
Ensuring Secure Health Data Exchange across Europe.
SHIELD Project
Borja López-Moreno
1
, David Martín-Barrios
2
, Ivan Revuelta-Antizar
1
, Santiago Rodríguez-Tejedor
3
,
M. Luz del Valle
1
and Eunate Arana-Arri
1
1
Biocruces Bizkaia Health Research Institute, Plaza Cruces 12, Barakaldo, Spain
2
Ibermatica, Derio, Parque Tecnológico de Bizkaia Ed. 501A, Spain
3
Cruces University Hospital, Osakidetza, Plaza Cruces 12, Barakaldo, Spain
Keywords: Secure Health Information Exchange, General Data Protection Regulation, e-Consent, Electronic Medical
Records, Patient Summaries, eHealth Interoperability.
Abstract: Nowadays, many people move from one country to another for various reasons: tourism, work, studies, etc.;
even with chronic or multi-pathological diseases. The main objective of SHIELD project is to create open
and extendable security architecture with supported privacy mechanisms and trust of citizens, to provide
systematic protection for the storage and exchange of health data across European borders. epSOS is a
European project funded and finished dealing with security and interoperability of eHealth data is, that
result in an OpenNCP (National Contact Point) architecture. In SHIELD project for the initial validation
framework two OpenNCP virtual nodes would simulate the real nodes between Italy and Spain. Validation
scenarios (realistic use cases) have been developed in three different member states (Italy, United Kingdom
and Spain). The first scenario is an Italian citizen traveling to Spain that has an acute emergency episode
(e.g. stroke) and loses consciousness. Spanish emergency department suddenly assists that patient and
doctor wishes to check patient´s health record. Results of the first round of validation frameworks of
SHiELD project have been made successfully and presented to the European Commission. Security
challenges need to be addressed when assessing eHealth solutions. Among others, the challenges are:
interoperability, confidentiality, availability, integrity, privacy, ethics, regulations and eHealth data. Which
data are going to be shared and by which mean? The first validations will be useful as the basis for both the
“in depth” requirements analysis as well as setting the main pillars for the SHIELD architecture detailed
design.
1 INTRODUCTION
The development of new eHealth tools and the
implementation of new policies in the European
Union (EU) can help to guarantee more efficient and
sustainable health services, and with this increase
the safety in the management of patients. In
addition, all this guarantees a better communication
between different professionals, end-users and other
decision makers. In the first e-Health Action Plan of
the European Commission (EC) (2004) these
benefits were fully recognized. Since then, the
Commission has made an important effort to
promote and develop specific political actions in this
context (European Commission, 2011; European
Commission, 2012).
Security is one of the main challenges when applied
to eHealth and is crucial in the transmission of
required data about patients and citizens when
traveling around the world.. Thus, there is a growing
need of rapid and secure access to clinical data
between different healthcare systems, at the national
and international levels.
The potential value of health data is huge, both in
traditional health sectors (e.g. for medical research
such as drug design) and in new sectors, such as
personalised health and lifestyle management
services based on wearable devices. Recent
estimates indicate that person’s health data is 50
times more valuable than their financial data (Minor,
2017). Unfortunately, health data is not only valued
highly by potential legitimate users. Cyber criminals
also regard health data as between 20 and 50 times
422
López-Moreno, B., Martín-Barrios, D., Revuelta-Antizar, I., Rodríguez-Tejedor, S., Valle, M. and Arana-Arri, E.
Ensuring Secure Health Data Exchange across Europe. SHIELD Project.
DOI: 10.5220/0007524004220430
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 422-430
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

more valuable than financial data, mainly because it
allows them to create very convincing false
identities based on individual personal histories
(Luna, 2016). Stealing credit card details provides
only a limited window of opportunity for criminals
before the card is cancelled by its rightful owner.
However, health records cannot be cancelled, and
provide criminals with opportunities for identity
theft over a long period. There are also dangers from
the use of health data by legitimate businesses.
That is why another of the great challenges is to
comply with the General Data Protection Regulation
(EU) 2016/679 (EU, 2016) also known as GDPR on
processing of personal data and on the free
movement of such data (Pocs, 2012).
The main objective of the SHIELD project is to
create an open and extendable security architecture
supported on security and privacy mechanisms to
provide systematic protection for the storage an
exchange of health data across European borders,
while improving patients trust in the security of their
data.
