
Empowering Translation of New Ideas - A EIT Health ClinMed
Summer School Overview
Sofia Ribeiro
1,2*
, Mariachiara Ricci
3*
, Albert Von Der Lieth
4*
, Yves Bayon
1
, Dimitrios I. Zeugolis
2,5
,
Sylvia Pelayo
6
, Isabelle Marque
7
and Lionel Pazart
8
1
Medtronic, Sofradim Production, Trevoux, France
2
Regenerative, Modular and Developmental Engineering Laboratory (REMODEL),
National University of Ireland Galway (NUI Galway), Galway, Ireland
3
Department of Electronic Engineering, University of Rome “Tor Vergata” (Rome), Rome, Italy
4
Medical Center Hamburg-Eppendorf (UKE), University of Hamburg, Germany
5
Science Foundation Ireland (SFI), Centre for Research in Medical Devices (CÚRAM),
National University of Ireland Galway (NUI Galway), Ireland
6
Univ. Lille, INSERM, CHU Lille, CIC-IT 1403 - Centre d'Investigation Clinique, EA 2694, F-59000 Lille, France
7
Inserm CIC 1406, F-38000 Grenoble, France
8
Inserm CIC1431, University Hospital of Besançon, EA481 - Integrative and Clinical Neuroscience Laboratory,
University of Burgundy-Franche Comte, France
Keywords: ClinMed Summer School, Innovation by Design, EIT Health, Medical Device, Training.
Abstract: Translational research training is crucial to convert academic research ideas into efficient real-life solutions.
In this paper a summer school supported by EIT Health is presented. Its main goal is to integrate clinical
knowledge in the development of new medical devices, from ideas to post-market approval, in the clinics.
Students were immersed in clinical centres where they had close contacts and engaged discussions with
clinicians and patients to identify and assimilate clinical unmet needs. From this immersive stage resulted
innovative solutions that were further investigated with the support of plenary lectures and by interaction with
experts of the medical field, from clinicians to Medtech company representatives. This experience proved to
have a positive impact on the student’s understanding of the clinical development life cycle from research
findings or new ideas into medical devices.
1 INTRODUCTION
Despite the ground-breaking innovations that have
been made in many clinical indications less than 5 %
of all medical findings made in academia are
translated into commercially available solutions, such
as new medication, diagnostics or devices. And with
each passing year the gap between the biomedical
research and the clinical applications fields increase
(Gehr and Garner, 2016). The reason for the low rate
of translation comes down to the struggle to transform
innovative ideas from publicly founded academic
research into commercially available products
manufactured by the industry (Duda et al., 2014). A
medical device must be conceptually
1
designed to
*
These authors contributed equally to this work.
satisfy a real clinical need identified by end-users in
the field. Its product development must be aligned
with the market expectations and be realistically
designed, producing a scalable, manufactory robust,
cost-effective and user-friendly product while
fulfilling its intended clinical function (De Pieri et al.,
2018).
While clinicians and academic researchers have their
competence in identifying clinical needs and finding
conceptual and technical approaches, companies have
an established experience in product development,
large clinical trials, regulations and manufacturing.
Already at this background collaborations and
exchanges of knowledge between the private and
public stakeholders seem to be reasonable and
Ribeiro, S., Ricci, M., Von Der Lieth, A., Bayon, Y., Zeugolis, D., Pelayo, S., Marque, I. and Pazart, L.
Empowering Translation of New Ideas - A EIT Health ClinMed Summer School Overview.
