
Run-time Support to Comorbidities in GLARE-SSCPM
Alessio Bottrighi, Luca Piovesan and Paolo Terenziani
DISIT, Universita’ del Piemonte Orientale, Viale Teresa Michel 11, Alessandria, Italy
Keywords: Clinical Guidelines, Comorbid Patients, Interaction Analysis and Management.
Abstract: Comorbidities play a relevant role in healthcare, so that, in the last years, several approaches Medical
Informatics and Artificial Intelligence have developed software tools to support physicians in the treatment
of comorbid patients. Computer Interpretable Guidelines (CIGs) are consolidated decision support tools to
help physicians, but they are devoted to provide evidence-based recommendations for one specific disease.
In order to support the treatment of patient affected by multiple diseases, challenging additional problems
have to be addressed, such as (i) the detection of the interactions between CIG actions, (ii) their
management, and, finally, (ii) the “merge” of CIGs. Several CIG approaches have been recently extended in
order to face (at least one of) such challenging problems, and one of them is GLARE (GuideLine
Acquisition Representation and Execution). However, such approaches have mostly focused on the “a-
priori” treatment of such problems, while addressing them “run-time” (i.e., to support physicians during the
execution of the CIGs on a specific patient) involves additional challenges, and requires additional
methodologies. In this paper we take advantage of previous extensions of GLARE (to cope with issues (i),
(ii), (iii)), and propose a new knowledge-based, “focused” and interactive management of comorbid
patients.
1 INTRODUCTION
The term comorbidity indicates the co-occurrence of
more than one disease in a patient. They are quite
frequent (an average of 25% of the population), thus
constituting an important problem from different
viewpoints.
Evidence-based decision making is a quite
consolidated practice in healthcare, since it exploits
the evidence and knowledge provided by clinical
trials, and by previous experiences. One of the main
methodologies to put evidence-based medicine into
practice is the development of Clinical Practice
Guidelines (CPGs). CPGs are defined as
“systematically developed statements to assist
practitioner and patient decisions about appropriate
health care in specific clinical circumstances”
(Institute of Medicine (US), 1990). Generally, CPGs
are elaborated by national or international teams of
specialists, and collect and organize in a textual form
the knowledge available in literature to manage a
specific clinical circumstance. They play a major
role in modern healthcare, and thousands of CPGs
have been devised in the last few years. For instance,
the Guideline International Network, which groups
97 organizations from all the continents, provides a
library of more than 6000 CPGs.
Additionally, in the last 30 years or so, the
research in Artificial Intelligence and in Medical
Informatics has shown that software tools can be
designed to increase the practical impact of CPGs in
healthcare. Specifically several software tools have
been devised in order to acquire, represent, execute
and reason with the so-called Computer-
Interpretable Guidelines (CIGs henceforth; see, for
example, the surveys (Peleg, 2013; Ten Teije et al.,
2008)).
1.1 CIG and Comorbidities
Unfortunately, CPGs provide evidence-based
information of interventions, but only on individual
pathologies. The simple solution of applying
multiple CPGs (one for each disease) to a patient
does not work: the treatments recommended by
different CPGs may interact with each other, and
such interactions may be (very) dangerous for
patients. The approach of considering all the
possible combinations of diseases is not only
difficult, but also impractical. Such considerations
498
Bottrighi, A., Piovesan, L. and Terenziani, P.
Run-time Suppor t to Comorbidities in GLARE-SSCPM.
DOI: 10.5220/0007685004980505
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 498-505
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

highlight the importance of developing
methodologies to merge CPGs for single disease
interventions to provide professionals' assistance to
comorbid patients (Riaño and Collado, 2013). The
development of such methods has been identified as
one of the “grand challenges” for clinical decision
support (Sittig et al., 2008). Since the early 2010's,
the research in Computer Science has been very
active in such a challenging area of research.
1.2 State of the Art
In general, the approaches devised in such an area
have specialized on the treatment of two different
subproblems:
(i) the detection of interactions between CIGs, and
their management (i.e., how to “solve”
interactions), and
(ii) the “merge” of CIGs.
Issue (i) above has been faced by relatively few
approaches in the CIG literature. In particular, the
approach in (Zamborlini et al., 2014) provides a
knowledge-based solution. It proposes a CIG-
independent conceptual model for medical actions
and reasoning forms operating on it, as well as
domain-independent rules to identify different types
of interactions on the basis of such a knowledge. A
similar approach has been pursued in the GLARE
approach (see Section 2 and (Piovesan et al., 2014),
(Piovesan and Terenziani, 2015)).
