
H-Links: Supporting Physicians with Objective Pain Monitoring for
the Comfort of Patients at Homes
Safae Moustakim
1
, De Jonckheere Julien
2
, Anna Holubová
3
, Aditi Shenoy
4
,
Daniela Carsch
5
and Jacques Battaglia
6
1
Institut Supérieur d’Ingénieurs de Franche Comté, Univ. Bourgogne Franche-Comté, 25030 Besançon cedex, France
2
CIT 1403 « Biocapteurs et eSanté », CHU de Lille, 59000 Lille, France
3
Czech Technical University in Prague, Faculty of Biomedical Engineering, 10000-199 99 Prague, Czech Republic
4
Health Informatics Department, Karolinska Institute, 171 77 Solna, Sweden
5
Leibniz-Institut für Polymerforschung, 01067 Dresden, Germany
6
Université Pierre et Marie Curie, 75000 Paris, France
Keywords: Telemedicine, mHealth, Pain Management, Pain Monitoring, Ambulatory Care, Pediatric Surgery.
Abstract: The following study focus on pain management during post-operative surgery treatment, it describes a
solution for measuring the pain level for pediatric surgery patients at home since hospital care is trending
towards ambulatory care. This solution can decrease the time in pain and optimize dosing of drug intake,
and thus, improve the effectiveness of the treatment, reduce the time of post-surgery recovery and reduce
the risk of chronic pain development. This can, in a global way, increase the overall quality of life of both
patients and their family members. From the parent’s point of view, better acceptance of ambulatory surgery
can be expected together with reduced stress caused by a fear of pain management at home. From the
hospital’s point of view, we expect an increase in the rate of ambulatory surgery, and thus, an increase of
beds availability.
1 INTRODUCTION
In the last couple of years, the hospital care is
trending towards the ambulatory care because of the
hospital charges for patient staying in hospital after
surgery and the bed availability. Indeed, for many
years, the number of hospitals beds available across
the EU has decreased : available beds fell from 2.93
million in 2004 to 2.65 million by 2014, a relative
decrease of 9.6% while the number of beds per 100
000 inhabitants fell from 592 in 2004 to 521 in
2014, a decline of 12% with the EU’s population
growth. Besides, the hospital charges for a patient in
ambulatory care is lesser than the one for a patient in
non-ambulatory care. For example, the patient
having a unicompartmental knee arthroplasty in
ambulatory care is charged $20,500 less than the
patient that is not and who has to pay in average
$46,845. Moreover, the average reimbursement was
55% of charges, or $25,550 for the patient staying in
hospital while it was 47%, or $12,370 for the patient
in ambulatory care (Richter, 2017). But this
tendency gives new challenges. It implies that all the
time that nurses and physicians used to spend with
patients face-to-face for monitoring or guiding them
will lessen, and also that post-operative care at home
should become more usual.In this context, the
communication between patient and physicians
worsen beyond what it already was (Kyle, 2014).
For example, according to a AAOS (American
Academy of Orthopaedic Surgeons) survey, 75% of
the orthopaedic surgeons believed that they
communicated satisfactorily with their patients but
only 21% of the patients reported satisfactory
communication with their doctors. Additionally, the
pain for patient will also worsen since acute pain is
followed by chronic postoperative pain for 10 to
50% of the patients, pain cannot be measured
objectively and there is no standard for feeling pain
and administering appropriate amounts of drugs
(Chou, 2016).
2 STATE OF THE ART
The presented solution is aiming to support post‐
operative surgery treatment in general and for the
first step measuring the pain level for pediatric
Moustakim, S., Julien, D., Holubová, A., Shenoy, A., Carsch, D. and Battaglia, J.
H-Links: Supporting Physicians with Objective Pain Monitoring for the Comfort of Patients at Homes.