DOI: 10.5220/0007696606030610
In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2019), pages 603-610
ISBN: 978-989-758-353-7
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
603
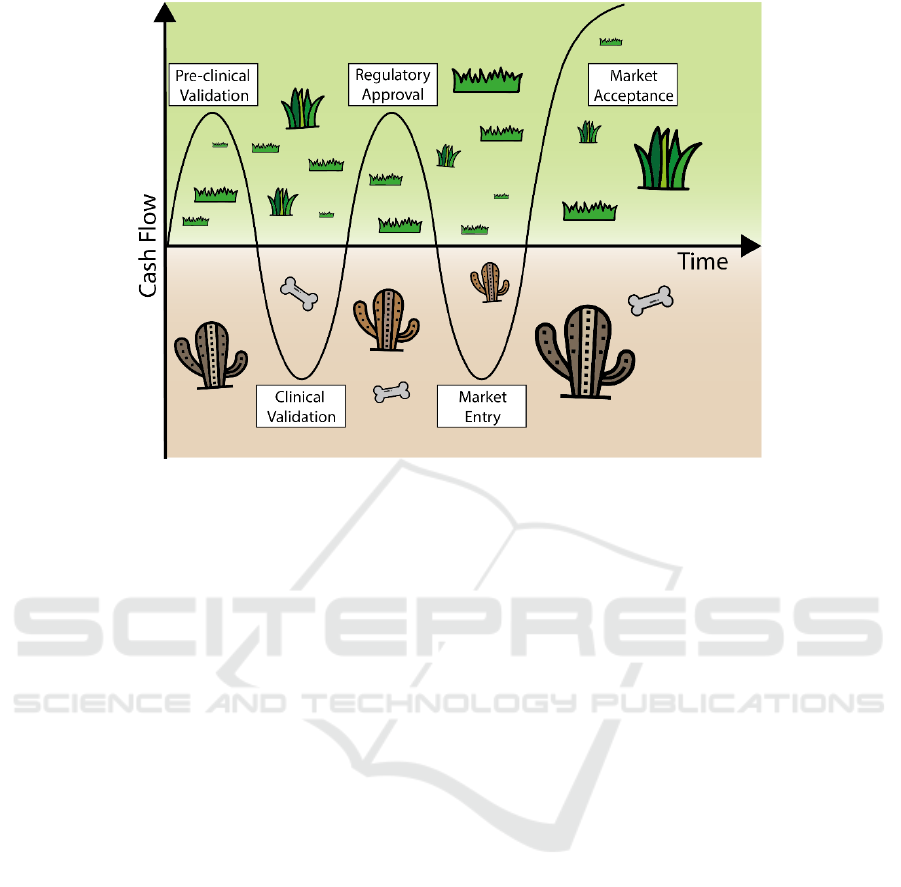
Figure 1: Valleys of death in translational research. The figure illustrates the two valleys of death that can occur during
medical device new product development.
necessary to improve translation. A study from 2016
showing the relative contribution of industry,
academia and private-public-partnerships in
regulatory approvals of medical devices underlines
this idea. With 82% of the medical devices obtaining
approval after clinical trials, the majority were
developed by the industry. However, the importance
of collaborations between academia and industry
showed to be relevant since they had a better
regulatory approval rate (13%) than the devices
developed by academia alone (5%) (Marcus et al.,
2016).
During the commercial development of a medical
device it is common to reach a stage referred to as
‘valley of death’ at an early point when the idea
begins to be translated into potential clinical solutions
(Figure 1). The main hurdle to overcome at that point
is the lack of financial resources, mainly for very
innovative projects (Farragher et al., 2015). A second
valley of death may come after pre-clinical and
clinical validation of the product and the completion
of the regulatory approval process. This second valley
comes from the difficulty to gain market acceptance
and reimbursement and it is the main cause for the
failure of 42% of start-ups in the medical devices
area. To overcome both valleys of death is it crucial
to precisely define the unmet need and to pave out a
clear path to both clinical acceptance and
reimbursement (Murphy and Edwards, 2003).
Therefore, paying a great attention to unmet clinical
needs and the product usability and acceptance from
the very early stages improves the chances of success
and allows for a reorientation of product development
early if needed. Nowadays there is an increased
difficulty to develop an innovative solution due to an
increased number of regulations and the fact that
complex solutions require more and more
sophisticated technology and knowledge.
New translational research programs have been
created in the last decade with the goal to educate
scientist and clinicians on product development and
how to make their research attractive to warrant
further development and commercialization (Gehr
and Garner, 2016). In the US several project-oriented
educational programs have emerged inside the
universities to install entrepreneurship in academia,
an understanding of drug development in industry and
project-management skills. In one of these
educational programs, SPARK-Stanford, the success
rate is high with more than 55% of projects/year being
licensed, entering the clinic or becoming
commercialized (Gehr and Garner, 2016).
