
Identification for a Large-volume Food-borne Bacteria on a Fully
Integrated Portable Centrifugal Disc
Hau Van Nguyen, Van Dan Nguyen, Eun Yeol Lee and Tae Seok Seo
Department of Chemical Engineering, Kyung Hee University, Yongin 17104, South Korea
Keywords: Centrifugal Microdevice, Portable Genetic Analyser, Loop-mediated Isothermal Amplification, Colorimetric
Detection, Super Absorbent Polymer.
Abstract: Herein, we present a fully integrated portable centrifugal microsystem for multiplex detection of food
poisoning bacteria with a large volume of sample up to 1 mL. The microsystem consists of a portable rotary
genetic analyzer and a fully integrated lab-on-a-disc device. The portable rotary genetic analyzer is equipped
with a couple of heating blocks, a motor and a UV-Vis optical detector. The device was designed with two
units: a 3D printed solution-loading cartridge and a centrifugal microfluidic disc. All the essential solutions
for the LAMP reaction (a sample, a washing, an elution and a LAMP cocktail solution) are stored inside the
cartridge, and orderly released into centrifugal microdevice by a rotation program. Each unit of the device
was designed with 20 reaction chambers for simultaneously detecting 19 kinds of food poisoning bacteria in
one test. To increase the amount of a sample to 1 mL, we incorporated the super absorbent polymer (SAP) in
the waste chamber to absorb the sample and washing solution during the device operation. The whole process
was automatically conducted from bead-based DNA extraction to isothermal DNA amplification by EBT-
mediated LAMP reaction to colorimetric and UV-vis detection of amplicons in 60 min to identify three kinds
of bacteria (Escherichia coli O157:H7, Salmonella Typhimurium, and Vibrio parahaemolyticus).
1 INTRODUCTION
Point-of-care testing (POCT) is recently blooming up
and plays a vital role in supporting immediate
treatment. The central laboratories are equipped with
high cost and automatic diagnostic platform for
highly sensitive, precise and accurate testing.
However, due to the bulkiness of the analytical
instruments, they are not adequate for on-site
diagnostics. On the contrary, the POC testing allows
simple and rapid analysis, which can help the doctor
to make a timely decision on the treatment method for
the patients. Also, the POC testing offers a user-
friendly prototype suitable for the un-trained worker,
minimal operation steps to reduce analysis time,
minimizing sample storage and transportation, and
cost-effective treatment in resource-limited
environments. The POC testing system includes a
paper-based microfluidic device (PPM) (Choi, 2015;
Ye, 2018), a lateral flow assay (LFA) (Deng, 2018;
Takalkar, 2017), a microfluidic device (DuVall,
2017; Zhang, 2017), a miniaturized PCR platform
(Guarnaccia, 2017; Liu, 2017), a smartphone-based
device (Berg, 2015; Priye, 2016; Stedtfeld, 2012;
Wang, 2017). Among these POCT approaches, a
microfluidic device has attracted huge attention over
decades, and, in particular, a centrifugal microfluidic
was considered as a promising candidate for complex
diagnostic purposes. Centrifugal microfluidics have
demonstrated the high fidelity for the unit operation
and integration on a single device such as sample
loading and reagent storage (Stumpf, 2016; van
Oordt, 2013), serial dilution (Kim, 2018), metering,
aliquoting, mixing, and incubation (Jung, 2015; Oh,
2016; Park, 2017), and detection (Andreasen, 2015;
Martin, 2017; Schwemmer, 2016). However, the
major challenge in the centrifugal microdevice is the
low volume of sample pretreatment, which could
affect the limit-of-detection level. In this study, we
proposed a prototype of a fully integrated centrifugal
device for the POC testing, which is capable of
multiplex bacteria detection with a large volume of
the sample up to 1mL. developed for multiplex
bacteria detection in a large volume of the sample up
to 1mL. In addition, this sample-to-answer platform
can fulfill all the requirements for on-site nucleic acid
analysis since the DNA solid-phase extraction by
glass bead, isothermal amplification by LAMP
114
Van Nguyen, H., Nguyen, V., Lee, E. and Seo, T.