2 METHODS
The exchange of health data is already possible, but
rarely happens in practice because it is hard to
ensure that the combined ‘end-to-end’ system will
be secure and comply with data protection laws.
SHiELD will address these security and compliance
challenges:
providing models and analysis tools for
automated identification of end-to-end security
risks and compliance issues and supporting
privacy ‘by design’;
defining an open and extensible data exchange
architecture based on epSOS (epSOS, 2012),
able to support security measures to address
these risks;
developing security mechanisms to deal with
new and emerging risks, such as inference
attacks on sensitive data, and risks from
relatively unprotected mobile edge devices;
providing faster and more cost-effective
methods to verify and monitor compliance with
multiple sets of applicable regulations.
SHiELD aims address security and regulatory
compliance challenges in two distinct situations:
where a business needs access to health data to
develop or operate a high value health or
lifestyle related product or service, including
wearable devices and associated services;
when a citizen´s health care is needed in one
Member State, and care givers need access to
their health (or lifestyle) data which may be
stored in a different Member State.
The validation case studies are designed to cover
both these situations, both separately and in
combination.
SHiELD case studies will address cross border
scenarios in which a citizen needs health care while
in one Member State, and care givers need access to
their health data from different Member State.
SHiELD will also consider how commercial
providers of lifestyle services or wearable sensors
may be involved in such data exchanges. SHiELD
will thereby also create opportunities for using
health data to create such products and services
addressing the common European market. SHiELD
will provide guidance in best practice to achieve
end-to-end security and data protection compliance
in health and health related applications.
2.1 Pilot Case Studies
Validation scenarios (realistic use cases) have been
developed in three different member states (Italy,
United Kingdom and Spain). In all scenarios, we
assume that a citizen travels abroad and needs health
care. The foreign health care professional needs to
access and/or manage patient’s health record.
Results of the first round of validation frameworks
of the SHiELD project have been made successfully
and presented to the European Commission.
Three use cases have been prepared with
different characteristics. Different levels of need for
attention have been developed: a case in which the
patient can’t consent because he or she is
unconscious, but it is a vital emergency; another
case in which the patient consents to what
information he wants to share and the third case,
requires exchange between more than two countries
and also adds data from devices provided by the
patient.
2.1.1 Use Case 1: “Break Glass”
Circumstance
An Italian citizen travelling in Spain incurs a stroke
and is taken to the nearest Spanish hospital. While
receiving first aid from the Emergency medical
services (EMS), the coordination center informs the
EMS in which hospital the patient should be taken
to. At the same time a message is sent to a
workstation located in the emergency department of
the hospital responsible for alerting the first-aid unit.
Ensuring Secure Health Data Exchange across Europe. SHIELD Project
423

As soon as the message is received a medical
team is created for the stroke assistance.
For this purpose, different physicians are
summoned: emergency physicians, neurologist.
neuroradiologist and anaesthesiologist.
In order to ensure the best assistance, the medical
staff wishes to check the patient’s Electronic Health
Record (EHR) to know their medical history (e.g.
their epSOS patient summary). Since the patient is
foreign, this is possible thanks to the SHiELD
platform, which ensures the communication between
NCPs of different countries within Europe in a
secured manner.
This is fundamental, not only to discover
possible illnesses or chronic conditions, but also to
ensure that the patient does not suffer from allergies
to drugs; also if the patient receives treatment for a
chronic condition, that should be relevant in order to
be able to perform a therapeutic management as
efficiently as possible.
Indeed, the first aid protocol for a stroke may
vary in case of other pathologies or allergies. For
example, in case of renal failure the cranium
computed tomography scan (the traditional
examination in case of stroke) can be replaced with
an magnetic resonance imaging in order to avoid
contrast agent, which can aggravate kidney
conditions. The fibrinolytic treatment has shown an
important reduction in mortality and morbidity in
patients with stroke, but all treatments may have
contraindications when applied, and it is so
important to know about them in order to not
generate iatrogenic damage in the patient. Examples
for such contraindications are oral anticoagulant
treatment, recent history of severe bleeding, severe
liver disease, hemorrhagic retinopathy, etc.
It could be possible that the patients receive
endovascular treatment. This case needs general
anesthesia in an operating room, and having access
to patient´s EHR for the anesthesiologist could be
vital.
