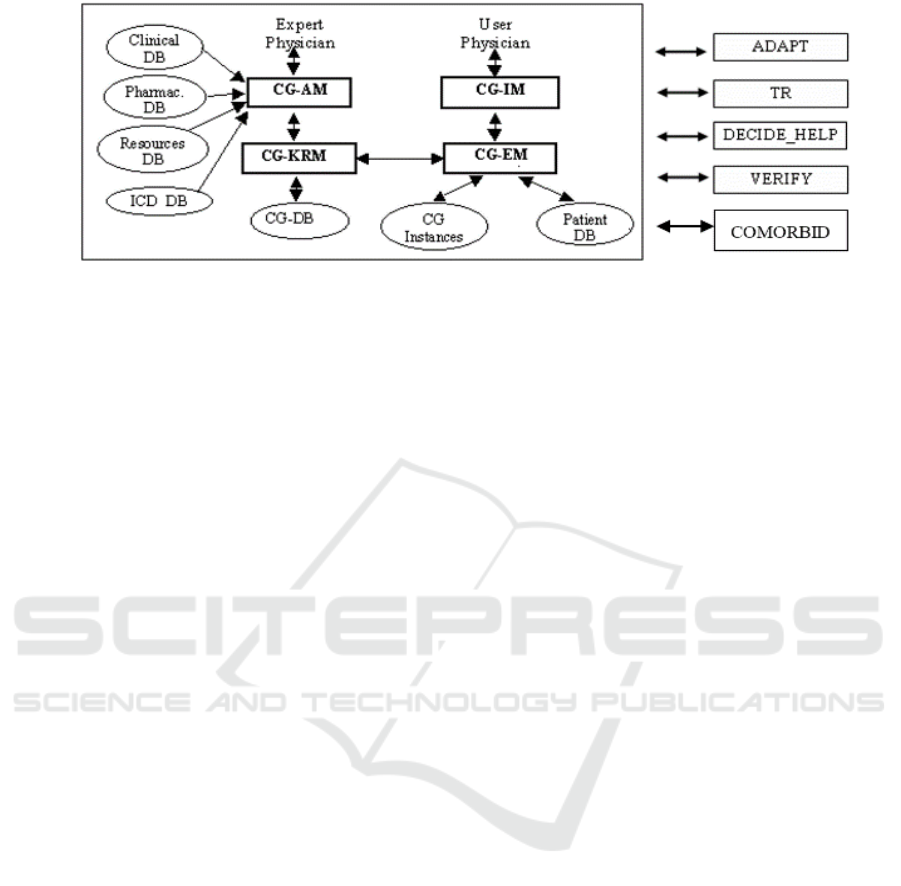
Figure 1: Architecture of GLARE. Rectangles represent computation modules, and ovals data/knowledge bases.
agent (e.g., student, practitioner) is given a patient
(in the form of the history of the evolution of the
patient’s data), and the CIG system can be used to
show how the GL would recommend to operate on
her\him (while, of course, no action is “physically”
executed: it is just a simulation).
Notably, since it is a simulation, the presence of
“physical” patients is not required: all that is needed
is the evolution of patients’ clinical data. As a
remarkable consequence, teachers may propose to
students (the data of) “significant” patients, and\or
invent (the data of) patients in such a way to force
students to explore the diagnosis/treatment of most
important (and\or problematic) cases.
This contribution may provide, in our opinion,
crucial benefits in the area of education, to
complement textbooks and traditional courses with
“practical” examples (through simulation) of how to
apply medical knowledge on specific patients.
Indeed, different modalities of education through
simulations can be provided:
(i) “standard” simulation, in which the CIG
system is used to show students how the (real
or invented) patient should be treated, step-by-
step, given the CIG recommendations
(ii) “second-opinion” simulation, in which a
student has to indicate how s\he would treat a
(real or invented) patient, and the CIG system
is used to indicate to the student where s\he has
followed the recommendations of the CIG,
and where s\he has violated them (with
additional explanations).
Notably, the educational approach we propose can be
strongly based on the facilities provided by currently
existing CIG systems. In particular, the acquisition of
CIGs can be done through the acquisition tools
already provided by such systems, with usually
automatically interact with patient clinical records.