Identification for a Large-volume Food-borne Bacteria on a Fully Integrated Portable Centrifugal Disc.
DOI: 10.5220/0007690401140118
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 114-118
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
reaction, and amplicon quantification by a UV-Vis
detector can be performed on the integrated
centrifugal microdevice in a portable genetic analyser
system.
2 EXPERIMENTAL
2.1 Design of the Centrifugal Device
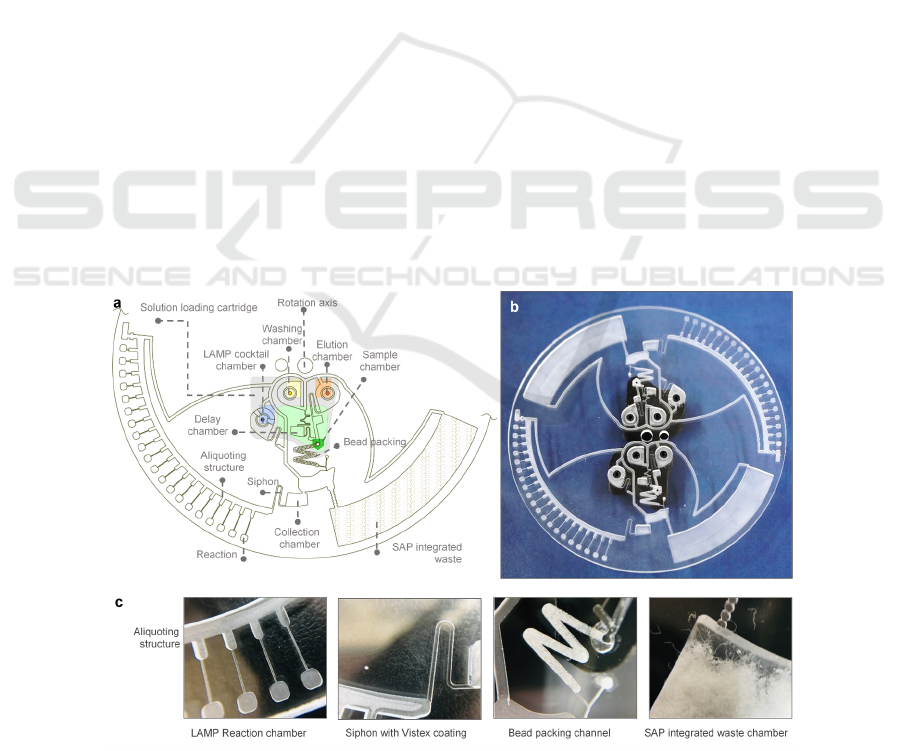
The disc was designed to perform the DNA solid-
phase extraction and high-throughput LAMP assay
with 20 reaction chambers. We proposed the device
with two units: a centrifugal microdevice for DNA
extraction and LAMP reaction, and a 3D-printed
cartridge for solution loading. The centrifugal
microdevice was designed with AutoCAD, and
etched in a 3.0 mm thick poly(methylmethacrylate)
(PMMA) plate using a CNC machine. All the siphon
channels were coated with a hydrophobic reagent,
Vistex 111-50. The positive reaction chamber was
coated with a primer set of the target bacteria. The
waste chamber was integrated with super absorption
polymer (SAP) from baby diaper for utterly absorbing
1 mL sample solution. A pressure sensitive adhesive
(PSA) foil layer was applied to seal the disc. The acid
wash glass bead (150-212 μm, Sigma) was then
packed into the DNA extraction channel. Finally, the
bead-packed channel was incubated in 6M Gu-HCl
for 30 min to enhance the DNA capture capacity.