This is just to demonstrate the importance of the
patient clinical history; the epSOS clinical record
summary with the mandatory basic dataset will be
enough to perform an appropriate management at the
time of the incident. It could be possible to extend
this information to other examinations (e.g. blood
tests, bio images etc.) made in the 60 days preceding
the “break glass” circumstance, that are usually
sufficient to give a general overview of the clinical
condition. This means that the chance of patient
survival increases if the physician has access to the
patient's clinical record as quickly as possible.
Consequently, a better patient response is expected,
the faster the therapy is provided. In the
management of stroke in the emergency services
there is a saying that “time is brain”.
Figure 1: Use Case 1 graph.
2.1.2 Use Case 2: Surgical Intervention
A Spanish patient has had a surgical intervention
(e.g. urological surgery) and he is planning to travel
across the EU within two months of the surgery.
The patient wants to have details of the surgical
intervention at his disposal in case it is needed for
medical assistance during the travel abroad. At this
scope the patient, together with the Italian urological
surgeon, decides - using the mobile interface of the
“SHiELD” platform - which information would be
useful to share with a foreign doctor during the trip.
They decide to share part of the hospital discharge
letter, including detailed information about the
patient’s clinical history and the recent surgery. The
SHiELD solution will also give the possibility to
hide sensitive information capturing patient consent.
Moreover, the patient, using the “SHiELD
platform”, can make the decision, relevant for
privacy issues, of when and where to share this
information. This is meant to limit the availability of
the shared information in time and location (e.g. “in
Milan for the next 2 weeks”). Access preferences
will be integrated into the access model to ensure the
balanced concerns of patient privacy and treatment
need.
In case of post-surgical complications during the
trip, after providing first aid, the emergency
physicians must have access to the EHR, including
the most recent clinical and surgical steps.
Initially, the doctor has access to the epSOS
Patient Summary with basic information, in order to
discover the type of surgical procedure performed;
then, they want to access detailed information about
the surgical procedure itself, all the complementary
tests carried out in the process, and therefore decide
to visualise the extract of the discharge letter shared
by the patient.
The patient and his doctor agree on the contents
to be shared on the platform, in order to be available
to a third party, (e.g. a foreign medical professional).
HEALTHINF 2019 - 12th International Conference on Health Informatics
424
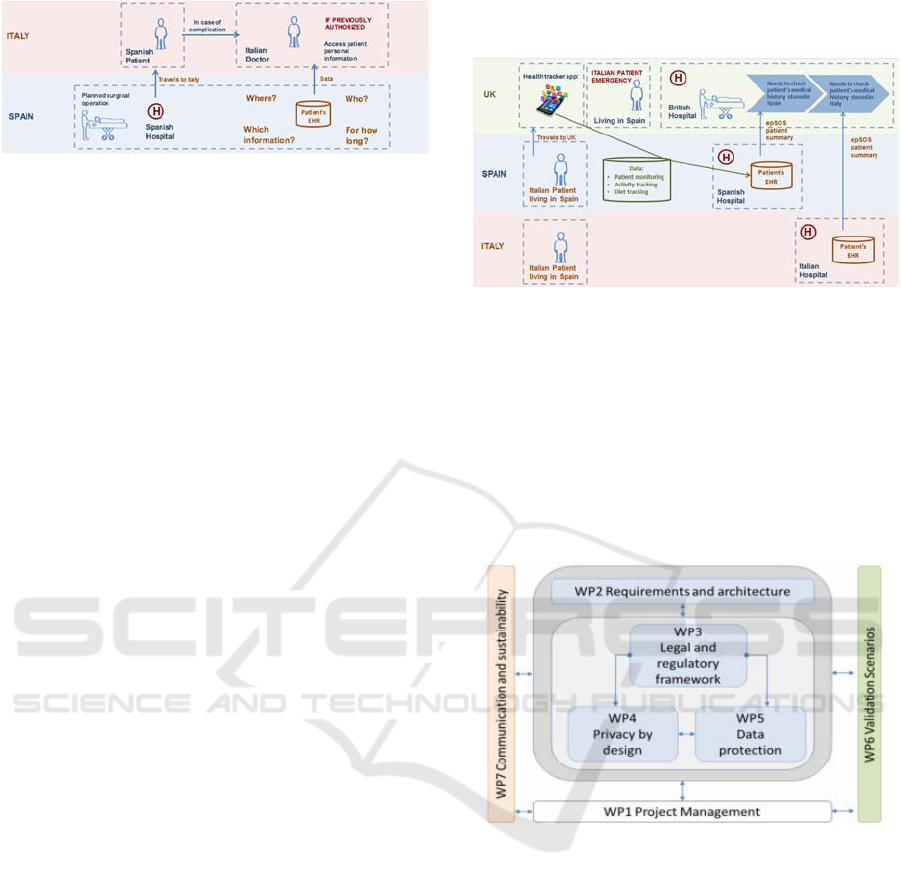
Figure 2: Use Case 2 graph.
