
A Practical Medical Experience of Successfully Mixing Model-Driven
Paradigm and Business Process Management Principles
V. Cid-de-la-Paz
1
, L. Morales
1
and J. M. Ramos
2
1
IWT2 Group, University of Seville, Spain
2
SOLTEL S.A., Spain
Keywords: Clinical Decision Support, Model Driven Engineering, Clinical Practices Guidelines.
Abstract: The Model-Driven Paradigm has been successfully used in several different software contexts and there are
a lot of literature offering approaches, techniques and tools to guarantee its application in different areas,
such as software design, software testing, and so on. But, this paradigm can be also used in other contexts
offering very good results. In this paper, we illustrate the power of using models and transformations to
make an effective and efficient management of clinical guides in medical environments. The paper shows
how using business process management to represent clinical guidelines, principles of Model-Driven
paradigm can be successfully used. The paper presents the experiences in the IDE4ICDS, which is framed
into the medical context to provide a solution to manage the life cycle of clinical guidelines. This project
presents a methodology that allows the management of clinical guidelines to be automated, as well as a
software platform to support it. This platform has been validated with health professionals from the Hospital
Virgen del Rocio (Seville), obtaining promising results. Nowadays, this platform is been validated by
healthcare professionals of Primary Care with patients suffering from Diabetes Mellitus Type 2.
1 INTRODUCTION, CONTEXT
AND NEED
BPM (Business Processes Management) could be
considered as a management strategy that includes
methods, techniques and tools to support the
business processes lifecycle, which includes design,
enactment, management and analysis of operational
BPs (Van-der-Aalst.2002). BPM aims to reduce
costs and improve processes (through a cycle of
continuous improvement) in many organizations
(PMI.2008). In fact, some studies, such as
(ISO/IEC.ISO 9001.2008), conclude that the reasons
for adopting BPM can be grouped into three main
needs: (i) understand and assimilate the intrinsic
knowledge of processes; (ii) know the employees’
performance during the execution processes; and
(iii) monitor and measure processes. Controlling
these needs improves ROI (Return on Investment)
parameter through reducing production costs
(Trkman P. 2010) in any kind of organization.
There are also many institutions that promote, by
means of their standards and guidelines, the
application of BPM as a process-oriented
mechanism to improve productivity,
competitiveness, quality and efficiency at
organizations (OMG.2011, Martinez-Ruiz T et al.
2008, ISO/IEC.ISO/IEC TR24744.2007, Ponce J et
al.2013). Such parameters have been followed by a
large number of companies in all areas of business.
Healthcare organizations are not an exception and,
in fact, the Healthcare Process (HP) management is
essential to ensure adequate patient care, as well as
facilitate the work of healthcare professionals in an
area where it is essential to take of decisions based on
the best available biomedical knowledge. This
practice is known as Evidence-Based Medicine
(EBM) (Tonelli M et al.2018) and it is applied within
a framework of quality of care, patient safety and
efficiency. The management of HP is also closely
related to the term of clinical guidelines, which are
usually are textual and systematic statements of
information on HP, clinical records, recommendations
and clinical decision rules.
The Clinical Guidelines (CG) themselves aim to
improve the quality and safety of patient care,
reduce variability in clinical practice and reduce
healthcare costs. In this context, in recent years,
different research groups in the field of medical
410
Cid-de-La-Paz, V., Morales, L. and Ramos, J.
A Practical Medical Experience of Successfully Mixing Model-Driven Paradigm and Business Process Management Principles.
DOI: 10.5220/0007484704100416
In Proceedings of the 7th International Conference on Model-Driven Engineering and Software Development (MODELSWARD 2019), pages 410-416
ISBN: 978-989-758-358-2
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

informatics have developed conceptual models to
represent executable CG. However, the
implementation and maintenance of these conceptual
models in software systems are hard, complex and
expensive tasks because these systems are usually
designed and developed ad hoc what implies
hindering interoperability among organizations, the
lack of standardization, and increasing the inter-
center or even inter-professional variability.
Moreover, at present, there are not
methodological frameworks that define how
managing comprehensively all phases of the CG
lifecycle: from its modeling (including its HP,
clinical records, clinical rules, etc.) to its execution,
monitoring, evolution, and integration with
Healthcare Information Systems (HIS) and Clinical
Devices of Patients (CDP, such as glucometer, blood
pressure monitor, clinical sensors, etc.). For this
reason, designing and developing software systems
to CG management are complex task and, in many
cases, these systems are developed ad hoc by each
health organization that decides to implement a
specific CG what implies that the same CG can be
developed (and evolved) in different ways in
different health organizations.