2.2 Portable Rotary Platform with
UV-Vis Detector
To adapt our system for POC testing, we also
proposed a portable compact and small size rotary
platform for operating the disc. The rotary platform
consists of: (1) a spindle motor, (2) a couple of Minco
heater and (3) a UV-Vis optical detector. The UV-Vis
optical system is composed of a yellow LED 570 nm
and a red LED 650 nm directed toward LAMP
reaction chambers through an optical fiber. A filter
was used for eliminating the interference from excited
light and an aspheric lens for reducing optical
aberrations. The transmittance light intensity was
then measured by a CMOS camera sensor and
converted into relative absorbance.
2.3 Procedure for the on-Chip Genetic
Analysis
Firstly, 1 mL of sample lysis mixture was prepared
containing 500 µL of bacteria sample, 250 µL of AL
buffer (Qiagen, Netherlands), and 250 µL of 6 mM
Gu-HCl (Thermo Fisher Scientific, USA). Sample
lysis mixture, a washing solution (70% Ethanol), an
elution solution (DNase/RNase water), and a
LAMP/EBT cocktail solution were then injected into
the cartridge at the injection hole. An in-house
program automatically performed all the operation
steps including spinning for solution transferring,
Figure 1: (a) Schematic illustration of the integrated centrifugal microdevice. (b) Digital images of the disc. (c) Components
of the microdevice, (i) Aliquoting structure and LAMP reaction chamber, (ii) Siphon channel coated with Vistex, (iii)
Glassbead-packed channel for DNA extraction, and (iv) Super Absorption Polymer (SAP) integrated waste chamber.
Identification for a Large-volume Food-borne Bacteria on a Fully Integrated Portable Centrifugal Disc
115

Figure 2: (a) Digital images of the portable rotary platform. (b) UV-Vis detector for measuring absorbance of reaction
chamber. (c) A couple of Minco heater. (d) Digital images of the portable rotary platform with closed lib. (e) Schematic
illustration of the rotary platform with a centrifugal motor, a couple of Minco heater, and a UV-Vis detector with two light
emitting diode (LED) light source at 640 and 570 nm.
shaking for mixing, heating for proceeding LAMP
reaction, measuring solution absorbance in real-time,
and data production. The absorbance at 640 nm
(Abs640) and 570 nm (Abs570) were recorded at a 5
min interval time during 60 min of LAMP reaction.
The ratio of Abs640 to Abs570 (Abs640/Abs570) was
then calculated. The real-time curve was plotted
between Abs640/Abs570 ratio and time.
3 RESULTS AND DISCUSSION
3.1 Optical Real-Time Sensing LAMP
Reaction Amplicon by in-House
Building System
We recorded the UV-Vis absorption spectrum of the
LAMP mixture before and after LAMP reaction. The
color of LAMP mixture changed from violet to sky
blue during the process of a LAMP reaction with the
change of maximum absorption wavelength from 570
nm to 640 nm, respectively. Therefore, we designed
the UV-Vis detector on the portable rotary platform
with the two LED light source at 570 and 640 nm. We
measured the relative absorbance at 640 nm and 570
nm of negative (NC) and positive (PC) chamber with
5 min interval time during the LAMP reaction. The
NC chamber has no change in the Abs640/Abs570
ratio. In contrast, for the PC chamber, the
Abs640/Abs570 ratio has a change when the LAMP
reaction occurs at 40-50 min and became saturated at
55-60 min. These results are in a good agree-ment
with UV-vis absorption spectrum of the NC and PC.
Therefore, the Abs640/Abs570 ratio could be used as
the criteria to identify a positive result of which
Abs640/Abs570 ratio is higher than 1.0.
3.2 Singleplex and Multiplex Detection
on the Integrated Portable System
The disc was designed for processing parallel 2
samples in one run and in 20 reaction chambers for
each sample. Theoretically, up to 20 kinds of
foodborne pathogens can be simultaneously detected.