In the EU similar programs, educational
curriculums and summer schools have been
developed. European Institute of Innovation &
Technology (EIT) Health is responsible for
innovative collaborations between research,
education and industry with over 140 partners. EIT
Health has supported 122 start-ups that have attracted
€27.9M in investment, has trained over 8 thousand
graduates, professionals and executives and has
launched 10 products or services on the market. It is
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
604

based in three program areas. Accelerator supports
innovation and business, Campus was designed for
education and Innovation Projects is to build new
ideas and collaborations.
The European regulation 2017/745 imposes the
obligation to produce clinical data for the CE marking
of any type of medical device. But during the year
2016, more than 13,000 new medical devices were
registered with the CE marking, while only 1,600
clinical studies were registered on a medical device in
Europe. In 95% of the cases, European medical
device companies are SMEs and start-ups. They lack
the skills and resources to respond to this increased
regulation. Filling this gap is an integral part of the
EIT Health core mission.
ClinMed summer school is a project developed
with support of EIT Health to address the gaps
previously presented regarding the need for
translational research program derived from the
heavier regulation imposed on medical devices
development in the present time.
2 METHODOLOGY
2.1 The Genesis of the Summer School
To maintain the competitiveness of European medical
device companies, the challenge of this summer
school is to strengthen internal skills of companies
and/or to recruit knowledgeable staff on the clinical
evaluation. A needs analysis focusing on the market
of Medical Devices and e-Health applications was
performed at European level during the FP7 European
ITECH project (2014-2016) co-lead by INSERM
CIC-IT network. The ITECH project describes the
process of going from research to market; identifying
the gaps and barriers existing at all stages. The
summer school benefits from results and
recommendations of the ITECH project; specifically,
to quickly reinforce clinical study capacities and
Human Factors Engineering.
Within the Tech4Health network of F-CRIN (the
French branch of European Clinical Research
Infrastructure Network) French partners of this
proposal have organized 3 annual training sessions
(2015, 2016 and 2017) on "Specificities of clinical
research for medical devices". Two-days training
courses were designed for academics, hospital staff
and industrials. But these short courses unfortunately
didn’t trigger the opportunity to set up formal
collaborative projects.
ClinMed is a summer school of EIT Health co-
organized by public and private partners: INSERM
(Public, France), Karolinska Institutet (Public,
Sweden), University of Grenoble-Alpes (Public,
France), University of Lisbon (Public, Portugal),
Medtronics (Private, Ireland), Becton Dickinson
(Private, France) and Madopa (Private, France). The
great variety of clinicians, academics and industry
representation from start-up and large companies is a
unique aspect to the ClinMed summer school. It was
intentional from the part of the committee to make
sure the participants had interactions and knowledge
from all players of the medical device sector.
This summer school is extending these initiatives
with an action-based training and the use of
innovative educational methods, tools and
pedagogies such as experiential learning, co-design
and teamwork based on mixed-skills. EIT Health
would give credibility and open up this international
training, especially for all European stakeholders.
The ClinMed summer school aims to train
participants on the technological innovation in health
care by providing a global vision of the maturation
cycle of a medical device, i.e. from the idea to the
market, using the concept of experiential learning.
As declared by the operational committee, the
summer school was organized “to identify new
challenges on unmet needs, to co-design new
solutions and to implement realistic and feasible
projects to solve important health problems”.
The link to other CAMPUS activities is bi-
directional: the ClinMed project can benefit from the
Innovation and Accelerator EIT Health pillars, and
those pillars can also take advantage of the summer
school. More particularly, projects arising from the
summer school can be implemented either in the
VALIDATE EIT Health program if the project is
already well defined, or within the Innovation
Journey program for innovative ideas that have
emerged from the summer school. The participants of
ClinMed also have the possibility to register in the
EIT Health Alumni network, which connects alumni
from the different EIT Health programs of Campus,
Accelerator and Innovation projects with one another,
partners and entrepreneurs.