DOI: 10.5220/0007696106370644
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 637-644
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
637

surgery patients. After Surgery, depending on kind
of surgery, the health care professional will regularly
check the health status of the patient (usually three
times a day). This examination is usually paper
based evaluation of questionnaires or simple
patient ́s observation. When the patients are sent
home, the health care professionals will inform the
patients about the medical prescriptions and arrange
additional appointments to check on the healing
process and to detect possible complications that
could occur. Typically, the nurse will call the patient
regularly and ask about the pain level. In hospitals,
the post‐operative pain level is measured usually by
observation and questionnaires.
2.1 Market Positioning
Automatic physiological pain measurement systems
exist but are exclusively dedicated to pain evaluation
of patients during surgery under general anesthesia
or in intensive care unit (Jeanne, 2014, Broucqsault
Dédrie, 2016). These devices are based on
measurement of heart rate variability, skin
conductivity or pupillary dilatation (De jonckheere,
2015). With respect to the telemonitoring systems
used for post ‐ surgery care, there are already
different solutions on the market available to
connect doctors and patients before and after surgery
(Yoon, 2016). The solutions differ with respect to
the field of activity, the measure of pain level,
hardware integration, degree of doctor interaction
and targeted patients. Examples of competitive
solutions in comparison to the project idea are
SeamlessMD or Kardia. One identified solution,
which addresses this patient group, is “Surgery
Connect”. However, this service focuses only on
informing the parents about the process of surgery.
There is no existing solution on the market for
objective pain assessment, with additional
physiological information from hardware devices for
patients at home after surgery. Products that measure
the pain level rely on information provided by the
patients only. However, at the University Hospital in
Lille the ANI monitoring system used for pain level
measurement in unconscious patients during surgery
has been already tested also on conscious patients
during the first 2 hours right after surgery, and thus,
approved as capable of measuring on conscious
patients as well (Jeanne, 2014). Therefore, the
unique selling proposition of the project idea
addresses the integration of hardware information
together with information from the patient. The
project idea focuses in the first step on children
which undergo surgery.
2.2 Market Potential
Different trends, like the encouragement by
government bodies for digital technology in
healthcare or increasing awareness of mobile based
medical devices support the annual growth of the
mobile health market (CAGR 32.3% until 2025),
which was 33.59 billion dollar in 2018 worldwide.
The market can be segmented by technology
(Telehealthcare, Telecare, Telehealth, mHealth,
Health analytics, Digital health system) but the
project idea includes technologies from many
segments and therefore the number of all patients
undergoing a surgery as well as patients under 14
years in Europe was taken into account to get a
clearer idea of the market. The countries with the
most surgeries performed per year in Europe in
million are 1. Germany (16.8 | 1.4) 2. UK (7.9 | 1.4)
3. France (5.0 | 0.9). To estimate the market volume,
we assumed to collaborate with three hospitals in
France for the first year, 5 in France and one each in
Germany and UK for the second year and for the last
year with 10 hospitals in each country. We identified
the average surgeries performed each year per
hospital and linked them with the cost savings we
could archive with our product. The cost savings are
based on the results of a survey, which indicates a
saving of three days of hospital stay with a mobile
application for postoperative monitoring after
discharge. We assume to archive an acceptance level
for each hospital of 70% (based on results of
SurgeryConnect).
3 SOLUTION SPECIFICATIONS
In order to address the problem with inability to
measure pain objectively among post-operative
surgery patients, the team has identified medical
device management systems from medical device
companies which could provide the software with
suitable physiological signals required to identify
pain level for physicians, who can then monitor
patient ́s health state remotely. Our team would
launch the first product module focused on pain
management with eventually spanning out to other
disease areas and suitable management of those
diseases.
The solution will enable to:
Provide hospitals sufficient data (both subjective
and objective) related to pain management online
from patients´ homes online,
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
638
Use notifications and reminders for inter and
intra-day situations where needed,
Reduce the amount of manually register data
needed to be provided by parents hanks to the
connected wearable technology,
Facilitate communication between
patients/parents and hospital staff,
Facilitate dosing of pain killers,
Discover pain level variability both during a day
and during the night also for further research
purposes.