On the other hand, issue (ii) has been faced by
several CIG approaches. It is possible to distinguish
between the approaches aiming at achieving
“conservative” CIGs, and those that do not. The
approach in (Sánchez-Garzón et al., 2013), for
instance, belongs to the latter category. It builds ad-
hoc CIGs from scratch, using an agent-based
approach. Agents with hierarchical planning
capabilities represent experts in the treatment of
specific diseases. The CIG coping with the
comorbidity is obtained through the coordination of
all the agents. However, the mainstream is
constituted by conservative techniques, attempting
to merge existing CIGs with limited changes since,
in the real medical practice, physician need to follow
as much as possible evidence-based
recommendations, such as the ones proposed in the
CPGs (and, thus, CIGs) in the literature. The
approaches in such a mainstream mostly assume that
the possible interactions and their managements
have been defined a priori by physicians, and
focuses on CIG merge only. However, quite
different techniques have been proposed. For
instance, in (Wilk et al., 2013), constraint logic
programming (CLP) is adopted. A CLP is derived
from the CIGs, the interactions and their
managements, and a mitigation algorithm is
proposed in order to achieve the merge. Riaño and
Collado (Riaño and Collado, 2013) propose a
model-based approach for the merge. They model
treatments as oriented graphs composed by decisions
and actions. With the help of physicians, they define
a set of operators to merge decisions or actions. The
combination of the original CIGs is obtained through
the application of the operators. On the other hand,
in GLARE, the different management options
applied to independently solve the interactions are
merged through a conciliation module which is
based on CSP (Constraint Satisfaction Problems)
methodologies (Piovesan and Terenziani, 2016).
1.3 “Run-time” Support
Some of the above approaches can be used both (i)
“a-priori”, to analyse interactions between CIGs or
to merge them, without any reference to a specific
patient, and (ii) “run-time”, to support the execution
of CIGs on a specific patient. However, the “run-
time” application of the above methodologies
involves the resolution of new problems: when and
on which parts of the CIGs interaction detection has
to be performed? And the management of the
interactions? And the merge? Such problems are
still open problems in the specialised literature, and
the goal of this paper is to propose a general
methodology to cope with them, thus providing
physicians with an effective, user-friendly and
highly interactive approach supporting physicians in
the run-time treatment of comorbid patients.
Specifically, our approach grounds on GLARE, and
on its extensions (called GLARE-SSCPM) to deal
with comorbidities, which are briefly resumed in
Section 2). However, we emphasize that our
methodology is mostly system-independent, and can
be tuned in order to apply to other approaches to
comorbidities in the literature.
2 BACKGROUND: GLARE AND
GLARE-SSCPM
GLARE Support System for Comorbid Patient
Management (GLARE-SSCPM; (Piovesan et al.,
2018)) is an extension of GLARE (Terenziani et al.,
2001) to support the management of comorbidities,
and which takes into account both the (i) interaction
detection and management, and (ii) the CIG merge.
Run-time Support to Comorbidities in GLARE-SSCPM
499

In the following, we briefly resume such an
approach, which is the basis of the support to the
approach proposed in Section 3.
2.1 GLARE
GLARE (GuideLine Acquisition Representation and
Execution, (Terenziani et al., 2001)) is a well-known
CIG framework, designed in a long term cooperation
between the University of Eastern Piedmont and the
Azienda Ospedaliera San Giovanni Battista in Turin
(one of the largest hospitals in Italy), started in 1997.
The kernel of GLARE provides a formalism to
represent CIGs, a tool to acquire them, a mechanism
to execute a CIG on a specific patient. In GLARE,
CIGs are modelled as hierarchical graphs, in which
nodes represent actions or decisions and arcs
represent the control flow relations between nodes.
GLARE distinguishes between atomic actions
(simple steps in a CIG) and composite actions
(plans), which are defined in terms of their
components (thus supporting the definition of CIGs
at different levels of abstraction). Atomic actions
can be work actions (a procedure which must be
executed), pharmacological actions (a drug to be
administered), query actions (retrieval of
information from the clinical record/examinations)
or decision actions (choice among different
alternatives). In particular, GLARE distinguish
among diagnostic and therapeutic decisions (see the
discussion in Section 3.2).