2.2 The Pedagogical Logic Adopted
and Main Goals
ClinMed summer school was based on the
pedagogical concepts of experiential learning (Kolb,
1984), design thinking (Plattner, 2011) and
competency-based approach (Frank, 2010); concrete
ideas of innovative products from a first observation
was developed by teams through workshops with the
contribution of coaches and experts. It begun with an
Empowering Translation of New Ideas - A EIT Health ClinMed Summer School Overview
605

unprecedented immersive experience in a healthcare
service: subgroups of participants were invited to
have a fresh look, to identify problems, and needs that
innovative solutions could meet and later share their
ideas within the clinical setting, through meetings
with healthcare professionals and patients, and direct
observations.
The aim is twofold. The first one is to provide
multiple knowledge and skills necessary to develop a
new medical device into the market by giving lectures
in several thematic sessions. The second one is to
allow the different teams of participants to confront
their ideas and discuss problems with the clinicians
and potential users, to work on a project using the
knowledge acquired during the lectures and to carry
the project towards EIT Accelerator or within the
INSERM structures that supervise this school.
Another unique aspect of ClinMed summer school
was to communicate not only theoretical information,
but also practical accompaniment from the lecturers
after their session. The lecturers were encouraged to
go through the teams and give advice on the
development of their specific product. Moreover, the
teams benefitted from regular meetings with the
coach belonging to the immersive site.
The participants are supposed to learn, at the end
of the program, how to assess the clinical and market
need for the development of a new medical device;
understand the rules for protection and property;
know how to find the adequate regulations; define a
development plan; recognize the state of the art,
understand the objectives and methods of usability
studies, clinical investigations and post-marketing
follow-up studies and work in a multidisciplinary
team.
2.3 Summer School Organization
The ClinMed summer school was organized between
the 21
st
and the 31
st
of August 2018. The attendees
were divided in team of 4 to 6 participants and hosted
for 3-day in a hospital or living lab where they
interacted with healthcare professionals and patients
in order to understand unmet needs in a specific field
that requires technological innovation. Afterwards,
all the participants gathered at the main site of the
summer school, located in Villard de Lans, a village
located in the French Alps near Grenoble (France), to
develop the projects through lectures and coaching
from professionals in academic and industrial sectors.
2.3.1 Online Session
Students were invited to attend courses offered on a
private e-learning platform on the theme of Health
Technology Innovation before the beginning of the
summer school. These courses, exclusively in
English, have been developed by the CIC-IT network.
They are part of teaching since 2011 in 4 master's
courses in France (Besançon, Bordeaux, Grenoble
and Nancy), with more than 700 graduate students.
The first part focuses on translational research, the
core business of CIC-ITs, explained in the form of 2
conferences filmed during international congresses
and illustrated by 5 videos of 6 minutes each showing
examples lived in CIC-ITs (with testimonies from
clinicians, researchers and industrialists). A very
concrete example of a DM (insufflation mask) which
led to the creation of a start-up is developed more
precisely. The other courses explore different
innovative topics in the world of medical devices:
Computer Assisted Medical Interventions,
Biomaterials, evaluation of medical device safety in
MRI and Usability.
2.3.2 Immersive Stage
The ClinMed summer school began with an
immersive experience in a healthcare service or a
living lab. This unique experience allowed the
participants to share their ideas within the clinical
setting, through direct observations and meetings
with healthcare professionals and patients. This
exceptional contact between students and real-life
situations permitted to grasp a real-life clinical
problematic presented to them by the healthcare
providers.
Each location had a specific theme. The locations
and themes are displayed in Figure 2 and are: Lyon,
Garches Lisbon, Lille, Besaçon, Grenoble, Tours and
Nancy.