2.1.3 Use Case 3: Chronic Conditions +
Remote Monitoring
A 40 year old Italian woman with type 1 diabetes
mellitus under treatment with insulin stays in the UK
for work reasons for 3 month. She’s been living in
the Basque country for 10 years. The woman, prior
to her stay in the UK, gives consent to access her
medical history. In the Basque Health System the
EHR has the Health Folder. By the Health Folder the
patient can send and receive information from and to
her General Practitioner (GP). The patient will use
this resource to monitor her pre-prandial glycaemia,
as prescribed by her GP. During her stay in the UK,
she has agreed with her GP that, since this is another
country, she will record her eating habits as well as
her physical activity. She will send this information
by the Health Folder.
After a week in the UK, she begins to notice
dizziness accompanied by general discomfort and
sometimes nausea. As it does not happen every day
and the glycaemia is within normal range, she
decides to take care of her diet and continue with her
usual treatment schedule. She blames these episodes
on the stress caused by her new job. After several
days without any improvement of her symptoms,
being at work she presents a transient loss of
consciousness (syncope) with a fall to the ground
and a slight traumatic brain injury, with total
recovery of consciousness. Her colleagues decide to
take her to the nearest hospital emergency
department.
During the patient's anamnesis, she refers to a
brain surgery she had as a child in Italy, but she does
not know any details. This old episode could be of
great importance for the management of the
incident.
As in the other cases, in order to ensure the best
assistance, the medical staff wish to check the
patient’s EHR to access her medical history (e.g. her
epSOS patient summary), but in this scenario, the
accessibility to more than the patient summary could
be helpful for the medical staff. Since the patient is
foreign, this is possible thanks to the SHiELD
platform, which ensures the communications
between NCPs of different countries within Europe.
Figure 3: Use Case 3 graph.
2.2 Definition of Work Packages (WP)
In order to respond the multidisciplinary and
interrelated proposed approach, SHiELD proposes a
work plan covering all particular project aspects
(legal, security, privacy), considering the
relationship among all these aspects. The work is
divided into 7 work packages. Figure 4 shows the
complete picture of work packages.
Figure 4: Work packages relation.
WP1 consists in Project management and WP7 deals
with Communication and sustainability, WP3 Legal
and regulatory framework, so this document focus
on the work packages WP2, WP4, WP5 and WP6.
2.2.1 WP2 – Requirements and Architecture
The main objectives for this work package are to
design the overall architecture of SHiELD and to
design and continuously integrate the SHiELD tool
following an iterative, continuous integration and
continuous deployment in order to smoothly
integrate the different tools to be developed within
SHiELD.
The main result of this WP will be the SHiELD
architecture that will be validated in the case studies
Ensuring Secure Health Data Exchange across Europe. SHIELD Project
425

defined in this project, as well as the open
architecture and secure interoperability API.
2.2.2 WP4 – Privacy by Design
The objectives of this work package are to develop
models capturing potential threats to health data, to
develop models capturing health data protection
regulatory compliance requirements in at least three
European jurisdictions, to devise architectural design
patterns that are secure with respect to threats and
address regulatory compliance requirements and to
develop software tools that can use these models and
design patterns to automatically analyses the end-to
end security of health data and compliance
requirements for specific systems.
2.2.3 WP5 – Data Protection
The objectives of this work package are to develop
data protection mechanisms and tools, to develop
privacy protection mechanisms and tools, to
incorporate developed mechanisms and tools within
the SHiELD architecture and to address regulatory
compliance requirements.
