
A Clinical Decision Support System based on an Unobtrusive Mobile App
Ariella Richardson
1
, Avigail Perl
1
, Sapir Natan
1
and Gil Segev
2
1
Lev Academic Center, Jerusalem, Israel
2
BGSegev Ltd. (segevlabs.org), Jerusalem, Israel
Keywords:
Mobile Health, Digital Health, Digital Monitoring, Clinical Decision Support System (CDSS), Medical
Decision Support System (MDSS), Cardiovascular Disease, Silent Disease.
Abstract:
Clinical decision support systems typically rely on medical records and information collected in the doctor’s
office. We propose a clinical decision support system that uses data collected from patients continuously and
in an unobtrusive manner. The system uses data collected from a mobile app installed on the patient’s device
(such as a mobile phone, smart-watch etc). The app collects data without user interference and combines it
with conventional medical records. Our system uses machine learning methods to extract meaningful insights
from the data. The output from the learning process is then presented to the doctor in a clear and meaningful
fashion on a web based platform. This system can be used to assist effective treatment selection, enable early
diagnosis, trigger alarms in case of an emergency and provide a tool for disease monitoring. We describe our
clinical decision support system and directions for future work.
1 INTRODUCTION
Often patients visit their doctor when they feel unwell
searching for a diagnosis. Doctors then try to deci-
pher the cause of the patients feeling. However, they
do not always have all the data necessary for precise
diagnosis, and the data that they have is often unclear
and hard to integrate. As the amount of data avail-
able for patients is vast, the need for clinical decision
support systems - CDSS (also called medical decision
support systems - MDSS) is great (El-Sappagh and
El-Masri, 2014). CDSS have been developed for var-
ious settings and conditions. A detailed architecture
for integrating electronic medical records from multi-
ple sources is presented in (El-Sappagh and El-Masri,
2014) and (Kawamoto et al., 2005; Shibl et al., 2013)
survey others. Developing a CDSS that makes a con-
tribution to the doctors practice is complex. Many
of them do not improve clinical practice, as shown
by (Kawamoto et al., 2005) who present a survey of
several systems and discuss parameters that correlate
with improving clinical practice. Some systems are
simply not accepted by practitioners, factors are de-
scribed in (Shibl et al., 2013).
Alongside CDSSs, health related applications for
mobile phones and smartwatches are capable of im-
proving health monitoring and detection. The number
of health related apps available is astounding, approx-
imating 40,000 apps in 2013 (Boulos et al., 2014) and
165,000 in 2015 (Terry, 2015) and growing continu-
ously. While CDSS are typically based on electronic
medical records and targeted at assisting the practi-
tioners, mobile apps often aim at providing feedback
to the patients themselves. We propose a CDSS that
collects data on a patients’ mobile phone, and then
uses it as input to the CDSS presented to the doctor.
Our proposed system is a CDSS that integrates tra-
ditionally documented information alongside sensor
information collected by the patient app on a mobile
device, such as a phone or smart-watch. The com-
bined data is analyzed using machine learning meth-
ods and presented in a clear and meaningful fashion
to the clinician. This enables assistance in making
medical diagnostic decisions. The system can also be
used to detect and alert the doctor or patient in case of
an emergency or deterioration. An important feature
of our system is that data is collected not only in the
doctor’s office, but also between visits. This enables
performing a diagnosis based on a much more infor-
mation than is typically possible with other systems.
Our system overview appears in Figure 1. (patent re-
quest submitted (BGSEGEV, 2018)).
Among the surplus of medical apps, many require
the users to actively interact with the application in
order to achieve medical feedback, for example (Seo
et al., 2015; Zhang et al., 2015; Nam et al., 2014).
Richardson, A., Perl, A., Natan, S. and Segev, G.
A Clinical Decision Support System based on an Unobtrusive Mobile App.