In this experiment, we targeted three kinds of bacteria
(E. coli O157:H7, S. Typhimurium and V.
parahaemolyticus) as a model. While no color change
was observed in negative control chambers
(chambers from left 1,5,9,13,17), the rest chambers
with the coated primer for targeting E. coli O157:H7,
S. Typhimurium and V. parahaemolyticus exhibited
color change from purple into sky blue.
The absorbance of 20 chambers was also measured
during the LAMP reaction. The Abs640/570 ratio
after the LAMP reaction shows that all the PC
chambers with sky blue color had an Abs640/570
ratio higher than 1.0, and all the NC chamber with
violet color had an Abs640/570 ratio lower than 1.0.
Consequently, we demonstrated that the proposed
microdevice could simultaneously detect multiple
pathogen targets in 20 reaction chambers.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
116
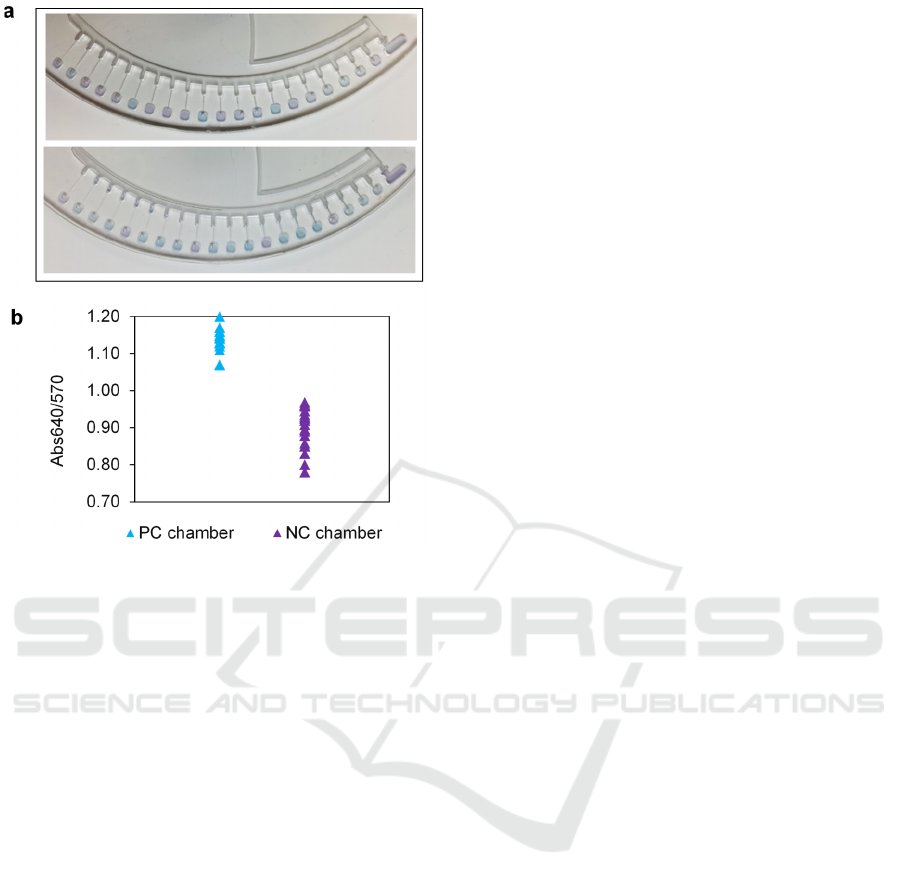
Figure 3: Multiplex detection of foodborne pathogens in
samples based on the prototype device. (a) Colorimetric
detection of single pathogen (E. coli O157:H7). (b)
Colorimetric detection of triple pathogens (E. coli O157:H7,
S. Typhimurium and V. parahaemolyticus). (c) The graph
of Abs640/570 ratio of negative and positive chamber.