We suspect that from the patient ́s point of view, this
solution can decrease the time in pain and optimize
dosing of drug intake, and thus, improve
effectiveness of the treatment, reduce time of post-
surgery recovery and reduce risk of chronic pain
development. This can, in global way, increase of
overall quality of life of both patients and their
family members. From the parent ́s point of view,
better acceptance of ambulatory surgery can be
expected together with reduced stress caused by a
fear of pain management at home. From the hospital
point of view we expect increase of the rate of
ambulatory surgery, and thus, increase of beds
available for patients. In addition, better awareness
of surgeons (and also other HCP) about patient ́s
post-surgery health state can bring them relief and
reduce time spend on face to face consultations, if
these can be replaced by telemedicine.
4 PROPOSAL OF INNOVATIVE
PRODUCT
4.1 Overview
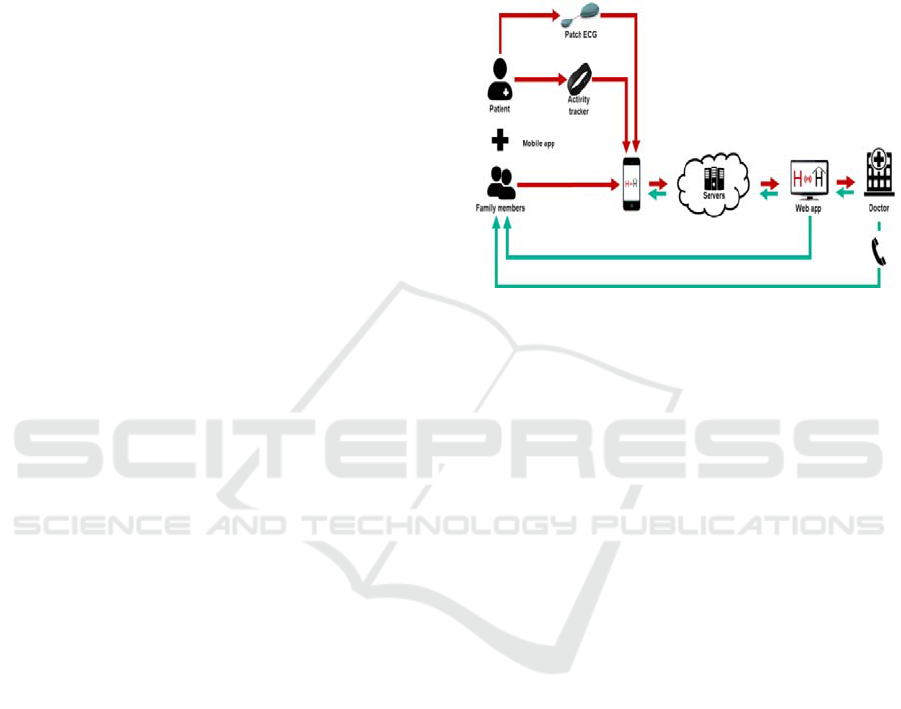
Our solution will serve as a home-based
telemonitoring platform used primarily for pain
management of children after ambulatory surgery.
The platform will enable the patients (or their
parents, respectively) to collect data needed for
proper control of the post-operative care, i.e.
manually registered information about patient ́s pain
and daily activities, and data automatically collected
through wearables, i.e. patch ECG monitor and
activity tracker (Ooley, 2018, Evenson, 2015), for
objective evaluation of pain level. In addition,
connection with a hospital through a mobile and web
application will be provided. The mobile app will
serve also as a bridge for the data measured by
wearable technology and transferred to a secured
server (Hassan, 2018; Boulos, 2014).
In that place, the data can be processed,
analyzed, and further displayed with a specific
interface to both the healthcare provider and the
patient/family member via the mobile or web
application. Notifications and reminders will be
included. Fig. 1 illustrates the whole concept
proposal.
Figure 1: System workflow and components diagram.
4.2 Connected Technology
The main wearable technology includes a patch
ECG monitor used (in connection with a pain
management system algorithm) for the objective
evaluation of patient´s pain, and an activity tracker
for automatic evaluation of patient´s intensity of
physical activity throughout a day and their sleeping
efficiency.