Arcs are used to represent the control flow
relations, and can be annotated with temporal
constraints. In particular, a sequence arc from node
N1 to N2 indicates that the action represented by N1
must terminate before the execution of N2. On the
other hand, constrained arcs represent complex
temporal relations between nodes (e.g., N2 during
N1), and can be used to enforce concurrent
execution of actions.
The kernel of GLARE consists of two main
modules: the acquisition module and the execution
one. The acquisition module proposes a user-
friendly graphical interface for the acquisition of
CIGs, and stores them in an internal format. The
execution module takes in input a CIG and the
clinical record of a specific patient, and supports the
“execution” of the CIG on the patient. The execution
module is based on the “agenda techniques”
(Terenziani et al., 2001): at each time during the
execution of a CIG, GLARE determines (in the
agenda) the set of current actions, each one paired
with a time window, indicating when the action has
to be executed (minimum and maximum time) to
comply the temporal constraints in the CIG.
Notably, GLARE supports concurrent actions.
GLARE’s architecture is open. In the latest
years, several new modules and\or methodologies
have been added to cope with automatic resource-
based contextualization (ADAPT module,
(Terenziani et al., 2004)), temporal reasoning (TR,
(Anselma et al., 2006)), decision making support
(DECIDE_HELP, (Montani et al., 2005)), and
model-based verification (VERIFY, (Bottrighi et al.,
2010)). Recently, GLARE has been extended to
cope with comorbidities (see below).
2.2 GLARE-SSCPM
GLARE-SSCPM (Piovesan et al., 2018) proposes a
set of user-friendly supports to the treatment of
comorbidities. It is a knowledge-based approach,
aimed to support step-by-step physicians in the
treatment of comorbidities.
Operationally speaking, GLARE-SSCPM is
based on a CIG-independent knowledge base of
clinical actions, effects, and interactions, and
supports three main tasks:
(1) The detection of interactions occurring
between CIGs
(2) The management of the interactions
(3) The final merging of the CIGs
Since, in the real practice, interaction occur in
time, all the above tasks can be achieved only if the
temporal dimension is taken into account. Therefore,
also (4) Temporal Reasoning is considered in
GLARE-SSCPM:
Knowledge Base and Reasoning Supports.
GLARE-SSPCM is based on a Knowledge
Manager, i.e., a module coping with additional
(CIG-independent) medical knowledge (Piovesan et
al., 2014). It adopts an OWL ontological model
developed in collaboration with expert physicians,
using Protégé and integrating part of medical
models, such as SNOMED CT and ATC. Each
action in GLARE can be associated with one or
more elements of the ontological model. Such a
knowledge base contains both a general ontology,
describing general notions such as actions, action
intentions\effects, time, interactions, as well as
domain-specific knowledge, such as possible
interactions between specific drug types. Moreover,
the Knowledge Manager module is provided with
standard OWL reasoners providing inferences. Such
inferential mechanisms are used to devise a tool that
navigates the knowledge base and detects which are
the possible interactions (if any) between actions’
effects.
HEALTHINF 2019 - 12th International Conference on Health Informatics
500

Interaction Detection. GLARE-SSCPM Interaction
Detection module (Piovesan et al., 2014) provides a
flexible and interactive focusing tool allowing
physicians to navigate through the different
abstraction levels in the CIGs, to identify the
“relevant” actions. Once the actions of interest are
identified though focusing, interaction detection is
automatic: GLARE-SSCPM exploits the knowledge
provided by the knowledge manager and the OWL
reasoner to retrieve all the interactions between the
intentions, effects and drugs prescribed (in case of
pharmacological actions) of the focused actions.
Interaction Management. Once detected and
analysed, interactions must be managed.
Management options are local (and as small as
possible) changes in the original CIGs, which make
the original GIGs executable, avoiding undesirable
interactions and promoting desirable ones. On the
basis of the medical literature, GLARE-SSPCM
propose a wide range of general (i.e., CIG
independent and domain independent) interaction
management options (Piovesan and Terenziani,
2015): Safe Alternative, Replanning, Temporal
Avoidance, Effect Monitoring, Dosage Adjustment,
Interaction Mitigation, Interaction Alignment,
Intention Alignment.
GLARE-SSCPM provides a facility to instantiate
each one of such options, i.e., to apply it to a specific
input interaction, and to modify the CIGs
accordingly. The idea is that, given a specific
interaction, the user-physicians may apply one of the
options, or even trying to apply more than one, in a
“what-if” modality, see what the consequences on
the CIG are, and finally chose an apply the preferred
option in a definitive way.