In this context, it is necessary to research,
propose and define mechanisms to ensure the correct
and successful execution and management of CG in
order to reduce developing costs of software systems
and reduce the variability of application of CG in
similar medical situations in different patients.
However, it is important to mention that although
these systems are defined correctly, it is necessary to
give solution to another important aspect related to
the maintenance of CG because this one evolves
frequently. Consequently, proper management of
change, maintenance of traceability between the
definition and implementation of CG, as well as the
achievement of effective continuous improvement,
become fundamental and key tasks within health
organizations.
This paper describes a practical experience in a
real R&D project which aims to propose a
technological solution to solve the previous need in
health organizations. This project (as well as its
support software platform) is named IDE
4
ICDS
(Integrated Developing Environment for Improving
Clinical Decision Support based on Clinical
Guidelines) and it is subsidized by the Ministry of
Economy and Competitiveness and co-financed with
FEDER funds, in the call Challenges-Collaboration
of the State Program of Research, Development and
Innovation Oriented to the Challenges of Society,
within the framework of the State Plan for Scientific
Research and Technique and Innovation 2013-2016.
The Consortium of public and private entities
that is carrying out the project are: the IWT2 Group
of the University of Seville, Soltel IT Software SLU,
Serviguide Consultoría S.L. and the GIT Group of
the FISEVI Foundation.
IDE
4
ICDS is based on the Model-Driven
Engineering (MDE) (Schmidt D.C.2006) paradigm
and defines a methodological framework to
comprehensively and collaboratively manage CG, as
well as systematic mechanisms for deploying and
maintaining these CG on a web platform. Moreover,
our experience in transferring knowledge to
companies confirms that naive MDE-based solutions
are more likely to succeed because they enable
designing and implementing transformation rules
(Escalona, M. J et al. 2007, García-García J.A., et
al.2015, Gutierrez, J.J, et al.2015, Dominguez, FJ et
al.2010, Dominguez, FJ et al.2014, García-García
J.A et al.2014, García-García J.A., et al.2017).
The features of IDE
4
ICDS are: (i) centralized,
i.e., a single nucleus of information is stored and
traceability maintained between all the components
of a clinical guide (processes, simple elements and
decision rules); (ii) integral, i.e., a single platform
(IDE
4
ICDS) provides modules to define, execute,
monitor and interoperate the CG with HIS and CDP;
and (iii) collaborate, i.e., if a health professional
considers it necessary to improve or evolve a CG
(based on his or her experience), he or she can do it
intuitively and friendly, and that modification could
be used by another health organization. In addition,
it is important to mention that this project will be
tested and validated in a real scenario of patients
with Type 2 Diabetes Mellitus
1
.
After this introduction, this paper is structured as
follows. Section 2 describes our proposal to clinical
guidelines management. Section 3 describes our
technological solution. Finally, Section 4 states
conclusions and introduces future lines of research.
2 THEORETICAL
FOUNDATIONS OF IDE4ICDS
BASED ON AN IMPROVEMENT
CONTINUOUS LIFECYCLE
From a general point of view, process management
could be considered a management strategy with a
1
Andalusian Regional Ministry of Health. Integrated
Welfare Process Diabetes Mellitus. Last access 2018.
A Practical Medical Experience of Successfully Mixing Model-Driven Paradigm and Business Process Management Principles
411

clear multidisciplinary nature, as it can be applied to
different contexts (e.g., healthcare domain) and can
be used by different user profiles (Hill J.B, et
al.2017). This situation has conditioned the
appearance of different views, definitions and
perspectives of management lifecycles as well as
their continuous improvement (Van-der-Aalst.2004),
which define a management model for continuous
business implementations and incremental problem
solving. Although clinical guidelines have more
feature than simple process, the Clinical Guideline
Management (CGM) is similar because both ones
need to be modelled, executed, orchestrated,
measured, improved, etc.
However, CGM has several particularities which
have to be properly supported by decision-support
software systems. As mentioned in previous section,
these particularities are related to improve the
maintenance and evolution of CG, streamline the
day of health professionals and reduce the variability
in the clinical practice and sanitary cost.