4.3 Data Collection and Analysis
For the patient's monitoring, both manually entered
and automatically registered data via wearable
sensors will be tracked.
Manually registered data contain the following
parameters:
Subjective pain evaluation (using standardized
scales for kids and adolescents)
Mood information
Acute pain detection needed to be immediately
suppressed by medication
Type of activity the patient performs during a
day and its duration (resting, playing, sleeping,
eating, walking)
Medication intake (dose, datetime)
Automatically registered data are displayed in Tab 1
together with the explanation of their role in pain
management. Based upon the results from the
clinical study during which all the parameters are to
H-Links: Supporting Physicians with Objective Pain Monitoring for the Comfort of Patients at Homes
639

be measured, the final composition of the sensors
needed to be tracked will be proposed.
Table 1: List of automatically collected data using
connected devices.
Parameter
Possible
device
Role in homecare
pain management
Heart rate
-Patch holter
-SmartWatch (if
the accuracy is
proved to be
sufficient)
Pain level monitoring
via pain management
systems ‘algorithms
Physical
activity
-Activity tracker
Automatic evaluation
of intensity of
patient´s daily activity
and sleep efficiency
Among to the data provided by healthcare
provide, the following informations are included:
Type of drug administration and its timing,
Drug prescription,
Visit appointments scheduling,
Notifications and comments on treatment.
The data transmitted to the server will be processed
using certain algorithms and displayed in form of
graphs and tables to the doctor via web portal. The
doctor will have the opportunity to go from the
general overview on the data collected into more
detailed characteristics.
Values out of the target range will be highlighted
and alarms implemented. Download of a report
(PDF) for upload into EMR capable of importing
PDF documents, or raw data (CSV) for further
analysis will be also an option for the web user.
4.4 Communication
The healthcare provider will be able to send a
notification to the patient through the connected web
app. Depending on the importance of the message,
three levels of notifications can be used (urgent
message, treatment change recommendation, general
information), whereas the notifications are
differentiated by a specific colour both on the
mobile app and web app the patient/parent can
connect to, using their unique credentials.
In case of emergency situation which needs to be
treated immediately, direct call can be performed by
both either the hospital or the parent.
4.5 Interoperability and Third-party
Integration
The data from wearable devices will be transmitted
to the server either directly (in case the
communication protocol is available) or through API
of given company. To create a secure and
interoperable health data exchange, the Continua
Design Guidelines will be followed. Following the
standards, the FHIR configuration will be
implemented for potential future connection with an
EMR system of a hospital.
4.6 Data Security
Three main principles will be followed to ensure
protection of the data: confidentiality, integrity and
availability.
5 PROJECT RISK ASSESSMENT
External Risks:
External risks include all influence which is
resulting from the environmental influence. One risk
is the development of the competitive landscape
during the project time and new technical solutions.
To address this risks a constant market analysis will
be implemented, to allow a imitate response and
agile adjustments in the project objectives, if
necessary.
Internal Risks:
The project requires certain competences to address
e.g. certification regulations but also knowledge
about country specific health care regulations and
systems. During the project period the specific
requirements will be identified and addressed by
external consulting services or the employment of
the suitable positions.
Financial Risks:
In the first time period to the breakeven point the
development of finance strategies for the project will
be necessary. This includes the identification and
implementation of the relevant financing options.
- Hospitals will not be willing to participate on the
expenses for the system
- Medical insurances will not participate on the
cost reimbursement
6 DEVELOPMENT PLAN
The development plan consists of 6 work packages
(WPs) summarized below, whereas each WP
consists of objectives, description and deliverables.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
640

WP1: Project Management
The management part goes across the whole project
lifecycle period and is responsible for leading the
whole project and its team members and
achievement of each particular WP to be done as
required and on time. In addition, financial and
administrative roles are included into this process, as
a subcategories.
To ensure seamless development of the project,
weekly report of the project monitoring evaluation
and control will be done.