Merge. Once the interactions have been identified,
and managed in isolation, the union of the original
CIGs with the applied managements is not yet an
executable CIG, since the management options lead
to changes to the original CIGs that are “locally”
consistent, but possibly not consistent with each
other. For these reasons, a final “merging” step is
required. In GLARE-SSCPM such a step is
performed by the CIG Conciliation module
(Piovesan and Terenziani, 2016), which provides as
output a “merged” CIG executable by GLARE.
Temporal Reasoning. GLARE-SSCPM provides
the Temporal module (Piovesan et al., 2015), to cope
with temporal constraints and to perform temporal
reasoning. Such a module operates as a knowledge
server: temporal problems may be demanded to the
Temporal module, which provides them a solution
(or report that there is no solution).
3 RUN-TIME SUPPORT TO
COMORBIDITIES
3.1 Philosophy of the Approach:
Focusing and Interactivity
As discussed in the Introduction, several approaches
in the literature focus on the a-priori “merge” of
CIGs, to avoid dangerous interactions. Such
approaches usually consider whole CIGs, and mostly
operate without interacting with physicians: given
two or more CIGs, to provide to physicians a new
CIG, avoiding dangerous interactions.
GLARE-SSCPM follows a different philosophy:
it provides a highly interactive approach, in which
physicians may (i) focus on specific subparts of the
CIGs, (ii) analyse possible interactions and (iii)
adopt GLARE–SSPCM to check the effects of
applying different management options to deal with
interactions (Piovesan et al., 2018).
In this paper, we propose a methodology to
extend the approaches coping with CIGs and
comorbidities with proper supports for “run-time”
execution, and we follow GLARE-SSCPM
“philosophy”: our methodology supports “focusing”,
and highly interactive with physicians.
Focusing is needed because, when executing
CIGs on a specific patient, physicians are not
interested with the whole CIGs, but only on the
subpart of them that is applicable to the given
patient, given the patient status. Indeed, focusing is
needed along two dimensions:
(i) The dimension of alternative paths in the
CIGs
(ii) The “temporal” dimension
Dimension (i) concern the fact that real CIGs usually
contain many (even hundreds) of different
alternative paths, depending on the different status
that the patient may assume during the CIG
execution. Obviously, only the paths that are
recommended (given the current status of the
patient) are interesting for physicians, and thus have
to be taken into account by decision support tools.
Dimension (ii) regards the fact that physicians do
not usually plan patient treatments far-away in the
future. They consider a limited “window” of the
CIG, usually not exceeding the next decision step in
the CIG. Indeed, taking “future” decisions on the
basis of the current status of the patient is nearly a
“bid”, which is rarely performed by physicians.
Interactivity is needed, in general, because we see
our approach as a support tool, which does not
substitute physicians, but helps them, by providing
Run-time Support to Comorbidities in GLARE-SSCPM
501
additional knowledge and recommendations.
Specifically, in the case of co-morbidities, while we
provide a fully automatic support to the detection of
possible interactions between (the “focused” parts of
the) CIGs, we want to be highly interactive in the
selection of the management options to treat such
interactions. In general, more than one option is
applicable, and we do not want to impose any
specific choice to physicians. On the other hand, we
want to support them in such a choice, by showing
them in an automatic way the consequences of
choosing a given option, or another.
3.2 Scheduled and Candidate Actions
From the practical point of view, a key issue to
realize the notion of “run-time” focusing is the
definition of scheduled (CIG) actions, and of
candidate ones. To propose such definitions, we first
have to point out the different nature of diagnostic vs
therapeutic decisions in CIGs. GLARE (as well as
several other CIG tools), clearly distinguishes
between diagnostic and therapeutic decisions.
Diagnostic decisions discriminate among different
diagnoses on the basis of the patient status,
considering a set of parameters (e.g., blood pressure,
fever, …), which vary from decision to decision. In
GLARE such decisions are represented as scored or
Boolean decisions. The decision criteria are
described within the decision action, and are
automatically evaluated by GLARE, considering the
clinical record describing the status of the patient.
Though in GLARE diagnostic decisions are taken in
a semi-automatic way (since GLARE allows
physicians to over-rule the decision taken
automatically by the system, selecting diagnoses
different from the ones derived from the automatic
evaluation of the decision criteria on the basis of the
status of the patient), such decisions are strictly
related to the state of the patient (so that physicians
cannot freely choose among them, independently of
the patient’s status). On the other hand, in
therapeutic decisions physicians have to choose
among different therapies that are all recommended
(by the CIG) for the given category of patients.