4 CONCLUSIONS
We have developed a sample-to-answer disc for
multiplex food poisoning bacteria screening with a
large volume of sample (up to 1 mL). The system was
automatic and small suitable for POC testing. The
disc was designed with the solution-loading
cartridges to accomplish a full automation, and a
specific SAP integrated waste chamber for large
sample volume handling. All experimental processes
of the molecular diagnostics were integrated in a
single device including extraction, amplification,
detection, and data analyzing/reporting. The sample
and other essential solutions for LAMP assay are
loaded into the 3D printed cartridge, and orderly
released into the centrifugal microdevice by a specific
channel design and spinning program. The portable
genetic analyzer provides a user-friendly interface
and a simple operation protocol which is affordable
for less technical training staff.
ACKNOWLEDGEMENTS
This work was supported by the Engineering
Research Center of Excellence Program of Korea
Ministry of Science, ICT & Future Planning
(MSIP)/National Research Foundation of Korea
(NRF) (2014R1A5A1009799) and by a grant of the
Korean Health Technology R&D Project, Ministry of
Health & Welfare, Republic of Korea (grant no.
HI13C1232).
REFERENCES
Andreasen, S. Z., Kwasny, D., Amato, L., Brøgger, A. L.,
Bosco, F. G., Andersen, K. B., Svendsen, W. E.,
Boisen, A., 2015. Integrating electrochemical detection
with centrifugal microfluidics for real-time and fully
automated sample testing. RSC Advances 5(22), 17187-
17193.
Berg, B., Cortazar, B., Tseng, D., Ozkan, H., Feng, S., Wei,
Q., Chan, R.Y.-L., Burbano, J., Farooqui, Q., Lewinski,
M., Di Carlo, D., Garner, O.B., Ozcan, A., 2015.
Cellphone-Based Hand-Held Microplate Reader for
Point-of-Care Testing of Enzyme-Linked
Immunosorbent Assays. ACS Nano 9(8), 7857-7866.
Choi, J. R., Tang, R., Wang, S., Wan Abas, W. A. B.,
Pingguan-Murphy, B., Xu, F., 2015. Paper-based
sample-to-answer molecular diagnostic platform for
point-of-care diagnostics. Biosensors and
Bioelectronics 74, 427-439.
Deng, X., Wang, C., Gao, Y., Li, J., Wen, W., Zhang, X.,
Wang, S., 2018. Applying strand displacement
amplification to quantum dots-based fluorescent lateral
flow assay strips for HIV-DNA detection. Biosensors
and Bioelectronics 105, 211-217.
DuVall, J. A., Le Roux, D., Thompson, B. L., Birch, C.,
Nelson, D. A., Li, J., Mills, D. L., Tsuei, A.-c.,
Ensenberger, M. G., Sprecher, C., Storts, D. R., Root,
B. E., Landers, J. P., 2017. Rapid multiplex DNA
amplification on an inexpensive microdevice for human
identification via short tandem repeat analysis.
Analytica Chimica Acta 980, 41-49.
Guarnaccia, M., Iemmolo, R., Petralia, S., Conoci, S.,
Cavallaro, S., 2017. Miniaturized Real-Time PCR on a
Q3 System for Rapid KRAS Genotyping. Sensors
(Basel, Switzerland) 17(4), 831.
Jung, J. H., Park, B. H., Oh, S. J., Choi, G., Seo, T. S., 2015.
Integrated centrifugal reverse transcriptase loop-
mediated isothermal amplification microdevice for
influenza A virus detection. Biosensors and
Bioelectronics 68, 218-224.
Kim, T.-H., Kim, C.-J., Kim, Y., Cho, Y.-K., 2018.
Centrifugal microfluidic system for a fully automated
N-fold serial dilution. Sensors and Actuators B:
Chemical 256, 310-317.
Liu, W., Zhang, M., Liu, X., Sharma, A., Ding, X., 2017. A
Point-of-Need infrared mediated PCR platform with
Identification for a Large-volume Food-borne Bacteria on a Fully Integrated Portable Centrifugal Disc
117

compatible lateral flow strip for HPV detection.