WP2: Definition of User Requirements,
Technical, Clinical and Ergonomic Evaluations
Using a user-centred design approach, the user needs
would be evaluated using focus groups and creation
of personas. This would be done collectively for
each of the stakeholders of this application. Since
current standards of pain measurement are not well
defined, a proof of concept with a software
prototype would be designed for stakeholder’s
feedback. The application would initially be
developed for an Android OS to be freely available
for download for patients and their families. The
application designed for the healthcare professionals
would be connected with the hospital systems which
would enable a single e-health system.
WP3: Preclinical Study
During the analysis of suitable sensors that could be
used for monitoring of given parameter, the
following factors will be taken into consideration:
compatibility of medical device with software
application, quality of signal, wireless data
transmission, size of the device, it´s price and
duration of battery used to charge the device.
To get sufficient data for development of
algorithm, preclinical study will be performed on
20-30 subjects (children in post-operative state, age
of 2-8 years) for 2 days. In these patients heart rate
variability and physical activity will be monitored
via selected wearable devices.
In parallel to the automatic data transfer from the
wearables, patient´s manually registered information
about the pain and daily activities will be registered
using usual tool.
WP4: Development
First step of the development stage the software
architecture will be performed. Based upon the data
obtained from WP2 the design, concrete
functionalities and final interface of both the mobile
and web application will be made, before conducting
the validation phase of clinical study.
As a first concept of the telemonitoring system
we will implement the software part only, without
the data evaluation obtained from connected
devices.
After the validation of the accuracy of the
devices and the final device composition is resolved,
the upgrade version of the system, including the
hardware part of the project, will be implemented,
processed through the regulatory, and marketed in
parallel to the software solution already approved.
The algorithm used for automatic evaluation of
pain level will be developed in cooperation with the
company producing the pain management system.
The automatic evaluation of physical activity level
will be created in accordance to the data analysis
made after the preclinical study.
During the whole development period, we will
also actively consult particular issues with software
experts and regulatory affairs to ensure the final
product will meet all the essential requirements.
Requirements for quality management system will
be followed based upon the ISO 9001 standard.
Each update of the software versions will be
tested based on the testing processes described
within the Risk assessment section. As a final
control of the software testing phase, the verification
and validation is performed based on the IEC 62304.
WP5: Regulatory Assessment
Certification and Standards
The commercial product we create would have to be
classified as Class IIa medical device (COUNCIL
DIRECTIVE 93/42/EEC, Annex IX, Rule 10).
After receiving clinical validation, we would
improve the functionalities of our application to
produce automatic recommendation of medication
dosage to assist decision making based on multi
sensor data. This might require us to move to Class
IIb medical device certification (Directive
93/42/EEC, Annex IX, Rule 11).
Risk Analysis
For the methodology, the FMEA-based method is
used and ISO 13485 is applied.
The protection of personal data will be treated
based upon the EU legal framework, GDPR
(General Data Protection Regulation).
Identification of characteristics of a medical
device related to a usage that could have an impact
on safety are treated based upon the international
standard IEC 62366.
H-Links: Supporting Physicians with Objective Pain Monitoring for the Comfort of Patients at Homes
641

WP6: Validation and Usability Studies
A series of formative and summative evaluations
will be conducted for this project. The formative
evaluations planned for this prototype were selected
from ‘Product planning methods’ and ‘User research
and validation’ method. For analysing how the
system was designed, evaluating which aspects of
the system are lacking and identifying usability
problems, a heuristic evaluation will be used.
Subsequently, focus groups and observation
studies will help us identify the problems with
usability of the application for the physicians and the
patients before the first prototype is created and also
during the testing phase of the system.
For the technical part of the solution, validation
and verification will be performed during the last
phase of the development period.
As a part of the validation, clinical study will be
performed.
Deliverables:
Proof that our product can be used as intended,
brings benefit to the end user as expected and is ease
to use enough to encourage the end user for its long-
term use.