Physicians have usually the “full control” of such
therapeutic decisions, since all CIG alternative
treatments are usually “eligible” for patients. The
choice is done considering a given set of parameters:
effectiveness, cost, side-effects, compliance,
duration. Thus, in GLARE, a therapeutic decision
action is represented by a qualitative evaluation of
each one of the parameters above, for each one of
the alternatives. At run-time, GLARE presents such
evaluations to physicians, who are completely free
to choose among each one of the alternatives.
As a consequence, at any time during the execution
of a CIG on a patient, we distinguish.
(1) the set of current actions
(2) the set of scheduled actions
(3) the set of candidate actions
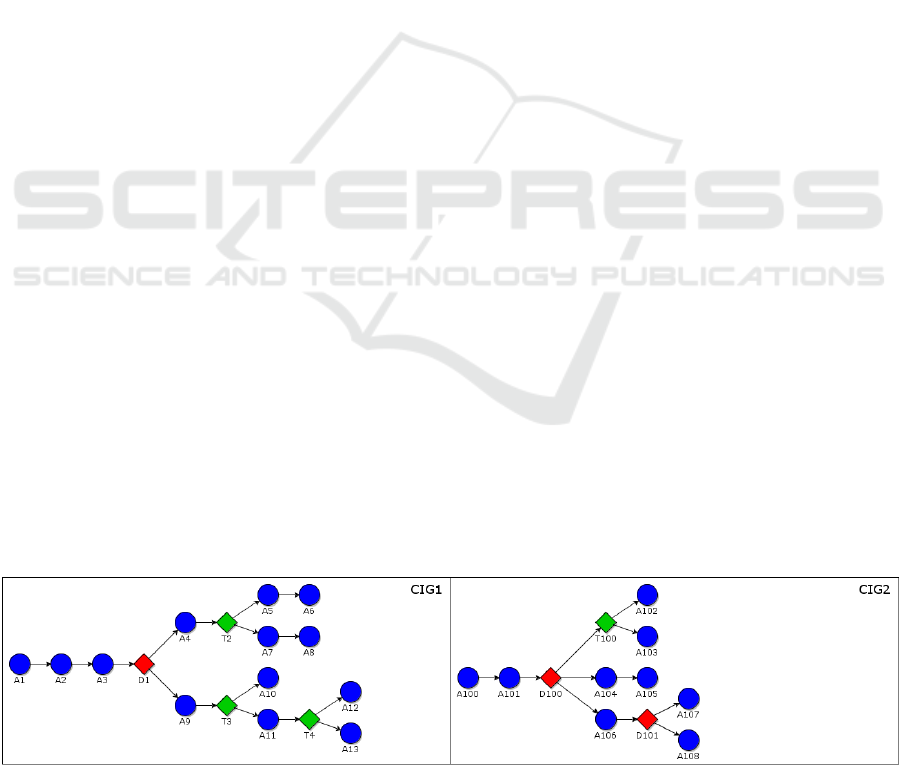
In Figure 1 in the following, we show a simple
example of GLARE CIGs, to exemplify the
definitions. For the sake of generality, we consider
an abstract example, instead of a concrete one. In the
example, round nodes represent work and
pharmacological actions, red diamond represent
diagnostic decisions, green diamonds represent
therapeutic decisions, and arcs represent the control
flow of actions. For the sake of simplicity, we only
consider sequence arcs (so that, at each time, each
CIG has only a current action).
Definition. Current Actions. The action to be
executed next (multiple next actions are possible, in
case of concurrency)
Example. For instance, in our example, we suppose
that the current action in CIG
1
is A
2
.
Definition. Scheduled Actions. Besides the current
action(s), the set of scheduled actions contains the
set of CIG actions which, if no failure or exception
arise, have necessarily to be executed next, and their
time window.
Specifically, the scheduled actions are all those CIG
actions that can be reached through chains of
Figure 1: Example CIGs. Round blue nodes represent work and pharmacological actions, red diamond represent diagnostic
decisions, green diamonds represent therapeutic decisions, and arcs represent the control flow of actions.
HEALTHINF 2019 - 12th International Conference on Health Informatics
502

sequence and constrained arcs starting from the
current actions, until a decision action is reached.
Example. In CIG
1
, if the current action is A
2
, the set
of scheduled actions is {A
2
, A
3
, D
1
}
Defintion. Candidate Actions. The set of candidate
actions contains the set of CIG actions which (if no
failure or exception arise), can possibly be scheduled
for execution, until a new therapeutic decision has to
be taken by physicians.