The participants’ goals were:
Observe the current situation;
Identify problems and needs;
Prioritize the issues to be addressed;
Formalize the technical specifications to be
achieved with a solution;
Gather information on the problem and existing
solutions.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
606
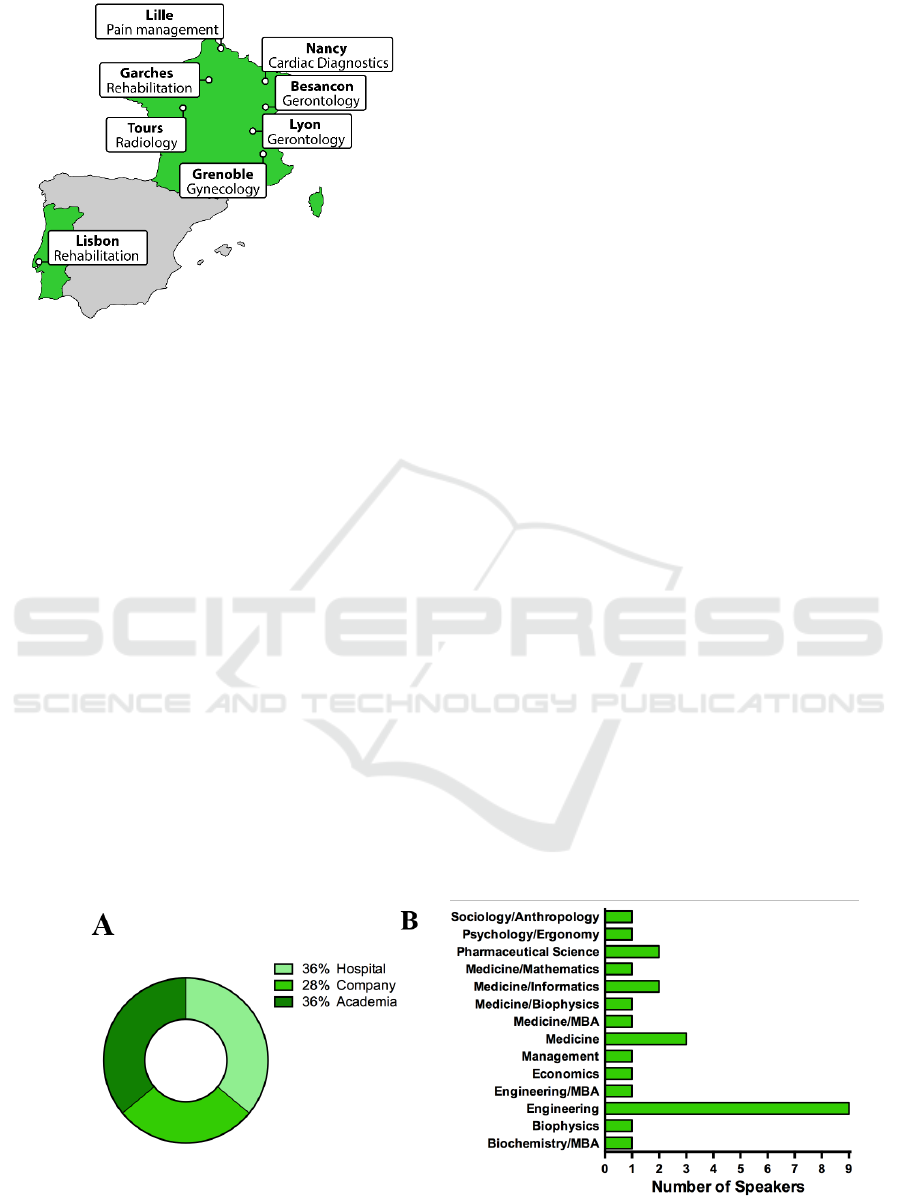
Figure 2: Location and corresponding themes for the
immersive stages possible during the ClinMed summer
school.
2.3.3 Plenary Sessions
After the immersive stage, participants met up at the
main site of the ClinMed summer school in Villard de
Lans to develop their projects through lectures and
coaching from lecturers and mentors.
The lectures were divided into thematic sessions
which were new product life cycle development,
regulation of CE marking, essential requirements,
risk analysis, pre-clinical testing, clinical
investigation, suitability for use, post-market
monitoring and the “market approach” (business
plans, protection and property management).
The daily agenda consisted of a first session of
lectures in the morning, followed by a period of time
dedicated for the participants to work on the project
assisted by the invited speakers and mentors. A
second session of lectures followed after lunch.
Before dinner the participants were encouraged to
continue the elaboration of their work or to participate
in social activities organized by the committee and
the participants to stimulate the networking between
participants.
During this second part of the summer school
students worked on the conceptualization phase of
their solution to the unmet need refining the problem
to be solved and identifying the missing skills if
necessary.