DOI: 10.5220/0007587001670173
In Proceedings of the 5th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2019), pages 167-173
ISBN: 978-989-758-368-1
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
167
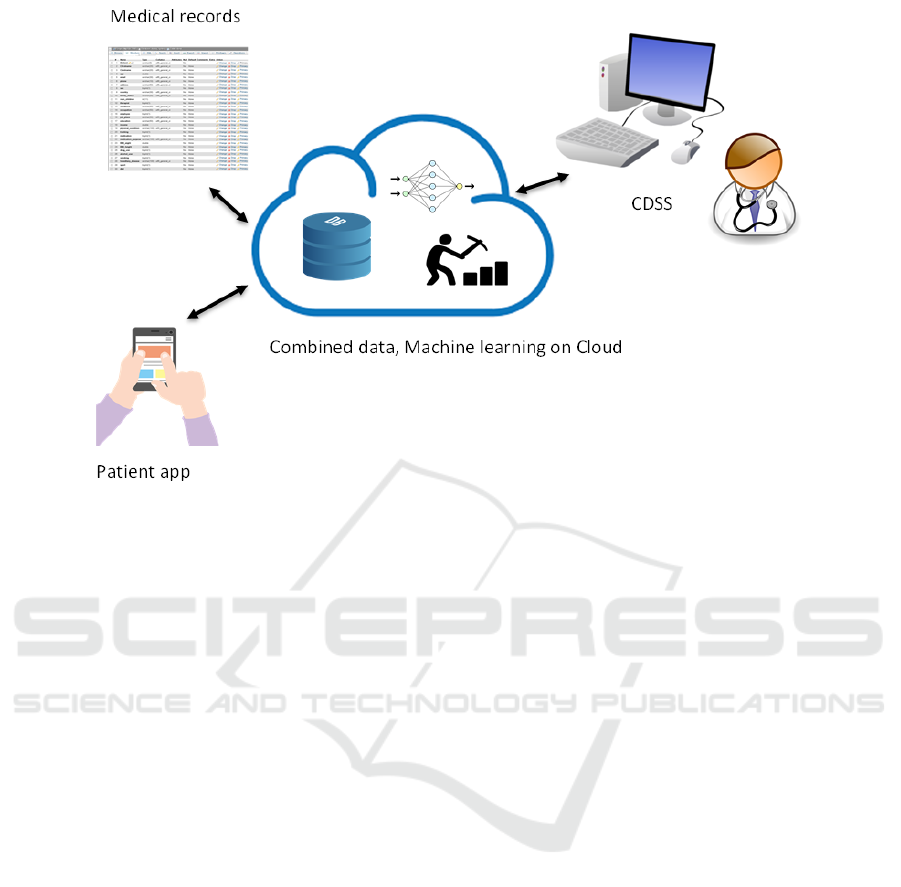
Figure 1: Overview of system.
In our system, we propose an app that will be able
to provide meaningful information, without requiring
user actions for data collection and input. Our CDSS
works in conjunction with an application that accom-
panies the patient and collects data on-line using a
mobile phone application. We collect data during reg-
ular phone usage, without the need for direct patient
involvement. This characteristic is what makes our
system different from many other health apps.
We currently focus on vascular diseases such as
stroke, heart and peripheral vascular disease but have
also been expanding our work to other conditions
where sensor data has predictive capabilities such as
cancer and osteoporosis. We collect data from many
sources, for example: typing patterns, voice record-
ings, pulse measurements, walking patterns and in-
jury related data.
Generally, during a doctor’s visit, the doctor re-
lies on descriptions of the patient’s subjective feeling,
and the patient’s description of his health difficulties.
These descriptions are often inadequate, and affected
by recent subjective feelings, rather than unbiased,
long-term and continuous health monitoring. Visits to
the doctor are sporadic and often far and between due
to the constraints of the health system. The physician
has no way of detecting deterioration in the patient’s
condition between visits. Patients often visit the doc-
tor only when the pain is unusual and sometimes this
is a sign that the disease has already progressed. Our
system has the benefit of being able to collect data
over time and between doctor visits. The use of such
data, may present a clearer picture of the patients con-
dition than data collected periodically during doctor
visits, or inexact patient descriptions of symptoms.
Moreover, the patient may not feel any symptoms
of a disease. It is known, for example, that many peo-
ple with PAD (Peripheral Artery disease) report a lack
of symptoms, including those with a relatively serious
illness (Criqui and Aboyans, 2015). With our CDSS,
diseases may be detected even before the patient be-
comes symptomatic. Identifying diseases in an early
stage is critical to treating them and may even prevent
their onset, hence the great contribution of our frame-
work. Obviously security measures must be consid-
ered when handling sensitive medical data, these are
out of the scope of this study.