As a consequence, the starting point of each
simulation could simply be the loading of
- an already acquired CIG
- the evolution of the data of a (real or
invented) patient.
As regards the simulation, “standard” simulation can
be performed by taking advantage of the execution
module already provided by most CIG systems. On
the other hand, the “second-opinion” simulation
require a modification of current execution tools, as
discussed in the next Section.
While in Section 4 we further elaborate such
issues, here we highlight that, for education, the
disease-independent CIG systems have a major
advantage with respect to the disease-based ones. In
fact, with such systems, one can acquire a library of
different CIGs (expressed using the same formalism,
the one provided by the CIG system), and provide a
unique software tool for simulating each of them.
Therefore, they allow one to develop a uniform
educational environment, in which all the CIGs are
homogeneously represented through the same
formalism, and the same simulation mechanism is
applied to all of them. On the contrary, the adoption
of disease-dependent CIG systems would force the
adoption of multiple different representation
formalisms and simulation mechanisms, one for each
different CIG. This move would force students to
learn different formalisms and to practice different
software tools for simulation, which would only be a
loss of time for medical students.
Though our position and the discussion until
now is fully general, in our future research we aim at
making it fully operative taking advantage of GLARE
(Guideline Acquisition, Representation and
Execution; (Terenziani et al., 2008)), and its
extension, META-GLARE (Bottrighi and Terenziani,
2016). Before highlighting how we plan to cope with
education through CIGs, in Section 3 we quickly
mention the current status of GLARE and META-
GLARE.
HEALTHINF 2019 - 12th International Conference on Health Informatics
404

3 STATUS OF GLARE PROJECT
GLARE is a disease-independent system for the
acquisition and execution of CIGs, which we are
developing since 1997, in collaboration with the
physicians of Azienda Ospedaliera San Giovanni
Battista in Torino, Italy (one of the major hospitals in
Italy). The core of GLARE (see the reference and the
box on the left of Fig. 1) is a modular architecture.
CG_KRM (Clinical Guidelines Knowledge
Representation Manager) is the main module of the
system: it manages the internal representation of GLs,
and operates as a domain-independent and task-
independent knowledge server for the other modules;
moreover, it permanently stores the acquired GLs in
a dedicated Clinical Guidelines Database (CG-DB).
The Clinical Guidelines Acquisition Manager
(CG_AM) provides expert-physicians with a user-
friendly graphical interface to introduce the GL into
the CG_KRM and to describe them. It may interact
with four databases: the Pharmacological DB, storing
a structured list of drugs and their costs; the
Resources DB, listing the resources that are available
in a given hospital; the ICD DB, containing an
international coding system of diseases; the Clinical
DB, providing a “standard” terminology to be used
when building a new GL, and storing the descriptions
and the set of possible values of clinical findings. The
execution module (CG-EM) executes a GL for a
specific patient, considering the patient’s data
(retrieved from the Patient DB). CG-EM stores the
execution status in another DB (CG Instances) and
interacts with the user-physician via a graphical
interface (CG-IM).
GLARE’s architecture is open. In the latest years,
several new modules and\or methodologies have been
added to cope with automatic resource-based
contextualization (ADAPT module, (Terenziani et
al., 2004)), temporal reasoning (TR, (Anselma et al.,
2006)), decision making support (DECIDE_HELP,
(Montani et al., 2005)), model-based verification
(VERIFY, (Bottrighi et al., 2010)), and comorbidities
(COMORBID, (Piovesan et al., 2014) (Piovesan et
al., 2015).
Representation Formalism. The core of GLARE
is the definition of the representation formalism used
to model GLs. Notably, a unique (disease-
independent) formalism is provided, and is used by
all the modules of GLARE. In GLARE, a GL is
represented through the set of actions composing it.
GLARE distinguishes between atomic and composite
actions. Atomic actions can be regarded as
elementary steps in a GL, in the sense that they do not
need a further decomposition into sub-actions to be
executed. Composite actions are defined in terms of
their components (atomic or composite actions), via
the “has-part” relation. GLARE adopts four different
types of atomic actions. Work actions represent basic
atomic actions which must be executed on the patient,
and can be described in terms of a set of attributes,
such as name, (textual) description, cost, time,
resources, goals. Query actions are requests of
information, which can be obtained from the outside
world (physicians, Databases, knowledge bases).