Candidate actions include all the actions that can be
reached from scheduled actions until a therapeutic
decision has to be taken. The idea is that, while
therapeutic decisions have to be taken by physicians,
diagnostic decisions depends on the status of the
patient. Therefore, the outcome of a future
diagnostic decision cannot be known a-priori, and
physicians may want to consider also the actions that
have to be taken after such decisions.
Example. In CIG
1
, if the current action is A
2
, the set
of candidate actions is {A
4
, T
2
, A
9
, T
3
}
3.3 GLARE Extensions
GLARE supports the execution of multiple CIGs.
For each CIG to be executed on a patient, GLARE
provides physicians with an Executor module
supporting the execution. In our approach to run-
time management of comorbidities, GLARE
executor has been enriched with the possibility of
sending and receiving messages to\from a
Comorbidity Master Module, and to activate
GLARE-SSCPM Interaction Management Module
and Conciliation Module.
In the following we consider a single patient (the
extension to multiple patients is trivial).
3.4 Extensions to the Executor
The modifications to GLARE’s original Executor
module are quite limited: it is extended to
communicate with the Comorbidity Master Module.
In particular, the Executor module sends to the
Comorbidity Master Module a message
(i) when it is created (i.e., when the execution of a
new CIG is started on the patient)
(ii) when the execution of a CIG action is
(successfully) terminated. In case the action is
a decision, also the selected path is sent to the
Comorbidity Master Module.
It receives from the Comorbidity Master Module a
message whenever
(iii) one or more interactions have to be managed
When the Executor receives a message that there are
interactions between scheduled actions, the standard
execution is stopped, until all interactions have been
managed. On the other hand, the treatment of
interactions between candidate actions is not
necessary, since such actions will not necessarily
have to be executed on the patient (their execution
depends on the future status of the patient, after the
execution of the scheduled actions). However, it is
important that physicians are notified soon that such
interactions may have to be faced in a near future.
3.5 Treatment of the Interactions
The management of interactions is performed by the
physicians with the support of GLARE-SSCPM
Interaction Management module. Given an
interaction, such a model provides physicians with
the possibility of choosing the most appropriate
management, and helps them in its application to the
original CIGs. Specifically, a result of the
application of the Interaction Management module,
the physician can see how the original CIGs are
modified when applying the chosen interaction
management operation to the given interaction. Such
a process can be iterated, until one of the possible
management is chosen by the physicians.
Notably, in case more than one interaction has to
be managed, the Conciliation Module is invoked, in
order to check the consistency of the different
modifications to the original CIGs. In case they are
consistent, the CIGs in CIG
pat
are updated with the
selected managements, and the Executor Modules
can re-start execution on the updated CIGs. In case
they are not consistent, physicians are requested to
backtrack to the management of the interactions to
consider alternative management options, until a
consistent set of managements is determined.
3.6 Comorbidity Master Module
A new dedicated module has to be introduced, in
order to support the run-time management of
comorbidities. In the following, we informally
describe it (called Comorbidity Master module).
The Comorbidity Master Module takes in input
(1) the clinical record of the patient
(2) the CIGs currently under execution (indicated
by CIG
pat
henceforth)
(3) the current action in each CIG in CIG
pat
and manages for each CIG
i
in (2), two local
data structures:
(4) the set SA
i
of scheduled actions
(5) the set CA
i
of candidate actions
Run-time Support to Comorbidities in GLARE-SSCPM
503

When the Comorbidity Master Module receives in
input (from the Executor of one of the CIGs) a
message that the execution of an action A
h
in the
CIG CIG
k
in CIG
pat
has terminated, and the action
A
h
is not a decision action, it simply updates the set
SA
k
by deleting A
h
from it.
On the other hand, in cases
(i) it receives in input a message that a new CIG
CIG
k
has been activated on the patient (so that
CIG
k
is added to CIG
pat
)
(ii) it receives in input a message that the execution
of an action A
h
in the CIG CIG
k
has
terminated, and A
h
is a decision action, and
Path
j
has been selected
several operations have to be performed, for the
“run-time” identification and resolution of possible
interactions. In such cases, the Comorbidity Master
Module
1. evaluates the new set SA
i
of scheduled actions
(and their temporal windows)
2. evaluates the new set CA
i
of scheduled actions
(and their temporal windows).