There is need for a system that will provide a
maximal response to all of its needs without requir-
ing active involvement from the patient during data
collection. Our CDSS offers state-of-the-art manage-
ment, documentation and user interface platform that
receives constant feedback from the patient’s applica-
tion about dynamic data collected in the background
and processed using data mining. Our proposed sys-
tem is autonomous, and therefor reduces the number
of required doctor visits, reduces human error, and
enriches the medical diagnosis and monitoring pro-
cess with information from new sources such as the
smartphone sensors. The data mining performed in
the system will provide rich output to the doctor and
patient, and may also be used for real-time events and
for alerting caregivers.
ICT4AWE 2019 - 5th International Conference on Information and Communication Technologies for Ageing Well and e-Health
168

2 RELATED WORK
We consider two types of studies to be relevant to our
work. The first are studies on clinical decision support
systems. The second set of studies are mobile appli-
cations for healthcare, as our CDSS is heavily based
on a mobile application for data collection.
CDSSs cover a broad variety of topics. Some
systems are specific to a condition such as diabetes
(Weymann et al., 2016) or retinal disease (Bourouis
et al., 2014). Many others are aimed at assisting di-
agnosis based on the vast amount of medical infor-
mation available. (El-Sappagh and El-Masri, 2014)
present an architecture for combining electronic med-
ical records into a single DSS to assist clinicians. A
survey of CDSSs along with a detailed discussion ex-
plaining the need for such systems can be found in
(Castaneda et al., 2015). Although CDSSs are plen-
tiful, they are often slow to be accepted and do not
always improve medical practice. (Shibl et al., 2013)
survey several CDSS and discuss factors that impact
system acceptance. One of the main parameters found
to affect acceptance was whether the system inter-
fered with the regular work-flow. Systems that re-
quired a break in the normal work-flow were not usu-
ally accepted. Surprisingly, ease of use was consid-
ered less important. Clinicians claimed that if a sys-
tem was helpful they would be prepared to make an
effort to use it. Other parameters are described in
the paper. (Kawamoto et al., 2005) perform a simi-
lar survey to identify features that impact the success
of CDSSs. They claim that clinicians were found to
adopt systems, where the information was provided
automatically. Clinicians were less likely to use sys-
tems that required an active search for information.
Similarly, systems that used a computer to generate
decision support were more effective than those that
required manual intervention. The studies provide
an indication, that our proposed system that requires
minimal intervention on the side of the patient, and
highly automated and clearly presented integrated in-
formation to the clinician, will have a high chance of
acceptance.
Similar to our framework is the work by (Artikis
et al., 2012). They propose a CDSS that integrates
data from sensors collected on the patient, alongside
other medical data, just as we do. The focus of their
work is on dementia and depression, whereas we are
looking at cardiovascular disease and other conditions
with physical deterioration. To the best of our under-
standing their work was not expanded.
Another system that must be discussed in close re-
lation to our work is that of the MobiGuide Project
(Peleg et al., 2014; Peleg et al., 2017). MobiGuide fo-
cuses on arterial fibrillation, and gestational diabetes.
The proposed system is targeted strongly at supply-
ing the patient with a DSS system to help manage
the disease. Although doctors also use the system,
it seems that focus of this large impressive study is
different to ours. We are less concerned with provid-
ing a DSS system for the patient. Our concept uses
the data collected from the patient as input to the clin-
icians decision. Our system collects as much data as
possible without inconveniencing the patient, in order
to supply the doctor with as much information as pos-
sible. The information is beneficial, as it is collected
between the office visits, and we hope it will assist
in making a decision that is supported by long term
conditions, as opposed to solely relying on recent in-
formation reported by the patient in the office.
Aside from CDSSs, it is important to consider mo-
bile health applications that are relevant to our frame-
work. The number of mobile applications for health-
care is vast and constantly expanding (Boulos et al.,
2014; Terry, 2015). Most mobile health apps are tar-
geted at monitoring and rehabilitation such as (Seo
et al., 2015) who tested the feasibility of a mobile app
for patients who had suffered a stroke. The app was
aimed at managing risk factors such as blood pressure
and diabetes management. Other applications (Zhang
et al., 2015; Micallef et al., 2016), accompany the pa-
tient by encouraging exercises, following up on taking
pills, and logging mood reports.