Conclusions represent the output of decision actions.
Decision actions are specific types of actions
embodying the criteria which can be used to select
alternative paths in a GL. They are crucial also to the
education task, so that they are detailed in the
following subsection.
Control relations establish which actions might
be executed next and in what order. We distinguish
among four different control relations: sequence,
constrained, alternative and repetition. Temporal
constraints between actions (e.g., overlaps, during)
are also supported.
Testing. GLARE has been applied to different
medical domains, including bladder cancer, reflux
esophagitis, heart failure, and ischemic stroke.
META-GLARE
In the last years, we have defined a new GL system,
META-GLARE (Bottrighi and Terenziani, 2016), on
top of GLARE. META-GLARE has been designed
to make extensions of CIG formalisms easy to
implement-manage, so that its availability is very
important, to support the extensions of GLARE with
the constructs needed to support educations.
However, META-GLARE is a support for system
designers, while users (e.g., physicians, or students)
never have to directly interact with META-GLARE.
3.1 Decisions in
GLARE\META-GLARE
Decisions are probably the most crucial aspect of
GLs, since they allow user-physicians to
discriminate among alternative actions. GLARE
supports two different types of decisions: diagnostic
and therapeutic decisions.
Diagnostic decisions consider a set of
parameters (to be evaluated on the basis of the status
of the patient) to discriminate among different
diagnoses. Of course, different parameters have to be
considered, depending on the specific diagnostic
decision. In GLARE, we support “scored” diagnostic
decisions: for each one of the relevant parameter (e.g.
Towards an “Operational” Educational Model in Healthcare: Exploiting Computer-Interpretable Guidelines
405
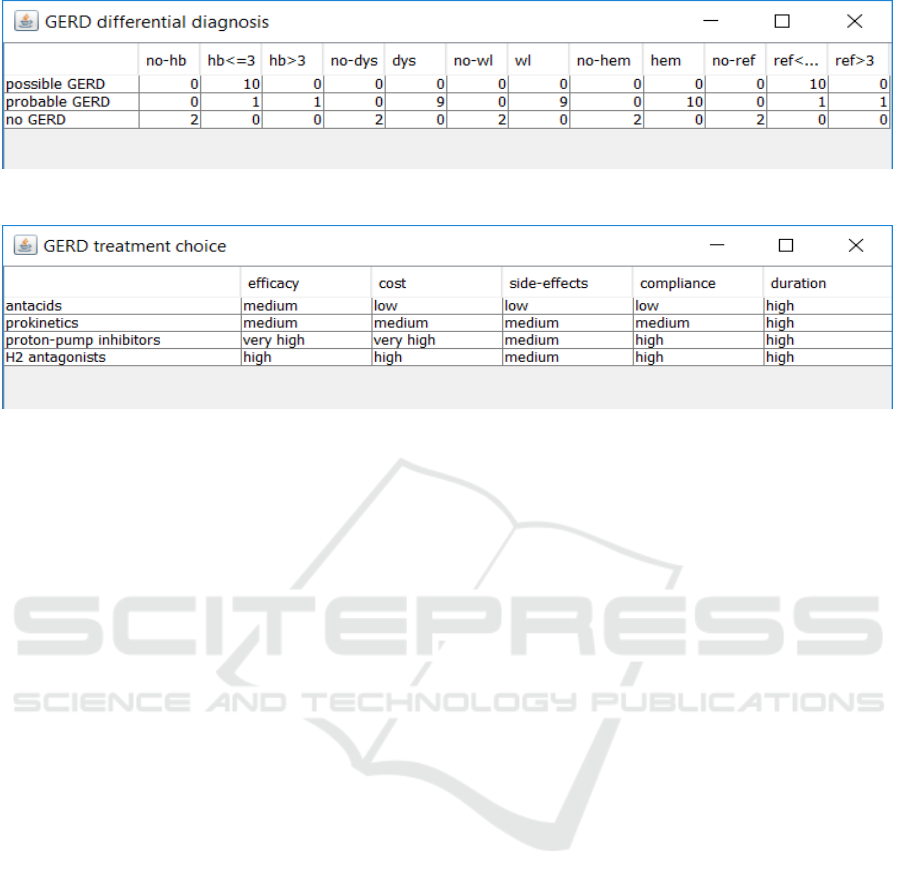
Figure 2: Tabular representation of a diagnostic decision for GERD, as shown in GLARE acquisition module.