For this purpose, the project defines a model-driven
theoretical framework to support a continuous
improvement lifecycle of CG based on four phases
which are:
1. Modeling Phase. In this phase, healthcare
professionals can model his/her CG in a
structured manner (i.e., identifying roles,
activities of the healthcare process, clinical
rules, clinical recommendations, for instance).
For this purpose, we have carried out different
studies of international clinical guidelines to
extract and analyse its textual structure. Once
analysed health documents, we have defined a
simple, flexible and highly-semantic
metamodel (which takes the form of a MOF-
compliant metamodel) to model any aspect of
CG. MOF (Meta-Object Facility) is a set of
standard interfaces that can be used to define
and manipulate a group of interoperable meta-
models and the corresponding models.
We offer a flexible language to model CG with
two main goals: (i) facilitate the application of
MDE-based mechanisms and extensible of our
metamodel in future; and (ii) reduce users'
cognitive overload when they are utilizing our
modeling language. From a MDE perspective,
this simplicity helps us to successfully apply
our solution to health service and open new
research lines related to testing and simulating
health processes. This simplicity is not seen as
a drawback since our proposal has extension
mechanisms.
Due to the complexity of CG modelling
metamodel, below we are going to briefly
describe show the most important metaclasses.
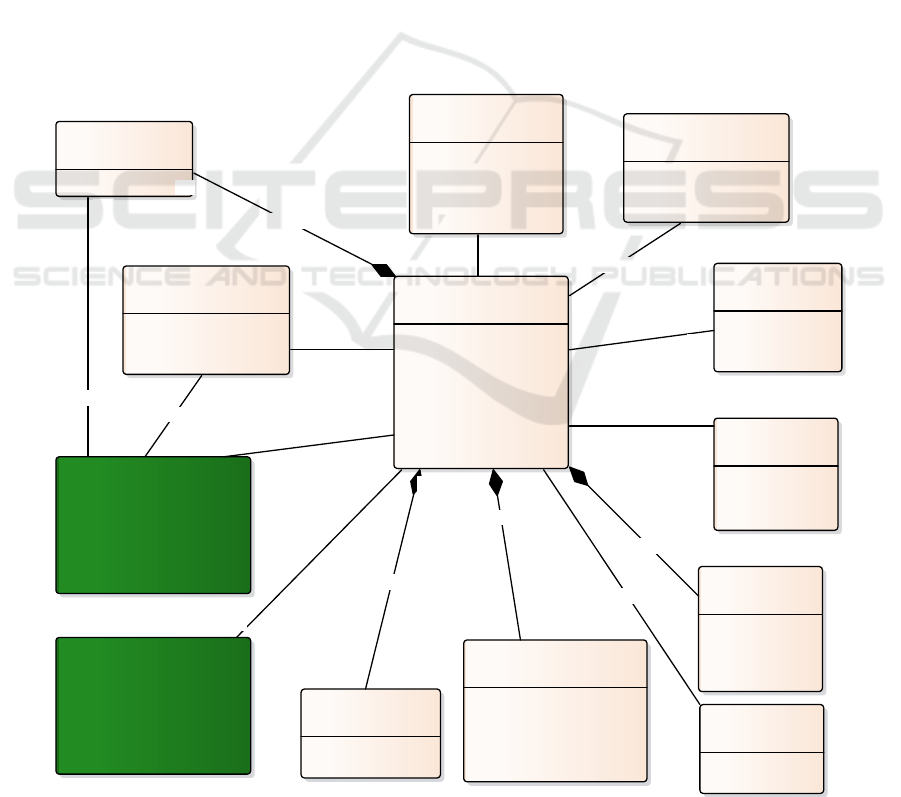
However, Figure 1 shows the general
metamodel of CG. This metamodel is related to
2 secondary metamodels: «ClinicalProcess»
and «ClinicalRule». These metamodel are not
explained in detail but can be consulted in
(García-García et al. 2015; García-García et al.
2018).
Before going further, it is worth clarifying that
the syntax used is not enough to semantically
define our metamodel. In fact, we use OCL
(Object Constraint Language) (28) to add
formal semantic constraints that validate
process models.
The metaclass «ClinicalPracticeGuideline» is
the epicenter of the metamodel and is the
element around which the rest of the elements
of the metamodel are orbiting. With this
metaclase it is possible to represent any CG.