3. Invokes the interaction detection module on the
sets of scheduled actions of the CIGs in CIG
pat
(not considering the decision actions). In case
some interaction is detected, the set INT_sched
of such interactions is sent to the Executors of
the CIGs in CIG
pat
, with the indication that
such interactions occur between scheduled
actions.
4. Invokes the interaction detection module on the
sets of candidate actions of the CIGs in CIG
pat
(not considering the decision actions). In case
some interaction is detected, the set INT_cand
of such interactions is sent to the Executors of
the CIGs in CIG
pat
, with the indication that
such interactions may occur between candidate
actions.
Notably, the detection of interaction is based on the
Knowledge base, and is fully automatic.
In the following, we show two examples of Steps 1
and 2 above. Concrete examples of the management
of CIG interactions have been reported in (Piovesan
and Terenziani, 2015; Piovesan et al., 2018).
Example. Suppose that the CIG
1
is being executed
on patient 1, and that, when A
2
is under execution (is
current), the treatment of a new disease, through the
CIG CIG
2
, is started. The start of the execution of
CIG
2
triggers the Comorbidity Master Manager for
patient 1. CIG
pat1
={CIG
1
,CIG
2
}, and the set of
scheduled and candidate actions are valuated as
follows:
SA
1
={A
2
,A
3
,D
1
}, CA
1
={A
4
,T
2
,A
9
,T
3
}
SA
2
={A
100
,A
101
,D
100
}, CA
1
={T
100
,A
104
,A
105
,A
106
,
D
101
,A
107
,A
108
}
The Interaction Detection module is activated, and
interactions between A
2
,A
3
,A
100
,A
101
(if any) must
be managed by physicians (while the interactions
considering also A
4
, A
9
, A
104
, A
105
,A
106
, A
107
,A
108
(if
any) are pointed out to the physicians.
Example. Suppose that CIG
1
and CIG
2
are being
executed on patient 1, that the current actions in
CIG
1
and CIG
2
are A
3
and D
100
respectively. We
thus have
SA
1
={A
3
,D
1
}, CA
1
={A
4
,T
2
,A
9
,T
3
}
SA
2
={D
100
}, CA
2
={A
104
,A
105
, D
101
, A
106
,A
107
, A
108
}
Suppose then that the execution of decision D
100
give as result the path starting with A
106
. Then,
SA
2
={A
106
,D
101
}, CA
2
={A
107
, A
108
}
4 CONCLUSIONS
The CIG literature has devoted a considerable
attention to the treatment of comorbid patients.
However, the problem of supporting physicians in
the “run-time” detection and management of CIG
interactions has been quite neglected: in short,
(Zamborlini et al., 2014) copes with knowledge-
based interaction detection (but not with CIG
merge), while the other approaches discussed in
Section 1.2 focus on the merge of whole CIGs,
assuming to have a pre-defined set of possible
interactions, and of the way to treat each of them.
In this paper, we propose a comprehensive
approach to run-time comorbidity management,
based on GLARE and GLARE-SSCPM, which (i)
automatically detects the “relevant” parts of the
CIGs (i.e., scheduled and candidate actions), (ii)
automatically detects possible interactions between
them, (iii) supports physicians in the choice of the
most appropriate management of such interactions.
A prototypical implementation of the proposed
approach is under development. Future works
mainly concern a full realization of a tool, and an
extensive experimentation on different concrete
cases of comorbidity.
REFERENCES
Anselma, L., Terenziani, P., Montani, S., Bottrighi, A.,
2006. Towards a comprehensive treatment of
repetitions, periodicity and temporal constraints in
clinical guidelines. Artif. Intell. Med., Temporal
HEALTHINF 2019 - 12th International Conference on Health Informatics
504

Representation and Reasoning in Medicine 38, 171–
195. https://doi.org/10.1016/j.artmed.2006.03.007
Bottrighi, A., Giordano, L., Molino, G., Montani, S.,
Terenziani, P., Torchio, M., 2010. Adopting model
checking techniques for clinical guidelines
verification. Artif. Intell. Med. 48, 1–19.
https://doi.org/10.1016/j.artmed.2009.09.003
Institute of Medicine (US), 1990. Clinical practice
guidelines directions for a new program. National
Academy Press, Washington, D.C.