2.2.4 WP6 – Validation Scenarios
This Work Package targets the definition of a solid
methodology for the scientific, technical and legal
validation of the tools and prototypes developed in
the project. The challenges are to define realistic use
cases identifying real-life-strength scenarios, to
define suitable metrics and protocols supporting a
solid validation framework, to identify relevant use
cases for the scenarios of the project, to implement
the use cases and to evaluate the integration and
interoperability level of the architecture with other
tools.
3 RESULTS
One of the European projects funded and already
finished dealing with the security and
interoperability of eHealth data is epSOS project that
result in an OpenNCP (National Contact Point)
architecture and implementation. The OpenNCP
community has designed and developed a set of
Open Source Components based on the services
developed in epSOS. This can be used by
Participating Nations to build their local
implementation of an NCP. However, this has not
been validated and put into practice (epSOS, 2012).
In SHIELD project for the initial validation
framework experiments two OpenNCP virtual nodes
would simulate the real nodes between Italy and
Spain (Virtual Machines). For the secure exchange
of clinical health records different prototype tools
have been designed and are being developed: end-to-
end user interfaces for different health systems
profiles (administrative staff, nurses, physicians,
etc.), sensitivity tools, data hiding tools, consent
management tools, reports translation tools and
mobile devices tampering detection tools.
One of the main achievements to be fulfilled in
SHIELD is the end-to end systemic analysis of
potential risks to health data. This is being
performed by creating a knowledge base from
potential threats including ´classical´ cyber security
threats, emerging threats to personal data and
compliance threats. SHIELD will unlock the value
of health data to European citizens and other
stakeholders by overcoming security and regulatory
challenges that today prevent this data being
exchanged with those who need it, especially in
emergency situations.
3.1 Validation of WP2
3.1.2 Description of the OpenNCP
Architecture and Clinical Data
Interchange
In the initial validation experiments two OpenNCP
nodes would simulate the real nodes between Italy
and Spain. This deployment would fit the first Use
Case scenario.
The minimal infrastructure needed to simulate it,
are (Figure 5) (HL7, 2012):
Spanish OpenNCP Node:
o (Virtual Machine) Ubuntu Server 16.06
simulating Spanish OpenNCP Node.
o (Virtual Machine) Basque Health service
(that would implement the underlying
communication with the central patient
database on the Spanish side.
o (Virtual Machine) Ubuntu Server 16.06
simulating Italian OpenNCP Node (Italian
data underlying patient database are
simulated in this virtual machine).
Both OpenNCP nodes are Linux distributions
that contains some features to host the
OpenNCP core like: Mysql databases, Apache
Tomcat and JDK 1.8.
OpenNCP should be able to:
Communicate with their own services through
OpenNCP local node.
HEALTHINF 2019 - 12th International Conference on Health Informatics
426

Communicate with a remote node (from the
Spanish OpenNCP to the Italian OpenNCP).
Communcation backwards: communicate with
a source remote node (from the Italian
OpenNCP to the Spanish OpenNCP).
Clinical Document Architecture (CDA) has been
used to markup standard that specifies the structure
and semantics of "clinical documents" for the
purpose of exchange between healthcare providers
and patients (eHealth DSI Semantic Community.
2012, Boone, 2012). For the simulation of sending
clinical data between OpenNCP nodes, is needed the
creation of a fake patient with useful clinical data for
the Use Case, with its correct structure (XML HL7
CDA Level 3). The format of the Patient Summary
must be HL7, CDA Level 3: in the case of Osabide
Global (Basque Health service´s EHR) and unlike
other reports, CDA level 3 (HL7) will be required,
which indicates that both the header and the body
will be properly structured. That is, just as other
reports will be sent embedded in Portable Document
Format (PDF), in the case of Osabide Global, the
XML should be sent properly structured according
to the standard HL7 CDA level 3 coding.
As described before, the Basque Health service’s
web portal will provide the access OpenNCP
endpoints to lookup for patients on their origin
countries. It is the Doctor who will access to the
patient summary and who will obtain critical
information of the patient, like the past illness
history, the medication section or the allergies.
Moreover, in this implementation the doctor would
be also able to obtain other associated clinical
documents, like laboratory results, electro-
cardiogram or echocardiogram from the Spanish
patient. The Italian side would also provide
laboratory results as other associated documents.
Figure 5: OpenNCP nodes implemented in virtual
machines.