Figure 3: Tabular representation of the therapeutic decision for “possible GERD”, as shown in GLARE acquisition module.
fever) and considering the alternatives to be
discriminated, expert-physicians define a priori a set
of values/ranges (e.g., fever < 37, between 37 and 38,
between 38 and 39, > 39; or, qualitatively: no, low,
medium, high). For each one of the values/ranges of
the findings in each one of the alternatives, the
expert-physician defines a score. Moreover, a
threshold is fixed to separate recommended/disliked
actions. GLARE execution engine considers the
patient’s data to evaluate each parameter, and, for
each alternative diagnosis, sums up the scores. Only
the alternatives whose additive score is greater than
the threshold are recommended for selection to the
user. To summarize, a decision among a set of
diagnoses can be represented as an open set of triples
<diagnosis, parameter, score> (where, in turn, a
parameter is a triple <data, attribute, value>), plus a
threshold which is relative to the sum of the scores.
For example, Fig. 2 shows a diagnostic decision
within the Gastro-Esophageal Reflux Disease
(GERD) guideline, namely the “differential
diagnosis”, which allows the physician to
discriminate between “possible GERD”, “probable
GERD” and “no GERD” according to the values of
several parameters: heartburn absent (abbreviated by
“no-hb” in Fig. 2), or lasted not more than 3 months
(“hb≤3”), or lasted more than 3 months (“hb>3m”);
dysphagia absent (“no-dys”) or present (“dys”);
occurrence of weight loss (“wl”) or non-occurrence
(“no-wl”); absence (“no-hem”) or presence (“hem”)
of hematemesis; postural reflux absent (“no-ref”), or
lasted not more than 3 months (“ref≤3”) or more than
three months (“ref>3m”). The threshold for such a
decision (not shown in Fig. 2) is >9. E.g., given the
scores in Fig. 2, one should conclude “no GERD”
only if heartburn, dysphagia, weight loss,
hematemesis and postural reflux are all absent.
Notably, also Boolean diagnostic decisions are
supported by META-GLARE, and will be used in the
educational task.
On the other hand, in the therapeutic decisions,
physicians have to choose from different therapies
(treatments) according to a given set of parameters:
effectiveness, cost, side-effects, compliance,
duration, which have to be specified for each one of
the alternative therapies to be discriminated. Thus, a
decision action can be represented by the set of the
parameters above, for each one of the alternatives.
E.g., in Fig. 3 we show a possible way of
modelling the decision among four different
treatments in the case of “possible GERD”, in the
tabular fashion which is used by GLARE acquisition
module.
3.2 GLARE in Education: First Steps
GLARE has been already tested in the training of
physicians in the context of emergency medicine, to
cope with poly-trauma, within the ROPHS (Report on
the Piedmont Health System) project (Leonardi et al.,
2012). In such a project, GLARE has been used “as it
is” for educational purposes following the lines
suggested in Section 2, point (1). Specifically:
(i) together with expert physicians, we have
acquired a GL for polytrauma in GLARE
(ii) expert physicians have defined for us a set of
“typical” patients affected by polytrauma. We
HEALTHINF 2019 - 12th International Conference on Health Informatics
406

have stored the evolution of the data of such
patients in the DB of clinical records.
(iii) Expert physicians have used GLARE execution
module to provide a “standard” simulation (see
Section 2) of the treatment of the patients
The adoption of GLARE has supported the
physicians attending the course while learning “the
process”: we showed the simulation of the application
of the polytrauma CIG to different patients, showing
step-by-step the results of applying the CIG’s
recommendations. However, we did not have the
possibility to split the physicians attending the course
into two groups (one taking advantage of GLARE,
and the other not using it), so that we could not have
a practical analysis of the educational advantages
provided by the adoption of GLARE. However, this
has just been our first experience in adopting GLARE
(and META-GLARE) for educational purposes. Our
roadmap is much more ambitious, and it is briefly
presented in the following section.