Biosensors and Bioelectronics 96, 213-219.
Martin, J. W., Nieuwoudt, M. K., Vargas, M. J. T., Bodley,
O.L.C., Yohendiran, T. S., Oosterbeek, R. N.,
Williams, D. E., Cather Simpson, M., 2017. Raman on
a disc: high-quality Raman spectroscopy in an open
channel on a centrifugal microfluidic disc. Analyst
142(10), 1682-1688.
Oh, S. J., Park, B. H., Choi, G., Seo, J. H., Jung, J. H., Choi,
J. S., Kim, D. H., Seo, T. S., 2016. Fully automated and
colorimetric foodborne pathogen detection on an
integrated centrifugal microfluidic device. Lab on a
Chip 16(10), 1917-1926.
Park, B. H., Oh, S. J., Jung, J. H., Choi, G., Seo, J. H., Kim,
D. H., Lee, E.Y., Seo, T. S., 2017. An integrated rotary
microfluidic system with DNA extraction, loop-
mediated isothermal amplification, and lateral flow
strip based detection for point-of-care pathogen
diagnostics. Biosensors and Bioelectronics 91, 334-
340.
Priye, A., Wong, S., Bi, Y., Carpio, M., Chang, J., Coen,
M., Cope, D., Harris, J., Johnson, J., Keller, A., Lim,
R., Lu, S., Millard, A., Pangelinan, A., Patel, N., Smith,
L., Chan, K., Ugaz, V.M., 2016. Lab-on-a-Drone:
Toward Pinpoint Deployment of Smartphone-Enabled
Nucleic Acid-Based Diagnostics for Mobile Health
Care. Analytical Chemistry 88(9), 4651-4660.
Schwemmer, F., Blanchet, C.E., Spilotros, A., Kosse, D.,
Zehnle, S., Mertens, H.D.T., Graewert, M.A., Rössle,
M., Paust, N., Svergun, D.I., von Stetten, F., Zengerle,
R., Mark, D., 2016. LabDisk for SAXS: a centrifugal
microfluidic sample preparation platform for small-
angle X-ray scattering. Lab on a Chip 16(7), 1161-
1170.
Stedtfeld, R. D., Tourlousse, D. M., Seyrig, G., Stedtfeld,
T. M., Kronlein, M., Price, S., Ahmad, F., Gulari, E.,
Tiedje, J. M., Hashsham, S. A., 2012. Gene-Z: a device
for point of care genetic testing using a smartphone. Lab
on a Chip 12(8), 1454-1462.
Stumpf, F., Schwemmer, F., Hutzenlaub, T., Baumann, D.,
Strohmeier, O., Dingemanns, G., Simons, G., Sager, C.,
Plobner, L., von Stetten, F., Zengerle, R., Mark, D.,
2016. LabDisk with complete reagent prestorage for
sample-to-answer nucleic acid based detection of
respiratory pathogens verified with influenza A H3N2
virus. Lab on a Chip 16(1), 199-207.
Takalkar, S., Baryeh, K., Liu, G., 2017. Fluorescent carbon
nanoparticle-based lateral flow biosensor for
ultrasensitive detection of DNA. Biosensors and
Bioelectronics 98, 147-154.
Wang, K., Liang, R., Chen, H., Lu, S., Jia, S., Wang, W.,
2017. A microfluidic immunoassay system on a
centrifugal platform. Sensors and Actuators B:
Chemical 251, 242-249.
Zhang, L., Ding, B., Chen, Q., Feng, Q., Lin, L., Sun, J.,
2017. Point-of-care-testing of nucleic acids by
microfluidics. TrAC Trends in Analytical Chemistry 94,
106-116.
BIODEVICES 2019 - 12th International Conference on Biomedical Electronics and Devices
118