3.2 Validation of WP4
This work package is divided in two subsections:
- Security Modelling Tools: creates design-time
(“offline”) modelling tools to support the modelling
of health data being transferred as required by the
use cases described, later, in WP6. This report
describes the existing tool including some generic
improvements and initial versions of the extensions
to support modelling of regulatory compliance.
Here, it uses the “System Modeller” tool that
enables the user to create design-time models of IT
systems describing healthcare applications.
Additionally, to basic functionality such as signing
in and out, performing CRUD (Create, Read,
Update, Delete) operations on models and
import/export of models, it supports: validating a
model, i.e. generating a threat catalogue by matching
pre-defined patterns from the knowledge base in the
system asserting controls directly on assets or
applying control strategies to block threats accepting
threats, for example when they don’t have a control
strategy System Modeller relies on the security
knowledge base in order to perform any of these
tasks.
- Security Knowledge Base: captures potential
security and compliance threats in a knowledge
base. The initial threats are described by tool
owners, and explain how the tools can help to
manage the threats. The set of threats covered in this
deliverable also serves as an example to help use
case owners describe the threats they are typically
confronted with.
In its initial version, the security knowledge base
contains generic security threats, including but not
limited to remote exploits, such as denial of service
attacks, remote injections or snooping attacks
software bugs, causing a host to become unreliable
or unavailable unauthorised local access, where an
attacker gains physical access to hardware, enabling
them to steal data or alter processes or hardware
Furthermore, secondary threats are covered, i.e.
threats that appear when a precondition exists. These
secondary effects cause other assets to misbehave.
This means that they can be chained into “secondary
effect chains”, where a set of root causes can cause a
whole tree of secondary effects and misbehaviours
in related assets.
3.3 Validation of WP5
This work package is divided in three parts:
- Consent Management: aims to provide support for
initial evaluation of the architecture and
functionality. SHiELD will provide an integrated
system to manage and enforce patient consent
preferences. A decision engine and administration
point will allow authorization policies to be defined
Ensuring Secure Health Data Exchange across Europe. SHIELD Project
427
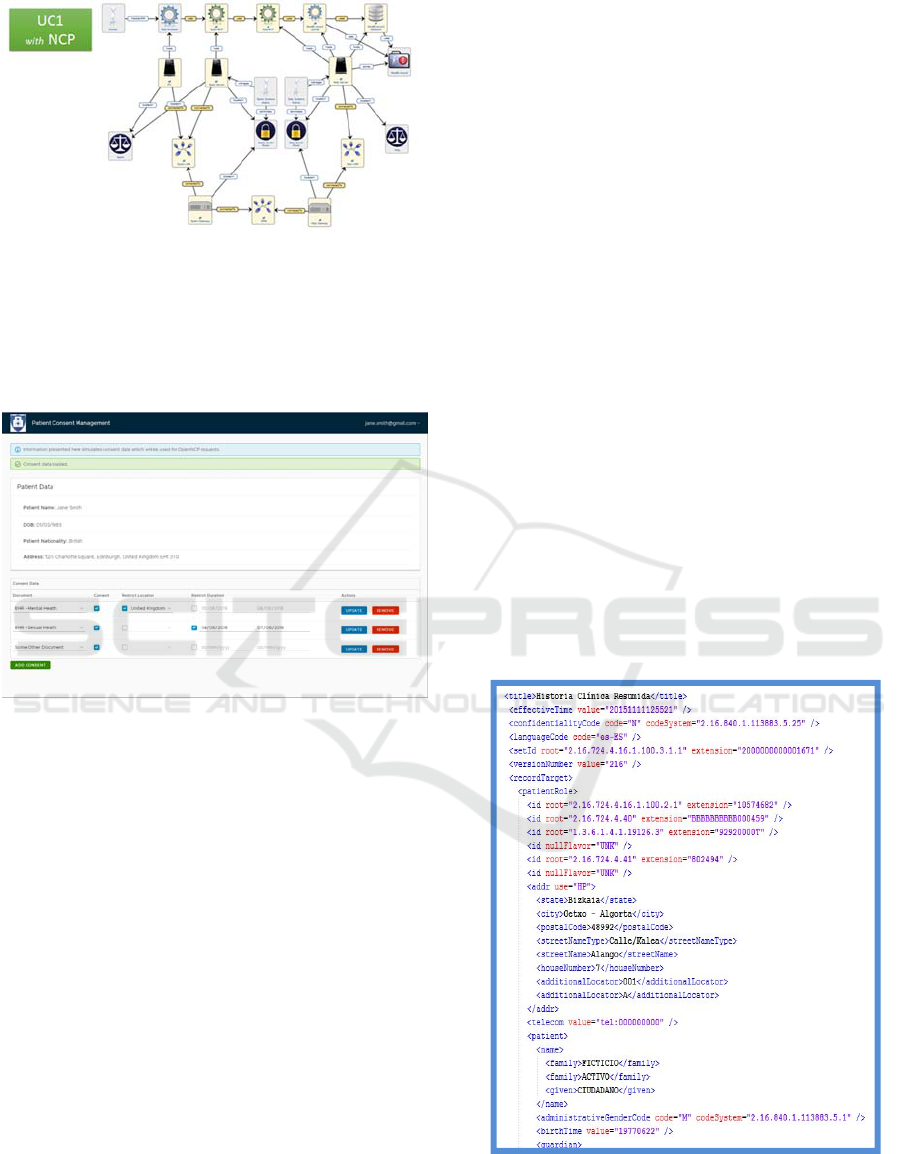
Figure 6: Use Case 1 represented in System Modeller tool.
and evaluated giving greater flexibility than
traditional authorization approaches. All this will be
done through consent UI and database will facilitate
the input and storage of patient consents at a fine-
grained level.
Figure 7: Consent Management User Interface.
- Sensitivity Tool and Data Hiding Tool: The process
of identifying sensitive data is a necessary step to be
able to address EU GDPR regulation which aims
primarily to give control to EU citizens and residents
over their personal data. The first step to address the