To describe the necessary actions since a
person, with a certain pathology, requests
assistance until it ends, each CG is composed
of a clinical process «ClinicalProcess». To
model these actions, we have activities (human,
automatic and complex) and gateways. In
human activities, the health professional
registers patient data. For it, we associate the
metaclass «Variable».
In addition, given that one of the objectives of
this project is to monitorize the CG, with the
metaclass «Indicator» we can associate
indicators to activities and processes. These
indicators, when the GC is running, will record
the values and will be displayed in the
monitoring module.
To help in making clinical decisions in
activities or to choice with path of actions the
patient should follow, we associated the
metaclass «ClinicalRule» to activity and
gateway. These rules are formed by clauses,
constituted in turn by the variable to be
evaluated, a mathematical operator and the
value with which to compare. Logical
operators are used to relate one clause to
another.
2. Execution and Orchestration Phase.
Nowadays, this phase could be considered as
critical and essential task because health
organizations are being driven by the need to
extensively and continually automate, evolve
and maintenance their CG. For this purpose,
MODELSWARD 2019 - 7th International Conference on Model-Driven Engineering and Software Development
412
CG have to include execution information (i.e.,
such as execution parameters for the communi-
cation and integration with external systems,
for instance). This information are essentials to
deploy and execute CG models into execution
engines, such as process engines or BPMS
(Business Process Management Suite (Meidan
A, et al.2017)), rule decision engines, etc.
IDE4ICDS provides MDE mechanism to solve
this situation. This mechanism allows systema-
tically generating the executable version of CG
from its model (previous phase). It is based on
model-to-text (M2T) transformation rules using
MOFM2T (OMG.MOF.2017). This transfor-
mation protocol will not be explained here,
since they are out of the scope of this paper and
it would become too extensive, but it is
possible to find further information of
application in other context into (García-García
JA, et al.2017). Anyway, we have been able to
generate executable code (based on BPMN-
XML and Java code) from the definition model
of the GC. On the one hand, BPMN-XML code
is generated because most BPMSs support this
standard format (Meidan A, et al.2017) and it
is used but the process engine selected in our
project (see Section 3). On the other hand, we
generate Java code to execute each clinical
decision rule (which is modelled in previous
phase) in the decision rule engine selected in
our project (see Section 3).
Although these kinds of code are related to our
design and technological solution, it is
important to mention that our MDE-based
framework is independent of the platform.
3. Monitoring Phase. Once CG and its
healthcare processes are modelled and
executed, it is time to evaluate its effectiveness.
This evaluation provides a granular view of the
overall productivity of each CG and it is based
on the definition of key performance
indicators.
Figure 1: Metamodel of Clinical Guideline.
«metaclass»
ClinicalPracticeGuideline
background: String
introduction: String
lastupdate: Date
scopeAndGoal: String
subtitle: String
title: String
version: Float
«metaclass»
Auth orshi p
category: CategoryKind
description: String
name: String
speciality: String
work ing Group: S tring
«metaclass»
Formu latedQuestion
description: String
«metaclass»
ScientistEvidence
assessmentCriteria: String
description: String
organization: String
«metaclass»
ClinicalR ule
«metaclass»
Disease
classification: String
definition: String
diagnosticCriteria: String
«metaclass»
Str ategy
description: String
name: String
type: StrategyKind
«metaclass»
Indicator
description: String
formula: String
name: String
«metaclass»
Reference
description: String
id: String
«metaclass»
Appendix
description: String
id: String
subtitle: String
title: String
«metaclass»
FutureWork
description: String
«metaclass»
Variable
value: float
variableDescription: String
variableName: String
variableTy pe: VariableKind
variableUnit: VariableUnit
«metaclass»
ClinicalPr ocess
1
1
1..*
isBasedOn
1..*
1
isAssociatedTo
1
1..*isProducedBy
1..*
1..*
isBasedOn
0..*
1
includes
0..*
0..*
isComposedBy
1
*
isComposedBy
1..*
1..*
isAssociatedTo
0..*
1
isAssociatedTo
0..*
1
follows
0..*
1
formulates
1..*
0..*
isComposedBy
1
0..*
isComposedBy
1
A Practical Medical Experience of Successfully Mixing Model-Driven Paradigm and Business Process Management Principles
413
In this case, we have included two mechanisms
in order to support this phase in the IDE
4
ICDS
platform. Firstly, our CG modeling metamodel
includes concepts (such as metric and
indicator) that help the healthcare managers to
measure each CG. Indicators are defined and
configured during the modeling phase.