4 ROADMAP TO A FULL
EXPLOITATION OF
META-GLARE FOR
EDUCATION
Besides the “conservative” application of GLARE for
education in the ROPHS project, in which GLARE
has been used “as it is” to show students practical
examples about how to operate on specific
(polytrauma) patients, our roadmap plans much more
ambitious applications, and consequently, a lot of
extensions. Notably, to achieve a fast prototyping of
such extensions, we will take advantage of META-
GLARE, which has been explicitly designed in order
to make extensions of CIG formalisms easy to
implement-manage.
4.1 Explanatory Annotations
For the sake of education, it is very important that
each action in the CIGs is carefully annotated with
detailed explanations and motivations. Indeed, GL
are based on best practices and medical evidence, and
such knowledge has to be explicitly stored in order to
support education. Of course, such an extension does
not require specific efforts from the computer science
point of view, but, indeed, the acquisition of such
annotation is a long and time-consuming task, to be
performed mostly by domain experts.
4.2 Propose & Check Execution
Modality
As discussed in Section 2, issue (ii), also “second-
opinion” simulation can play an important role in
medical education. However, to support “second-
opinion” simulation, GLARE execution engine must
be enriched with a new modality, in which GLARE,
instead of suggesting the most suitable choice for
diagnostic and therapeutic decisions, let the student
choose without any suggestion, and then compares
the student’s choice with the one that would have
been suggested by the CIG. Especially in case of
disagreement (but also in case the choice of the
student is the same that would have been
recommended by the GL) the motivations for the
choice recommended by the GL should be provided
to the students (e.g., by showing the logic underlying
the decision – see the discussion about therapeutic
and diagnostic decisions in Section 3.1).
4.3 Fake Decisions and Paths
By definition, GLs contain the “best practices” to
cope with specific diseases. However, during the
educational processes, diagnostic and therapeutic
problems might be made more complex for students
by adding wrong decisions leading to non-existing (in
the real GL) alternative paths into the GL (called fake
decisions and paths in the following). Fake decisions
and path can be used in the “second-opinion”
simulation, to increase the complexity of the problem,
especially in case such fake alternatives represent
cases of frequent\plausible medical errors. Of course,
the explanatory facility is fundamental in this context:
each fake path must be paired with exhaustive
annotations, detailing why such a path should not be
applied.
4.4 What-if Analysis
The “what-if” analysis in an important cognitive tool
in human reasoning in general, and in the medical
context in particular, since it allows one to analyse the
consequences of a given action or choice. GLARE
already supports a quite reach mechanism to support
what-if analysis (Terenziani et al., 2002). GLARE’s
“what-if” facility is the implementation of a form of
hypothetical reasoning. In particular, users are helped
in gathering the various types of information
necessary for discriminating among the alternative
paths of actions at any stage of the GL. Relevant
decision parameters (e.g., costs, resources, times) are
gathered from the alternative paths in an automatic
Towards an “Operational” Educational Model in Healthcare: Exploiting Computer-Interpretable Guidelines
407

way. At the end of this process, the tool displays the
values of the chosen parameters for each of the
alternative paths.
Such a mechanism can be used for education in
two different ways:
(1) In the “standard” simulation, to show to the
students the different consequences of
decision actions in the GLs.
(2) In the “second-opinion” simulation, to
support students’ choices. Before taking a
decision, students could use such a facility to
explore the consequences of choosing one
alternative or another.
4.5 Patients’ Data Generation
A central issue is the definition of patients. Indeed,
(the data of) a specific patient will be the starting
point of each educational session, in which students
will have to simulate the application of the GL “best
practices” on her\him. Since we are speaking of
education, we do not “physically” operate on real
patient. Therefore, while in a real application (of a
GL on a patient) the whole evolution of the data of
the patient cannot be known “a-priori” (but it is
discovered “step-by-step”, looking at the evolution of
the status of the patient), in the educational context
we only simulate an application, so that we can
hypothesize to know a-priori the whole evolution of
the patient. However, different forms of simulations
can be provided, depending on the model one adopts
to represent the patient data. At least two different
approaches are possible, that we call (i) deterministic
patient and (ii) probabilistic patient.