The participants’ goals at this stage were to get a
clear idea on the key components of clinical
translation process, such as:
Define the broad outlines of the development plan
for their new medical device;
Perform a state of the art;
Describe the market, the competition and the
means to afford the market;
Assess the possibility of technical, biological and
clinical equivalence of the innovation with an
existing product;
Define the class of the new medical device and
find the regulations and essential requirements
needed;
Perform the risk analysis of the product;
Define tests and experiments;
Precise the business plan, protection and property
management.
2.4 Lecturers, Mentors and Clinicians
Lectures and mentors were invited to share their
knowledge and expertise with the students. This
unique opportunity allowed the students to have a
close interaction with experts that are in the top of
their field and enhance their project.
The ClinMed summer school had 25 lecturers and
mentors, from 9 different countries, who are
professionals from hospitals, companies and
academia (Figure 3A). They have expertise in diverse
fields such as medicine, sociology, biomaterials,
Figure 3: Speakers background, A- distribution in percentage of professional backgrounds, B- distribution of speakers’ fields.
Empowering Translation of New Ideas - A EIT Health ClinMed Summer School Overview
607
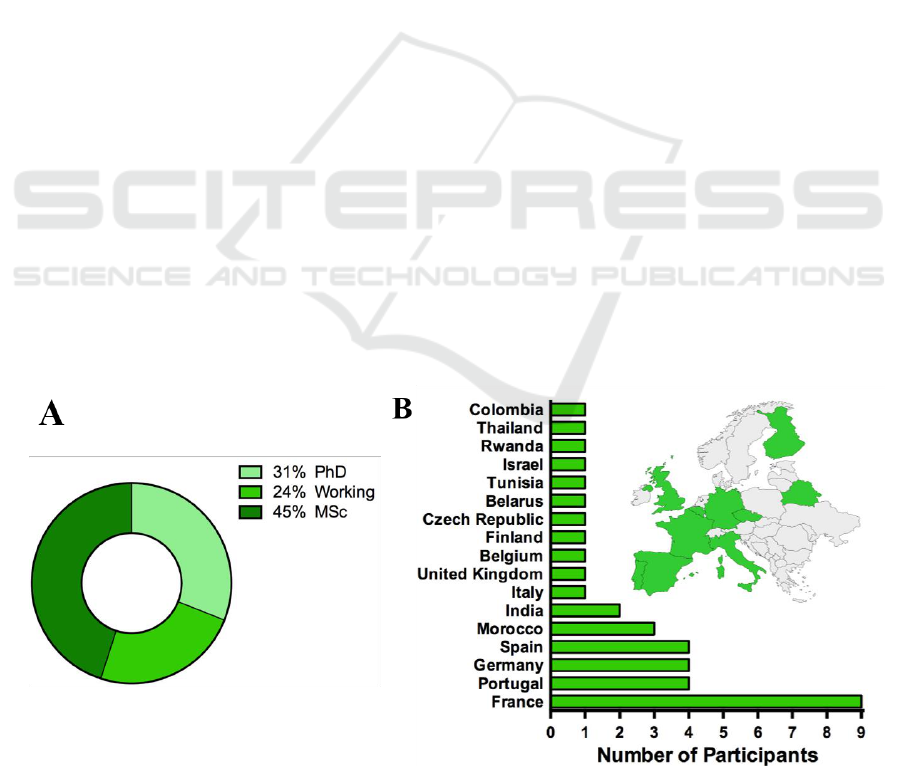
medical imaging, engineering, health informatics,
ergonomics, marketing and economy (Figure 3B),
which guaranteed a comprehensive approach.
It was also made possible to obtain a feedback
from clinicians within the EIT Health network. Each
group developed an idea to satisfy a clinical need.
This solution was shared with clinicians and their
feedback was taken into account for the development
of their projects.
2.5 Recruitment Procedure and
Participants
The summer school accepted 37 participants. A
selection committee evaluated the participants’
experience, academic performance and motivation.
The evaluation was based on their curricula and
motivation letter.
The aim of the selection committee was to put
together a group of attendees that came from diverse
educational and professional backgrounds with high
motivation in developing new solution in the medical
field.