Some of the apps are aimed at bridging the dis-
tance between the patients to medical assistance.
(Nam et al., 2014) provide a stroke screening appli-
cation. The application shows a set of cartoons repre-
senting stroke symptoms. Potential patients can fol-
low the cartoons and try to determine whether they
may be suffering from a stroke. (Mitchell et al., 2011)
bridge the gap by providing a teleradiology system
that enables a doctor to interpret a CT scan. (Demaer-
schalk et al., 2012) provide high-quality video tele-
conferencing.
Several surveys on the use of smartphones in
medicine (Ozdalga et al., 2012; Boulos et al., 2014;
Dobkin and Dorsch, 2011) show how smartphones
can be used for patient care, monitoring and rehabil-
itation alongside accessing clinical data such as elec-
tronic medical records CT scans etc. Most of these
systems require active and sometimes heavy user in-
volvement. Patients are expected to use the app fre-
quently and enter the relevant information for data
collection. We did not encounter a system that com-
bines both of an unobtrusive application on the pa-
tient side with a CDSS for the doctor, in the structure
of the system that we propose in this paper.
A Clinical Decision Support System based on an Unobtrusive Mobile App
169

3 DESCRIPTION OF OUR CDSS
The main focus of this paper is the CDSS for the doc-
tor. As shown in Figure 1 data from phone sensors
is aggregated with medical history. After this data is
analyzed using machine learning, a clear presentation
of the output including diagnostic suggestions is dis-
played to the doctor. We developed a prototype web
based application that presents this information to the
doctor, and describe it in section 3.1. We also devel-
oped a data collection system to collect labeled data
in order to train our system, described in Section 3.2.
3.1 CDSS
In designing the DSS for the doctor, we kept in mind
that the system should be designed in a convenient
and intuitive manner. Physician activity while us-
ing the system should not deviate much from regular
practice, as claimed by (Shibl et al., 2013). Improve-
ments will aim at saving time and improving diagno-
sis accuracy. We take User interface and experience
into account in our design. The CDSS has been de-
veloped as a prototype (in Hebrew), with the actual
machine learning engine left for future work.
We describe and display some of the more inter-
esting features of our CDSS. The first feature is the
disease simulator. The simulator automatically ticks
boxes that the system has detected from the app data.
The doctor can then discuss these choices with the
patient and change the selection of symptoms. Once
ticked symptoms are agreed on, the system outputs
suggestions for possible diagnosis. The diagnosis out-
put by the system takes into account both the data en-
tered by the doctor, but also the input collected auto-
matically by the app on the patients device. The in-
tegration of these two sets of data, both that inserted
by the doctor, and that automatically collected from
the patient app are what make this simulator unique.
An example of what this screen looks like is shown in
Figure 2. The simulator provides the doctor with a list
of possible symptoms, and the doctor inputs data in a
simple manner by ticking boxes. Examples to symp-
toms (that are ticked) are limping, slow ulcer heal-
ing, ulcers on legs and weight loss. After the aggre-
gation with the patient app data the diagnostic sug-
gestions are presented on the screen, and can be seen
in red. For example on the this screen the proposed
diagnoses are Peripheral Vascular disease (PVD) and
Osteoporosis.
One of the special features in our system, is plots
of the data collected by the patient app that can be
viewed in real time as the data accumulates or at a
later time. The doctor may choose to enter the CDSS,
Figure 2: Diagnosis simulator. Symptoms in tic boxes, Di-
agnosis in red.
Figure 3: Plots of patient symptoms, alongside proposed
diagnosis with confidence rating.
between office visits, to follow how a patient is per-
forming. The screen displays the name of the patient,
and a set of plots for graphs of ulceration information,
walking patterns, pulse measurements and sleep pat-
terns. These are all shown in Figure 3. The graphs
show the patient’s condition in the various profiles.
The graph is accompanied by a box with the sug-
gested diagnosis along with a degree of confidence
that this diagnosis is correct. Plots can also be be
printed or saved.
Alerting the doctor to suspicious events that re-
quire attention are an important part of our system.