In the “deterministic patient” model, we assume
that the clinical record of the patients contains, “a-
priori” (i.e., already at the beginning of the
simulation) the whole evolution of the patient’s data.
There is only one specific evolution, corresponding to
a specific path in the CIG, the one recommended for
such a patient. A temporal (relational) database is
used to manage such temporal data (as regards
GLARE, consider, e.g., (Anselma et al., 2018; Stantic
et al., 2012)). The deterministic patient model can be
used in the “standard” simulation (since the data
evolution corresponds to the path of actions that one
wants to show to the students). It may also be used for
the “second-opinion” simulation. However, in such a
case, whenever students deviate from the
recommended path, they have to receive a warning
(and exhaustive explanations), and then be forced to
continue the simulation considering the
recommended path (since only the data
corresponding to the recommended path are available
to the system).
On the other hand, the probabilistic patient model
represents the initial status of a patient, and the
probabilistic history of her\his evolutions, depending
on the GL actions performed on her\him. Obviously,
the definition of (“probabilistic”) patients is complex,
and requires the availability of a lot of knowledge,
e.g. (Dagan et al., 2007), (Real et al., 2015). However,
it supports also flexible “second-opinion”
simulations, in which students may also follow non-
recommended paths in the CIGs.
Finally, it is important to emphasize that, while
the whole history of the evolution of the (data of the)
patients must be provided as input to the simulation,
the students will operate “step-by-step” in the
application of the CIGs to the patients, so that, at each
step, they will only see the data holding at that point
of the execution, plus past data.
4.6 Experimental Evaluation
Needless to say, the experimental evaluation is
necessarily a cue issue in the educational context. One
of the major goals we have in this context is to be able
to establish partnerships or projects with educational
institutions, in order to be able to provide extended
and accurate experimental evaluations of the impact
of adopting CIG systems in medical education.
5 CONCLUSIONS
GLs and CIGs have a quite consolidated role in the
standardization and optimization of the healthcare
services. On the other hand, their potentiality in the
education of medical students and, more generally, of
healthcare agents has not been adequately explored.
In this position paper, we claim that CIG systems
have great potentiality in educations. Since they
support the “simulation” of GLs on specific patients,
they can be used to show students how to apply
medical knowledge and best practices on specific
patients, providing them an “operational
methodology” that, otherwise, they could only learn
from the medical practice.
In this paper, we have taken GLARE as an
example of CIG system, and we have addressed the
roadmap we intend to follow in order to fully exploit
its potentialities in medical education.
HEALTHINF 2019 - 12th International Conference on Health Informatics
408

REFERENCES
Anselma, L., Piovesan, L., Stantic, B., Terenziani, P., 2018.
Representing and querying now-relative relational
medical data. Artif. Intell. Med. 86, 33–52.
https://doi.org/10.1016/j.artmed.2018.01.004
Anselma, L., Terenziani, P., Montani, S., Bottrighi, A.,
2006. Towards a comprehensive treatment of
repetitions, periodicity and temporal constraints in
clinical guidelines. Artif. Intell. Med., Temporal
Representation and Reasoning in Medicine 38, 171–
195. https://doi.org/10.1016/j.artmed.2006.03.007
Bottrighi, A., Giordano, L., Molino, G., Montani, S.,
Terenziani, P., Torchio, M., 2010. Adopting model
checking techniques for clinical guidelines verification.
Artif. Intell. Med. 48, 1–19. https://doi.org/10.1016/
j.artmed.2009.09.003
Bottrighi, A., Terenziani, P., 2016. META-GLARE: A
meta-system for defining your own computer
interpretable guideline system—Architecture and
acquisition. Artif. Intell. Med. 72, 22–41.
https://doi.org/10.1016/j.artmed.2016.07.002
Corriere, M.D., Minang, L.B., Sisson, S.D., Brancati, F.L.,
Kalyani, R.R., 2014. The use of clinical guidelines
highlights ongoing educational gaps in physicians’
knowledge and decision making related to diabetes.