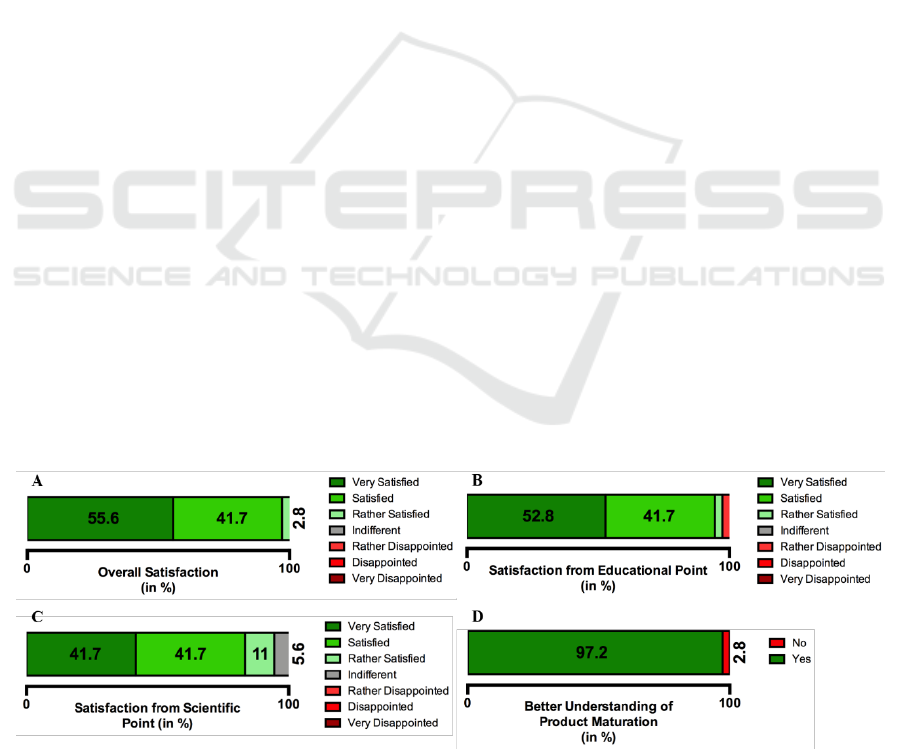
The participants had Bachelor’s or Master’s
degree in the fields of biomedical engineering,
medicine, biology, ergonomics, health informatics,
economy and pharmacy. Most of them came from
university whereas five students came from industry
(Figure 4A).
The participants came from all over the world,
with a total of 17 different countries, with the
majority coming from European countries (Figure
4B). The participants were divided in the following
groups at the start of the program: 4 students in Lyon,
4 students in Garches, 4 students in Lisbon, 6 students
in Lille, 5 students in Besaçon, 5 students in
Grenoble, 4 students in Tours and 5 students in
Nancy.
2.6 Evaluation
After the immersive stage and after an initial lesson
on how to pitch, the participants made their first day
in Villars de Lans a first pitch to an audience to
present their idea. Feedback was given regarding the
content and the efficiency of the pitch by other
participants and mentors. Afterwards it was
encouraged for the groups to keep working on the
pitch.
The participants performed another pitch to two
mentors with experience in industry in order to obtain
a more detailed and constructed feedback.
As a final evaluation each group presented a written
report and an oral presentation, in form of a pitch, in
front of an international jury of experts in the field
followed by Q&A session. Each group project was
evaluated according to the criteria used for the
assessment of European projects, i.e. excellence,
impact, quality and efficiency of the implementation,
quality and efficiency of the pitch.
3 LESSONS LEARNED
During the ClinMed Summer School 8 projects were
developed in the fields of home assisted health, self-
monitoring devices, rehabilitation solutions and
diagnostic products. All projects originated from a
Figure 4: Background of the participants of the ClinMed summer school. A- Distribution in percentage of the participants’
background; B- Countries of origin of ClinMed summer school participants.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
608
clinical need suggested by the clinicians involved and
further developed during the course of the summer
school.