Montani, S., Terenziani, P., Bottrighi, A., 2005. Exploiting
Decision Theory for Supporting Therapy Selection in
Computerized Clinical Guidelines, in: Miksch, S.,
Hunter, J., Keravnou, E.T. (Eds.), Artificial
Intelligence in Medicine, 10th Conference on
Artificial Intelligence in Medicine, AIME 2005,
Aberdeen, UK, July 23-27, 2005, Proceedings, Lecture
Notes in Computer Science. Springer, pp. 136–140.
https://doi.org/10.1007/11527770_19
Peleg, M., 2013. Computer-interpretable clinical
guidelines: A methodological review. J. Biomed.
Inform. 46, 744–763.
https://doi.org/10.1016/j.jbi.2013.06.009
Piovesan, L., Anselma, L., Terenziani, P., 2015. Temporal
detection of guideline interactions, in: HEALTHINF
2015. Presented at the International Conference on
Health Informatics 2015, Scitepress, pp. 40–50.
https://doi.org/10.5220/0005186300400050
Piovesan, L., Molino, G., Terenziani, P., 2014. Supporting
Physicians in the Detection of the Interactions between
Treatments of Co-Morbid Patients, in: Healthcare
Informatics and Analytics: Emerging Issues and
Trends. IGI Global, pp. 165–193.
Piovesan, L., Terenziani, P., 2016. A Constraint-Based
Approach for the Conciliation of Clinical Guidelines,
in: Advances in Artificial Intelligence - IBERAMIA
2016, LNCS. Presented at the Ibero-American
Conference on Artificial Intelligence, Springer
International Publishing, pp. 77–88.
https://doi.org/10.1007/978-3-319-47955-2_7
Piovesan, L., Terenziani, P., 2015. A Mixed-Initiative
approach to the conciliation of Clinical Guidelines for
comorbid patients, in: KR4HC 2015, Lecture Notes in
Artificial Intelligence. Springer International
Publishing, Pavia, pp. 95–108.
Piovesan, L., Terenziani, P., Molino, G., 2018. GLARE-
SSCPM: an Intelligent System to Support the
Treatment of Comorbid Patients. IEEE Intell. Syst.
https://doi.org/10.1109/MIS.2018.111144734
Riaño, D., Collado, A., 2013. Model-Based Combination
of Treatments for the Management of Chronic
Comorbid Patients, in: Artificial Intelligence in
Medicine. Springer Berlin Heidelberg, Berlin,
Heidelberg, pp. 11–16.
Sánchez-Garzón, I., Fernández-Olivares, J., Onaindía, E.,
Milla, G., Jordán, J., Castejón, P., 2013. A Multi-agent
Planning Approach for the Generation of Personalized
Treatment Plans of Comorbid Patients, in: AIME
2013, Lecture Notes in Computer Science. Springer
Berlin Heidelberg, pp. 23–27.
https://doi.org/10.1007/978-3-642-38326-7_4
Sittig, D.F., Wright, A., Osheroff, J.A., Middleton, B.,
Teich, J.M., Ash, J.S., Campbell, E., Bates, D.W.,
2008. Grand challenges in clinical decision support. J.
Biomed. Inform. 41, 387–392.
https://doi.org/10.1016/j.jbi.2007.09.003
Ten Teije, A., Miksch, S., Lucas, P. (Eds.), 2008.
Computer-based medical guidelines and protocols: a
primer and current trends, Studies in health technology
and informatics. IOS Press, Amsterdam.
Terenziani, P., Molino, G., Torchio, M., 2001. A modular
approach for representing and executing clinical
guidelines. Artif. Intell. Med. 23, 249–276.
https://doi.org/10.1016/S0933-3657(01)00087-2
Terenziani, P., Montani, S., Bottrighi, A., Torchio, M.,
Molino, G., Correndo, G., 2004. A context-adaptable
approach to clinical guidelines. Stud. Health Technol.
Inform. 107, 169–173.
Wilk, S., Michalowski, W., Michalowski, M., Farion, K.,
Hing, M.M., Mohapatra, S., 2013. Mitigation of
adverse interactions in pairs of clinical practice
guidelines using constraint logic programming. J.
Biomed. Inform. 46, 341–353.
https://doi.org/10.1016/j.jbi.2013.01.002
Zamborlini, V., da Silveira, M., Pruski, C., ten Teije, A.,
van Harmelen, F., 2014. Towards a Conceptual Model
for Enhancing Reasoning About Clinical Guidelines,
in: Miksch, S., Riaño, D., ten Teije, A. (Eds.),
Knowledge Representation for Health Care. Springer
International Publishing, Cham, pp. 29–44.
Run-time Support to Comorbidities in GLARE-SSCPM
505