Secondly, and once modelled CG and
indicators, these models are systematically
transformed to in executable code using M2T
transformation rules. These executable code is
composed of: (i) SQL (Structured Query
Language) scripts, which update the
measurement database of IDE
4
ICDS; and (ii)
code scripts, which connect each model of the
CG (i.e., definition model, execution and
orchestration model, indicator model, etc.)
between itself, and calculate each defined
indicator.
4. Continuous Improvement Phase. This phase
aims to achieve higher quality, efficiency,
effectiveness and performance levels during
CG execution what imply to improve the
patient assistant. This improvement of CG
could emerge from two situations. The first one
could success after evaluating CG performance
(through assessment indicators and metrics),
i.e., a healthcare organization could start an
internal improvement process to improve its
services to patients and increase or optimize its
resources. The second situation could append
when healthcare professionals identify any
improvement in the definition model of the
CG. Anyway, after appending any these
situations, organization could iterate over our
CG lifecycle as many times as necessary in
order to achieve its goals.
3 THEORETICAL
FOUNDATIONS OF IDE4ICDS
BASED ON AN IMPROVEMENT
CONTINUOUS LIFECYCLE
Previous section has briefly presented our theoretical
framework to make easier the CG management.
However, it is required to offer a tool-based
mechanism to support this framework in order to
reduce costs and improve its applicability in real
healthcare environments during the definition,
design, implementation and validation of clinical
guidelines. The IDE4ICDS platform has been
designed and developed to achieve the previous goal
under a service-oriented architecture with a user-
centered design. For this purpose, our platform
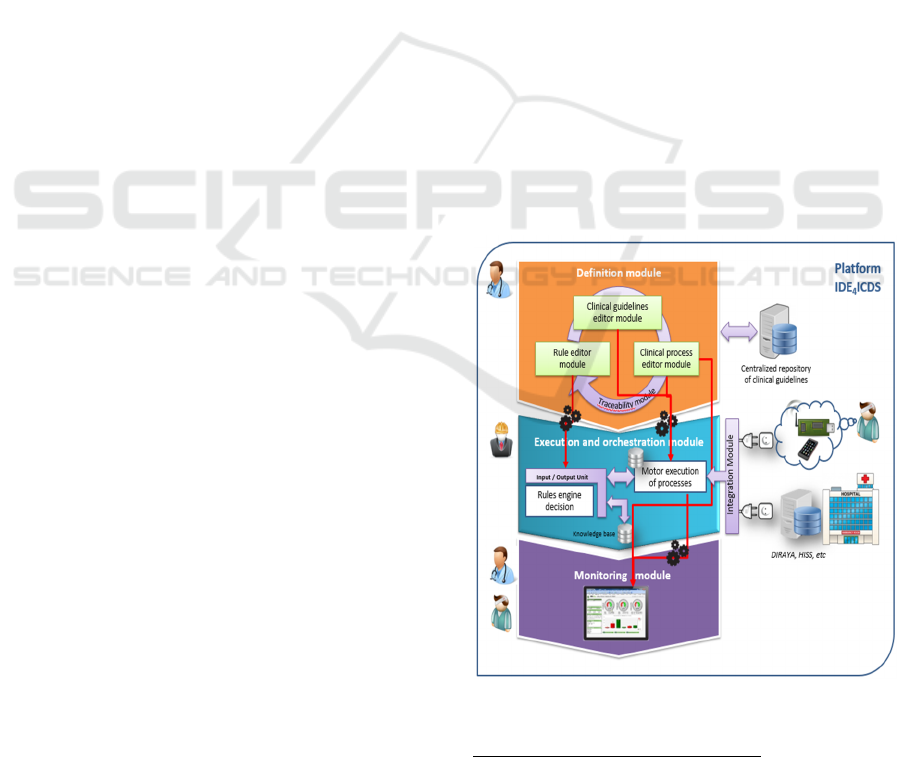
provides five functional modules (Figure. 2) as
follows.
The first one is the Definition & Traceability
Module (M1), which provides three graphic editors
to model each aspect of a GC (i.e., general
information, healthcare processes, clinical
information records and clinical decision rules).
These editors has been implemented as plugins on
Enterprise Architect (EA)
2
. These plugins have
different functionalities, such as, UML Profiles to
friendly instance our GC metamodel (Figure. 1),
plugin to guarantee well-defined models verifying
each OCL constraint of metamodel, plugin to
automatically execute each transformation rule, etc.