BMC Med. Educ. 14, 186. https://doi.org/10.1186/
1472-6920-14-186
Dagan, I., Gabay, M., Barnea, O., 2007. Fluid resuscitation:
computer simulation and animal experiments. Conf.
Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE
Eng. Med. Biol. Soc. Annu. Conf. 2007, 2992–2995.
https://doi.org/10.1109/IEMBS.2007.4352958
Field, M.J., Lohr, K.N. (Eds.), 1990. Clinical Practice
Guidelines: Directions for a New Program. National
Academies Press (US), Washington (DC).
Fridsma, D.B., 2001. Special Issue on Workflow
Management and Clinical Guidelines. J. Am. Med.
Inform. Assoc. 22, 1–80.
Gordon, C., Christensen, J. P. (Eds.), 1995. Health
telematics for clinical guidelines and protocols, Studies
in health technology and informatics. IOS Press,
Amsterdam, Netherlands.
Leonardi, G., Bottrighi, A., Galliani, G., Terenziani, P.,
Messina, A., Della Corte, F., 2012. Exceptions handling
within GLARE clinical guideline framework. AMIA
Annu. Symp. Proc. AMIA Symp. AMIA Symp. 2012,
512–521.
Montani, S., Terenziani, P., Bottrighi, A., 2005. Exploiting
Decision Theory for Supporting Therapy Selection in
Computerized Clinical Guidelines, in: Miksch, S.,
Hunter, J., Keravnou, E.T. (Eds.), Artificial Intelligence
in Medicine, 10th Conference on Artificial Intelligence
in Medicine, AIME 2005, Aberdeen, UK, July 23-27,
2005, Proceedings, Lecture Notes in Computer Science.
Springer, pp. 136–140. https://doi.org/10.1007/
11527770_19
Peleg, M., 2013. Computer-interpretable clinical
guidelines: A methodological review. J. Biomed.
Inform. 46, 744–763. https://doi.org/10.1016/j.jbi.
2013.06.009
Piovesan, L., Molino, G., Terenziani, P., 2015. Supporting
Multi-level User-driven Detection of Guideline
Interactions, in: Verdier, C., Bienkiewicz, M., Fred,
A.L.N., Gamboa, H., Elias, D. (Eds.), HEALTHINF
2015 - Proceedings of the International Conference on
Health Informatics, Lisbon, Portugal, 12-15 January,
2015. SciTePress, pp. 413–422.
Piovesan, L., Molino, G., Terenziani, P., 2014. Supporting
Physicians in the Detection of the Interactions between
Treatments of Co-Morbid Patients, in: Healthcare
Informatics and Analytics: Emerging Issues and
Trends. IGI Global, pp. 165–193.
Real, F., Riaño, D., Alonso, J. R., 2015. A Patient
Simulation Model Based on Decision Tables for
Emergency Shocks, in: Riaño, D., Lenz, R., Miksch, S.,
Peleg, M., Reichert, M., ten Teije, A. (Eds.),
Knowledge Representation for Health Care. Springer
International Publishing, Cham, pp. 21–33.
Stantic, B., Terenziani, P., Governatori, G., Bottrighi, A.,
Sattar, A., 2012. An implicit approach to deal with
periodically repeated medical data. Artif. Intell. Med.
55, 149–162.
Terenziani, P., Montani, S., Bottrighi, A., Molino, G.,
Torchio, M., 2008. Applying artificial intelligence to
clinical guidelines: the GLARE approach. Stud. Health
Technol. Inform. 139, 273–282.
Terenziani, P., Montani, S., Bottrighi, A., Torchio, M.,
Molino, G., 2002. Supporting physicians in taking
decisions in clinical guidelines: the GLARE“ what if”
facility. Proc. AMIA Symp. 772.
Terenziani, P., Montani, S., Bottrighi, A., Torchio, M.,
Molino, G., Correndo, G., 2004. A context-adaptable
approach to clinical guidelines. Stud. Health Technol.
Inform. 107, 169–173.
Towards an “Operational” Educational Model in Healthcare: Exploiting Computer-Interpretable Guidelines
409