Figure 4 is the description of the profile belonging to
a patient as shown to the doctor. The data includes the
patient ID, age, name, phone number, address, gender
etc. The data also includes medical information such
as smoking habits, alcohol consumption, medications,
sport, diet etc. In case of a suspicious event, an alert
is displayed on the top of the screen, as shown in the
figure. Then details of the patients condition can then
be seen in Figure 5, that shows an example of a re-
ICT4AWE 2019 - 5th International Conference on Information and Communication Technologies for Ageing Well and e-Health
170

Figure 4: Patient profile, with an alarm indicating a suspi-
cious event.
port that summarizes a patients data collection. Each
row has the date, type of activity performed, state de-
tected and the suspected condition. Events that have
been detected as suspicious are marked in red. Ulcers
are accompanied by a picture of the ulcer.
Our system also includes standard medical system
functionality such as the display of patient informa-
tion in a table form, as shown in Figure 6 that de-
scribes the patients information (name, ID, visit num-
ber) Then the diagnosis, the reason for the visit, extra
referrals and recommendations. Aside from the pa-
tient information, the doctor is offered links to extra
reading material as shown in Figure 7, enabling ac-
cess to the latest medical articles on relevant topics.
3.2 Labeled Data Collection
As we have stated the app used to collect patient data
is unobtrusive. Data is collected without the need for
user intervention. However, in order to build machine
learning models we need to collect labeled data for
the conditions we wish to diagnose. In order to for-
ward our research we developed a data collection app
for research. This application (that is different to the
application for data collection for the CDSS) requires
the user to describe the type of information being col-
lected, and then collect the information. Examples to
types of data that can be collected for research with
this app are various walking activities such as run-
ning, walking and even falling, or dropping the phone.
The are examples to activities that might play an im-
portant part in stroke detection. We can collect the
typing patterns that are created as patients type mes-
sages or other text into the phone. We also collect
voice recordings that are obtained during phone calls.
Figure 5: Example of visit report.
Figure 6: Patient data in tabular form, with suspicious
events marked in red.
Some of the application screens are shown in Figures
8 and 9. Figure 8 shows the screen where user infor-
mation is inserted. The user first defines a profile, by
entering a name, gender, and age. Then the user may
select a condition from a lost of conditions, such as
PVD, and continues by pressing the ”continue” button
on the bottom of the screen. This brings the patient to
the next screen, for recording the activity. This data
is used to identify the subject, as the app may be used
to collect data from many subjects. Figure 9 is the
screen used for recording an activity. The activity will
be recorded using all sensors, and labeled according
the the information provided in the first screen. First
the type of activity is selected (i.e. walking, climbing
stairs), then the phone position is selected, and then
recording is performed by pressing the red round but-
ton, and stopped by pressing the black square button.