In addition, it is important to emphasize that these
models are stored in a central repository of models
(supported on a MySQL database).
The second module is the Execution Module
(M2), where the executable version of the CG is
systematically deployed and, later, executed by
healthcare professionals. Once modelled a GC, the
deployment method of this one has to be carried out
in two steps. Firstly, process engineer applies
transformation rules from M1 to automatically
obtain BPMN-XML and Java code which are used
Figure 2: Conceptual model of the IDE4ICDS platform.
2
SparxSystems. Enterprise Architect. Website. Last
access 2018.
MODELSWARD 2019 - 7th International Conference on Model-Driven Engineering and Software Development
414

by BonitaOS
3
(process engine) and Drools
4
(decision
rule engine), respectively. These tools were chosen
according to the requirements of the project and
taking into account the characterization scheme
proposed in (Meidan A, et al.2017), which was very
useful. However, it is important to mention that our
MDE-based framework is independent of the
platform. Therefore, other engines could be chosen
within great efforts. Secondly, process engineer has
to configure and compile these codes manually. This
configuration includes to set execution parameters
with HIS and CDP. The configuration of this
information cannot be automated because it depends
on the specific system and chosen device.
Moreover, the fourth module is the Monitoring
Module (M3). This module has been specially
developed for the project using web technology
(cakePHP, HTML5, CSS3, jQuery, etc.). Once
modeled a GC using the M1 module, the process
engineer can automatically execute the set of M2T
transformations to generate cakePHP code from
definition models of the GC. This cakePHP code
allows calculating each indicator associated with the
healthcare process (which is defined in the clinical
guideline). For this purpose, the M3 module has to
be communicated to the M2 module in order to
calculate indicators during the execution of the GC
(such as number of patients attended, percentage of
emergencies, average time of patient care, maximum
time of patient care, etc.). Once calculated each
indicator, this module provides scorecards, timelines
with the evolution of each indicator, and
alarms/notifications, among other functions.
As mentioned above, M3 and M2 have to be
communicated. For this purpose, we have designed
the Integration Module (M4), which is developed
as an enterprise services bus based on OpenESB
5
.
M4 allows internally connecting each module with
another one, as well as externally communicating
the M2 module with HIS and CDP.
Finally, the last module is the Traceability
Module (M5). An important aspect when MDE is
used is to ensure traceability among generated
models. This is essential in the context of IDE
4
ICDS
because it allows identifying each GC in a unique
way in the platform and its modules. In addition,
traceability allows enhancement points such as
version management of a GC.
3
BonitaSoft. Website. Last access 2018.
4
Drools. Website. Last access 2018.
5
OpenESB. Website. Last access 2018.
4 CONCLUSIONS AND FUTURE
WORKS
Today’s world economic situation is ruled by issues
such as reducing cost, improving quality,
maximizing profit and improving and optimizing
processes at any kind of organization. In this
context, BPM have been confirmed as an essential
and successful strategy. However, over last years,
research community have started to combine the
process management and MDE within controlled
contexts as software testing processes, requirement
processes, etc.
However, the application of MDE in health
contexts is a work and research line that has aroused
the interest of research teams and organizations
interested in transferring research results to real
environments. In addition, the application of MDE
in health environments is also a new research line,
little treated over last years and innovative in terms
of potential results that allows its application. In this
context, this paper has presented the IDE
4
ICDS
project which proposes a MDE-based solution to
solve two main goals: (i) improving the application
and management of CG in real healthcare
environments; and (ii) reducing variability in clinical
practice during the application of a specific CG, as
well as reducing healthcare costs. At present, this
project will be tested and validated in a real scenario
of patients with Diabetes Mellitus, but we plan to
extend and apply our solution to other medical
pathologies as future works.
ACKNOWLEDGEMENTS
This research has been partially supported by
POLOLAS project (TIN2016-76956-C3-2-R) and
IDE
4
ICDS (RTC-2016-5824-1) of the Spanish
Ministry of Economy and Competitiveness, and by
the VI PPIT-US of the University of Seville (Spain).
REFERENCES
Domínguez-Mayo, F. J., Escalona, M. J., & Mejías, M.
(2010, July). QuEF (quality evaluation framework) for
model-driven web methodologies. In International
Conference on Web Engineering (pp. 571-575).