GDPR regulation is to find the sensitive/personal
data in the organization data stores. Once the
sensitive data has been identified, the organizations
can provide their customers/users the ability to
control (delete, modify etc.) their personal data.
The Data Sensitivity Analysis Tool addresses
this step. It finds the sensitive/personal data in
relational databases. For each column in the
database, the tool indicates if the column is sensitive
or not and provides a confidence score (a value
between 0.0 and 1.0). The confidences core indicates
how much the tool is confident that the specific
column is indeed sensitive. In addition, the tool
provides explanations why a specific column is
considered as sensitive. This is done by displaying
additional categories the column belongs too.
The tool itself is configurable. The tool contains
a library of data classifiers, each finds if a column
belongs to a specific category. In addition, it enables
adding additional categories by adding
corresponding data classifiers.
The users configure the sensitive classification
problem by selecting which categories are related to
the problem, and how they relate to the sensitivity
category. For example, a user may decide that a
column is sensitive if it is either email, or social-id.
In addition, the user declares a threshold. A column
belongs to a specific category only if its confidence
score is above the threshold. Then, data masking is
the process by which sensitive data is replaced,
possibly in a reversible manner, with data that is
unintelligible to receiver. The masked data is usually
sensitive data, such as personally identifiable
information, health information, names, addresses,
and so on.
The main purpose of data masking is to preserve
the data owner privacy enforce the data owners
consent and comply with legal regulation (such as
GDPR).So these figures that appear below (number
8 and 9), show how the fields of the Patient
Summary from the Spanish side are masked.
There is an output of the masking tool showing
how patient details -state, city, postal code, street
etc. - in the data (sample) can be masked (encrypted)
as well as unmasked (decrypted).
Figure 8: Spanish Patient Summary no masked.
HEALTHINF 2019 - 12th International Conference on Health Informatics
428
Figure 9: Spanish Patient Summary masked.
- Mobile Devices Security Prototype: In its current
state, the prototype uses hardware features to
demonstrate the ability to detect device tampering.
Moving forward, additional feature types will be
integrated to determine who is operating a device,
and in what context. The hardware features that will
be utilized are evolving, with the feature mappings
still being refined and improved upon. Further
methods of delivery are also continuously being
evaluated.
3.4 Validation of WP6
During the execution of WP6, a user interface has
been developed to simulate real-time access to
patient data, which is exchanged through the
OpenNCP nodes. The user interface has three
different roles for accessing to different level of
clinical data (Figure 10):
administrative staff; only has access to
administrative data;
nurse: only has access to patient summary;
doctor: has access to all clinical data that the
patient has consent to be exchange.