7 CLINICAL STUDY
The clinical study aim to evaluate the usability and
effectiveness of the whole system and the workflow
process, the main evaluations of this study are:
Comparison of the manually registered pain level
data with the automatic data obtained through the
wearables
Patient level of pain
Treatment adaptation
Physicians and parents feedback
Family quality of life
7.1 Criteria for Acceptance
- Age: 2-8 years old
- Orthopedic post-surgery
- No cardiac disease
- No neuropathic disorder
7.2 Clinical Study Performance
2 arms of patients (50-60 children, 2-8 years of age)
who undergo an ambulatory orthopedic surgery will
be recruited to the study, i.e. intervention and
control group.
Intervention Group:
The intervention group will get the sensors to
measure HR and physical activity. All the data will
be automatically transmitted wirelessly to the
connected mobile app, synchronized with the server
and displayed to a doctor in a hospital. In addition,
parents of the kids will provide manually registered
information to the app.
Both the parents and the doctor will have an
access to the data collected through a mobile/web
app.
The doctor will be able to send a notification to
the patient based upon the data obtained through the
connected web app.
The doctor will check the data approx. 30 mins
before patient ́s scheduled medication, and in
case of the need of the dosage change, he will
send the patient a message with the recommended
dosage.
The phone call can be used in case of emergency
situation.
Control Group:
As a control group the patients ́ results from the
preliminary study will be used. In case the treatment
processes will be changed until the beginning of the
clinical study the new arm of control group will be
performed.
The control group will be following the standard
healthcare methods used for ambulatory surgery.
The parents will follow the doctor's medications
prescriptions and will be able to contact a nurse 2
times a day to check the patient's health state. Paper-
based questionnaires and information about patient ́s
daily activities will be provided by the parents and
consulted at the face to face consultation with the
doctor.
8 BUSINESS PERSPECTIVE AND
ECONOMIC MODEL
The business model involves the software
application for patients and doctors, which is
connected to the database and also includes
information from wearable devices. The market
entrance strategy focuses primarily on the promotion
of the software to enhance the connection between
healthcare professionals and patients based on
subjective data.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
642

For the market entrance of the business model
the end user group will be parents, whose children
are under 14 years and underwent surgery.
Additionally to subjective information the main
hardware information will be an objective pain
measurement, which is already used during surgery.
For future portfolio-expansion different solutions in
different field of surgery and specifically chronic
pain monitoring will be considered. For the
expansion of the field of areas additional hardware
devices could be implemented and the related
information included into the database.
The main targeted customer groups will be
hospitals and health systems, where the biggest
benefit will be generated due to cost savings
regarding the reduce of the length of stay in
hospitals by around three days. There also exists
evidence, showing that 110 clinic spots became
available due to monitored patients at home after
surgery. Based on the saving of three hospital stays
per day a potential savings for Germany (1 billion
€), France (450 million €), and UK (3.5 billion €)
could be calculated. The main business core activity
will be to develop the software application but also
to manage the data transfer and storage. To process
and analyse the received data different algorithm
will be applied, which will be licensed. For the
provision of the hardware devices corporations with
manufacture of already existing medical device
solutions on the market will be targeted.
The marketing strategy will primary rely on
doctors as influencers. Therefore, the main efforts of
communication will target this group. Studies, which
quantify the benefits of the project solutions, will
have a high priority to raise the awareness level and
building trust. Nevertheless, the patients will be the
user to decide to download the software and will
therefore have a direct influence of the selling
outcome.
The most important geographical markets where
identified according to the number of surgeries
performed per year in Europe. As a first stage the for
the market launch the first customer will be the
leading hospitals in France (e.g. Centre Hospitalier
Régional Universitaire de Lille). For the following
year the market entrance to one hospital each in
Germany and France will be addressed. After
showing evident of the quantified data for the
financial benefit of the products health systems (e.g.
APICIL in France) can be addressed to cover the
costs. As a last step and long-time goal the data shall
be provided to research institutes and companies.