Springer, Berlin, Heidelberg.
Domínguez-Mayo, F. J., Escalona, M. J., Mejías, M.,
Ross, M., & Staples, G. (2014). Towards a
homogeneous characterization of the model-driven
A Practical Medical Experience of Successfully Mixing Model-Driven Paradigm and Business Process Management Principles
415

web development methodologies. Journal of web
engineering, 13(1-2), 129-159.
Escalona, M. J., Gutierrez, J. J., Villadiego, D., León, A.,
& Torres, J. (2007). Practical experiences in web
engineering. In Advances in Information Systems
Development (pp. 421-433). Springer, Boston, MA.
Garcia-Garcia, J. A., Meidan, A., Carreño, A. V., &
Risoto, M. M. (2017, October). A Model-Driven
Proposal to Execute and Orchestrate Processes:
PLM4BS. In International Conference on Software
Process Improvement and Capability Determination
(pp. 211-225). Springer, Cham.
García García, J. A., Escalona, M. J., Martínez-García, A.,
Parra, C., & Wojdyński, T. (2015). Clinical Process
Management: A model-driven & tool-based proposal.
Information Systems Development: Transforming
Healthcare through Information Systems. ISBN: 978-
962-442-393-8. 2015.
Garcia-Garcia, J. A., Enríquez, J. G., Garcia-Borgoñon, L.,
Arevalo, C., & Morillo, E. (2017, July). A MDE-based
framework to improve the process management: the
EMPOWER project. In 2017 IEEE 15th International
Conference on Industrial Informatics (INDIN) (pp.
553-558). IEEE.
García-García J. A., García-Borgoñón L., Escalona MJ,
Mejías M. A model-based solution for processes
modeling in practice environments: PLM4BS. Journal
of Software-Evolution and Process. pp.1-23.
https://doi.org/10.1002/smr.1982 2018.
García-García, J. A., Escalona, M. J., Domínguez-Mayo,
F. J., & Salido, A. (2014). NDT-Suite: A
methodological tool solution in the Model-Driven
Engineering Paradigm. Journal of Software
Engineering and Applications, 7(04), 206.
Gutiérrez, J. J., Escalona, M. J., & Mejías, M. A Model-
Driven approach for functional test case generation.
Journal of Systems and Software, 109, 214-228. 2015.
Hill J. B., et al. Cool Vendors in Business Process
Management, Gartner Research, 2007.
ISO/IEC.ISO 9001:2008. Quality management systems,
Requirements. International Organization for
Standardization, 2008.
ISO/IEC.ISO/IEC TR24744:2007 Software and systems
engineering lifecycle management guidelines for
process description. International Organization for
Standardization, 2007.
Martínez-Ruiz T., et al. Towards a SPEM 2.0 extension
define process lines variability mechanisms. Software
engineering research, management & applications,
115–130, 2008.
Meidan, A., García-García, J. A., Escalona, M. J., &
Ramos, I. (2017). A survey on business processes
management suites. Computer Standards & Interfaces,
51, 71-86.
OMG. BPMN, Business Process Modelling Notation,
Version 2.0. 2011.
OMG.MOF Model to Text Transformation Language
(MOFM2T). Website. 2017.
PMI. A Guide to Project Management Body of
Knowledge. ISBN: 978-1933890517. 2008.
Ponce, J., Borgoñon, L. G., García, J. G., Escalona, M. J.,
Domínguez-Mayo, F. J., Alba, M., & Aragon, G.
(2013). A model-driven approach for business process
management. Covenant Journal of Informatics and
Communication Technology, 2(1).
Schmidt D.C. Model-Driven Engineering. IEEE
Computer, 39, 2, 25-31, 2006.
Tonelli M., et al. The role of experience in an evidence-
based practice. The Medical Roundtable General
Medicine Edition, 2018.
Trkman, P. The critical success factors of business process
management. International Journal of Information
Management, 30(2),125-134. 2010.
Van-der-Aalst WMP. Business process management: a
personal view. Business Process Management
Journal, vol.10 (2), 2004.
Van-der-Aalst WMP. Making Work Flow: On the
Application of Petri Nets to Business Process
Management. LNCS, vol. 2360, Application and
Theory Petri Nets, pp 1-22. 2002.
MODELSWARD 2019 - 7th International Conference on Model-Driven Engineering and Software Development
416