A Clinical Decision Support System based on an Unobtrusive Mobile App
171
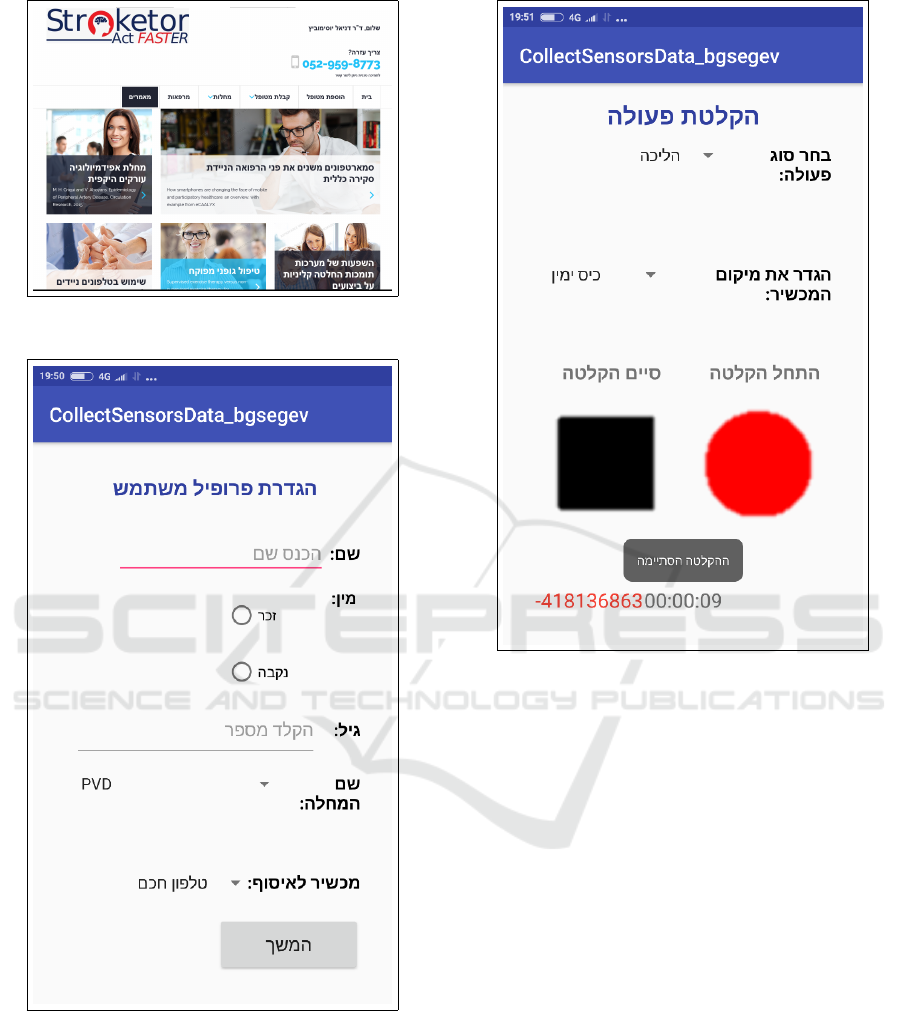
Figure 7: Extra reading material.
Figure 8: App for collecting patient information for re-
search - subject data.
Data were collected for patients with various car-
diovascular diseases: PVD, stroke, heart disease and
also for osteoporosis. Early detection is a common
necessity for all these diseases, and is important for
the effective treatment of the disease. The ability to
use the information obtained from the various sensors
of the application contributes greatly to carry out the
Figure 9: App for collecting patient information for re-
search - activity description.
disease detection. This app can easily be extended to
perform data collection for research for many more
conditions, and used for other research applications
as well.
4 DISCUSSION AND FUTURE
WORK
This study presents the development of an CDSS sys-
tem for a doctor that works together with the patient’s
app. The patient app collects data from the patient,
during regular phone usage, without requiring that
user actively assist the data collection (but obviously
with patient permission). The data is collected on the
cloud and used by the doctor CDSS system to mon-
itor and detect various diseases. It seems that the
structure of the system, which combines all the in-
formation from the patient, constitutes a significant
milestone in the management of the patient’s medi-
cal file, to the point of identifying diseases at an early
stage and even before their outbreak. The system re-
ICT4AWE 2019 - 5th International Conference on Information and Communication Technologies for Ageing Well and e-Health
172

lies on having successful data mining models, and a
description of these is left for future work, as it is a
separate module of the system. Using data mining to
analyze the data collected in the app brings the sys-
tem even further towards better disease management.
In the future, we will offer research and analysis of
data from other diseases, such as cancer that our team
has begun to study, and adapt the system accordingly.
We have begun studying various data mining meth-
ods (Richardson et al., 2019) in order to select the
most appropriate models from our CDSS and will re-
port on progress in future work. Future studies will
also involve testing the system with both patient and
caregiver subjects.
REFERENCES
Artikis, A., Bamidis, P. D., Billis, A., Bratsas, C.,
Frantzidis, C., Karkaletsis, V., Klados, M., Konstan-
tinidis, E., Konstantopoulos, S., Kosmopoulos, D.,
et al. (2012). Supporting tele-health and ai-based
clinical decision making with sensor data fusion and
semantic interpretation: The usefil case study. In
International workshop on artificial intelligence and
NetMedicine, page 21.
BGSEGEV (2018). BGSegev STROKETOR - stroke detec-
tor. https://stroketor.segevlabs.org/.
Boulos, M. N. K., Brewer, A. C., Karimkhani, C., Buller,
D. B., and Dellavalle, R. P. (2014). Mobile medical
and health apps: state of the art, concerns, regula-
tory control and certification. Online journal of public
health informatics, 5(3):229.
Bourouis, A., Feham, M., Hossain, M. A., and Zhang, L.
(2014). An intelligent mobile based decision support
system for retinal disease diagnosis. Decision Support
Systems, 59:341–350.
Castaneda, C., Nalley, K., Mannion, C., Bhattacharyya, P.,
Blake, P., Pecora, A., Goy, A., and Suh, K. S. (2015).