9 CONCLUSIONS
Opening the doors for both subjective and objective
evaluation of pain in homecare enables us to collect
big data related to pain management and explore our
solution to other healthcare sectors. The data
collected from both home and hospital environment
can give us the opportunity to explore our solution
and involve machine learning algorithms enabling to
provide better decision support and make some so
far manual processes fully automatic (e.g. automatic
recommendation of drug dose adjustment). This
could significantly reduce time spent on data
analysis on the side of HCP.
Healthcare providers can profit from the data in
order to proof their high quality and efficient
treatment practices. Researchers can get an
opportunity to discover new methods for pain
management treatment, and manufacturers can
obtain evaluation of effectiveness of their products
and tips for their further improvement.
Proving that the objective pain evaluation can
work for treatment of post-surgery homecare of
children, our future aim is to explore the solution to
adults -considering software adjustment due to a bit
different treatment procedures- and moreover, focus
on its potential use also in chronic pain
management, which represents a huge market
opportunities.
ACKNOWLEDGEMENTS
This work was supported by The EIT Health
Summer School CLINMED, INSERM CIC- IT 1403
of Lille, Bourgogne Franche-Comté University,
Karolinska Institute and Pierre and Marie Curie
University and Charles University.
The authors would like to thank the surgery
department at the University Hospital of Lille, and
Mdoloris Medical Systems based in Lille, France for
their collaboration.
REFERENCES
Boulos, M.N.K., Brewer, A.C., Karimkhani, C., Buller,
D.B., Dellavalle, R.P. Mobile medical and health apps:
state of the art, concerns, regulatory control and
certification. Online. Journal of Public Health
Informatics. 2014;5(3):229. doi:10.5210/ojphi.v5i3.
4814.
Broucqsault-Dédrie, C., De jonckheere, J., Jeanne, M.,
Nseir, S. Measurment of heart rate variability to assess
H-Links: Supporting Physicians with Objective Pain Monitoring for the Comfort of Patients at Homes
643

pain in sedated critically ill patients: A prospective
observational study. PloS One. 2016 Jan 25;11(1).
Chou, R., Debra, B. Gordon, Oscar A. et al. Management
of Postoperative Pain: A Clinical Practice Guideline
From the American Pain Society, the American
Society of Regional Anesthesia and Pain Medicine,
and the American Society of Anesthesiologists'
Committee on Regional Anesthesia, Executive
Committee, and Administrative Council. The Journal
of Pain [online]. 2016, 17(2), 131-157.
De jonckheere J., Bonhomme, V., Jeanne, M., Boselli, E.,
Gruenewald, M., Logier, R., Richebé P. Physiological
signal processing for individualized anti-nociception
management during general anesthesia: a Review.
Yearb Med Inform. 2015 Aug 13;10(1):95-101.
Hassan, A. Aziz: Integration of Wearable Technologies
into Patient's Electronic Medical Records. Quality in
Primary Care, 2018. Available from:
http://primarycare.imedpub.com/integration-of-wearab
le-technologies-into- patientselectronic-medical-record
s.php?aid=11118
Jeanne, M., Delecroix, M., De jonckheere, J., Keribedj A,
Logier, R, Tavernier B. Variations of the Analgesia
Nociception Index During Propofol Anesthesia for
Total Knee Replacement. Clin J Pain. 2014
Dec;30(12):1084-8.
Kyle, S., Shaw, D. Doctor–patient communication, patient
knowledge and health literacy: how difficult can it all
be?. The Bulletin of the Royal College of Surgeons of
England [online]. 2014, 96(6), e9-e13 [cit. 2018-08-
30]. DOI: 10.1308/rcsbull.2014.96.6.e9. ISSN 1473-
6357.
Richter, D. L., Diduch, D. R. (2017). Cost Comparison of
Outpatient Versus Inpatient Unicompartmental Knee
Arthroplasty. Orthopaedic Journal of Sports Medicine,
5(3), 2325967117694352. http://doi.org/10.1177/
2325967117694352
Yoon, S. et al. A Flexible and Wearable Human Stress
Monitoring Patch. Sci. Rep. 6, 23468; doi:
10.1038/srep23468 (2016).
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
644