From a Spanish hospital, to access the
application, the administrative staff must enter their
own credentials to access the system. This obviously
will be required for each healthcare professional
involved in the system (i.e., administrative staff,
nurse, medical doctor).
Once the professional is logged into the
application, he has to fill the patient's personal data
in the system for searching it in the Italian Health
System.
Then,
the italian OpenNCP request info to the
Figure 10: Access system screen.
Spanish OpenNCP and returns a list of patients from
Italian Health System.
The professional has to click in the button
“Watch patient” for seeing the clinical data related
to the patient from the Spanish System. Once the
patient is chosen, his/her personal information
appears in the SHiELD Application.
Apart from seeing the personal data, the
administrative has to click in the button “Generate
episode” for registering the patient in the Italian
system and then, the nurse is who do the triage (the
triage process, after some basic tests like taking the
temperature, blood pressure check and so on, the
patient gravity is rated to locate them in the system
with the proper priority).
The nurse would be able to check also the
patient’s Medical Prescriptions and Personal
History.
After this, the doctor can see which patient is
pending for consultation, order by the gravity.
The doctor can see the all clinical records not
only on the screen but also in PDF document.
4 CONCLUSIONS
Security challenges need to be addressed by the
SHIELD project for the eHealth domain. Among
others, the challenges are: interoperability,
confidentiality, availability, integrity, privacy,
regulations and eHealth data. Which data are going
to be shared and by which mean? The first
validations will be useful as the basis for both the
“in depth” requirements analysis for the platform as
well as setting the main pillars for the SHIELD
architecture detailed design.
SHiELD will unlock the value of health data to
European citizens and businesses by overcoming
security and regulatory challenges that today prevent
Ensuring Secure Health Data Exchange across Europe. SHIELD Project
429

Figure 11: Doctors’ view.
this data being exchanged with those who need it.
This will make it possible to provide better health
care to mobile citizens across European borders, and
facilitate legitimate commercial uses of health data.
REFERENCES
Boone, KW. The CDA TM book. Springer-Verlag
London: 2012.
eHealth DSI Semantic Community. Clinical Documents:
CDA Implementation Guides. https://ec.europa.eu/
cefdigital/wiki/display/EHSEMANTIC/Clinical+Docu
ments%3A+CDA+Implementation+Guides (accessed
on October 2018).
epSOS D3.2.2 Final definition of functional service
requirements- Patient Summary and Glossary of
terms.https://openncp.atlassian.net/wiki/spaces/ncp/ov
erview?mode=global (accessed on October 2018).
European Commission. Directive 2011/24/EU of the
European Parliament and of the Council of 9 March
2011, on the application of patients’ rights in cross-
border healthcare (OJ l 88, 4.4.2011, p.45), 2011.
European Commission. eHealth action plan 2012–2020.
http://ec.europa.eu/health/ehealth/docs/com_2012_736
_en.pdf (accessed on October 2018).
European Commission. GDPR, Regulation (EU) 2016/679
of the European Parliament and of the Council of 27
April 2016 on the protection of natural persons with
regard to the processing of personal data and on the
free movement of such data, and repealing Directive
95/46/EC (General Data Protection Regulation),
Official Journal of the EU. 2016 L 119, page 1.
Health Level Seven International - HL7 Implementation
Guide for CDA® Release 2: IHE Health Story
Consolidation, DSTU Release 1.1 (US Realm), Draft
Standard for Trial Use, July 2012.
https://www.hl7.org/implement/standards/product_bri
ef. cfm?product_id=258 (accessed on October 2018).
Luna R, Rhine E, Myhra M, Sullivan R, Kruse CS. Cyber
threats to health information systems: A systematic
review. Technol Health Care. 2016; 24(1):1-9.
Minor LB. Report Harnessing the Power of Data in
Health. Stanford University School of Medicine 2017.
https://med.stanford.edu/content/dam/sm/sm-
news/documents/StanfordMedicineHealthTrendsWhite
Paper2017.pdf (accessed on October 2018).
Pocs M. Will the European Commission be able to
standardize legal technology design without a legal
method? Comput Law Secur Rev. 2012; 28: 641-650.
HEALTHINF 2019 - 12th International Conference on Health Informatics
430