Clinical decision support systems for improving di-
agnostic accuracy and achieving precision medicine.
Journal of clinical bioinformatics, 5(1):4.
Criqui, M. H. and Aboyans, V. (2015). Epidemiology
of peripheral artery disease. Circulation research,
116(9):1509–1526.
Demaerschalk, B. M., Vegunta, S., Vargas, B. B., Wu, Q.,
Channer, D. D., and Hentz, J. G. (2012). Reliability
of real-time video smartphone for assessing national
institutes of health stroke scale scores in acute stroke
patients. Stroke, 43(12):3271–3277.
Dobkin, B. H. and Dorsch, A. (2011). The promise of
mhealth: daily activity monitoring and outcome as-
sessments by wearable sensors. Neurorehabilitation
and neural repair, 25(9):788–798.
El-Sappagh, S. H. and El-Masri, S. (2014). A distributed
clinical decision support system architecture. Journal
of King Saud University-Computer and Information
Sciences, 26(1):69–78.
Kawamoto, K., Houlihan, C. A., Balas, E. A., and Lobach,
D. F. (2005). Improving clinical practice using clin-
ical decision support systems: a systematic review
of trials to identify features critical to success. Bmj,
330(7494):765.
Micallef, N., Baillie, L., and Uzor, S. (2016). Time to ex-
ercise!: an aide-memoire stroke app for post-stroke
arm rehabilitation. In Proceedings of the 18th Inter-
national Conference on Human-Computer Interaction
with Mobile Devices and Services, pages 112–123.
ACM.
Mitchell, J. R., Sharma, P., Modi, J., Simpson, M., Thomas,
M., Hill, M. D., and Goyal, M. (2011). A smartphone
client-server teleradiology system for primary diagno-
sis of acute stroke. Journal of medical Internet re-
search, 13(2).
Nam, H. S., Heo, J., Kim, J., Kim, Y. D., Song, T. J., Park,
E., and Heo, J. H. (2014). Development of smartphone
application that aids stroke screening and identifying
nearby acute stroke care hospitals. Yonsei medical
journal, 55(1):25–29.
Ozdalga, E., Ozdalga, A., and Ahuja, N. (2012). The smart-
phone in medicine: a review of current and potential
use among physicians and students. Journal of medi-
cal Internet research, 14(5).
Peleg, M., Shahar, Y., and Quaglini, S. (2014). Making
healthcare more accessible, better, faster, and cheaper:
the mobiguide project. European Journal of ePrac-
tice: Issue on Mobile eHealth, 20:5–20.
Peleg, M., Shahar, Y., Quaglini, S., Broens, T., Budasu,
R., Fung, N., Fux, A., Garc
´
ıa-S
´
aez, G., Goldstein,
A., Gonz
´
alez-Ferrer, A., et al. (2017). Assessment of
a personalized and distributed patient guidance sys-
tem. International journal of medical informatics,
101:108–130.
Richardson, A., Shani Ben Ari, and Sinai, M., Atsmon, A.,
Conley, E. S., Gat, Y., and Segev, G. (2019). Mobile
applications for stroke: A survey and a speech classi-
fication approach. In ICT4AWE, page to appear.
Seo, W.-K., Kang, J., Jeon, M., Lee, K., Lee, S., Kim, J. H.,
Oh, K., and Koh, S.-B. (2015). Feasibility of using
a mobile application for the monitoring and manage-
ment of stroke-associated risk factors. Journal of Clin-
ical Neurology, 11(2):142–148.
Shibl, R., Lawley, M., and Debuse, J. (2013). Factors influ-
encing decision support system acceptance. Decision
Support Systems, 54(2):953–961.
Terry, K. (2015). Number of health apps soars but use does
not always follow. Medscape Medical News.
Weymann, N., H
¨
arter, M., and Dirmaier, J. (2016). Informa-
tion and decision support needs in patients with type
2 diabetes. Health informatics journal, 22(1):46–59.
Zhang, M. W., Yeo, L. L., and Ho, R. C. (2015). Harness-
ing smartphone technologies for stroke care, rehabili-
tation and beyond. BMJ innovations, 1(4):145–150.
A Clinical Decision Support System based on an Unobtrusive Mobile App
173