The evaluation process gave an overview of the
quality of the projects based on criteria related to i)
excellence (eg. relevance of the proposal, innovation
potential, credibility of the proposal, accuracy of risk
analysis);
ii) impact (eg. presentation of market, economic
viability, potential to improve healthcare);
iii) quality & efficiency of the implementation (eg.
co-design and interdisciplinary approach, clinical
impact, intellectual property management,
exploitation and dissemination of results);
iv) quality & efficiency of the pitch (eg. presentation
quality and answers to the jury’s questions, team’s
ability to convince).
3.1 Feedback
At the end of the summer school, students were asked
to fill a feedback survey. The questionnaires covered
topics of satisfaction of the program, organization,
accommodation, quality of lectures, educational point
of view (i.e. immersive experience, common core and
team project), social events and general aspects
related to the understanding gained through the
summer school. The questionnaires had both scale
questions (1. Very Satisfied-2.Satisfied-3.Rather
Satisfied -4.Indifferent -5.Rather Disappointed-
6.Disappointed-7.Very Disappointed) and free
commentaries.
In Figure 5A is represented the overall
satisfaction expressed by the participants. 55.6% of
the participants replied that they were very satisfied,
while 41.7% were satisfied and the remaining
percentage (2.8%) were rather satisfied. Some of the
positive comments refer to the high quality of
lectures, the comprehensive overview of the medical
devices field, the unique opportunity to work and
interact with people with diverse backgrounds, as a
few examples. A few more negative comments refer
that the time of the year (end of the summer) was not
ideal for the immersive stage due to the limited
number of people available, some of the lectures were
too detailed and extensive for the type of summer
school and there was not enough time to work on the
final report.
In Figure 5B is represented the satisfaction from
an educational point. 94.5% of the participants stated
they were very satisfied or satisfied. While from a
Scientific point of view (Figure 5C), 83.7% of the
participants were very satisfied or satisfied, 11% were
rather satisfied while 5.6% were indifferent.
When asked if the participant had a better
understanding of the maturation cycle of an
innovative medical device, the majority of the
participants replied yes (97.2%) (Figure 5D).
From the different concepts presented during the
summer school, the students singled out some as the
most difficult ones to comprehend, such as business
model and regulatory affairs. It is to be expected that
students that have different backgrounds would find
specific biomedical topics more difficult to grasp.
4 CONCLUSIONS AND FUTURE
PERSPECTIVES
Looking at the present view of translational research
it is clear that there are many challenges left to
overcome. Using research findings for improving
clinical medicine needs the combined expertise of
basic researchers, clinicians and the industry. At the
initial stage of the development of a medical device it
Figure 5: Representation of data (in percentage) obtained from the participants’ reply to a survey. A- Overall satisfaction; B-
Satisfaction from an educational point; C- Satisfaction from a scientific point; D- If the participant had a better understanding
of the product maturation.
Empowering Translation of New Ideas - A EIT Health ClinMed Summer School Overview
609

is crucial to clearly identify an unmet need and to
optimize the product usability in order to improve the
chance of success. This notion, as simple as it may
seem, needs to be thought through and put into
practice by young researchers and developers.
In this paper a report of the ClinMed summer
school was given. This program gave an up-to-date
general perspective of the life cycle of a medical
device: from the initial concept until it reaches the
European market. Its uniqueness came from the
immersive stage and the close contact between
participants and experts of the medical field. It main
goal was to empower students to become developers
and innovators.
According to the feedback obtained at the end of
the summer school, students believe that the
experience improved their knowledge of the medical
device field, broadened their comprehension of
possibilities for the development of devices. The
participants believe that the impact of this summer
school will be presented to them only in future
projects of innovation in medical devices.
ACKNOWLEDGEMENTS
This work received funding from EIT-Health campus
call (Project Grant Agreement n°18497).
REFERENCES
De Pieri. et al., 2018. Joint academic and industrial efforts
towards innovative and efficient solutions for clinical
needs. Journal of Materials Science: Materials in
Medicine. 29. 129
Duda, G. N. et al. 2014. Changing the Mindset in Life
Sciences Toward Translation: A Consensus. 6.
264cm12264cm12
European Institute of Innovation and Technology [Online]
https://www.eithealth.eu/ [Accessed 19-12-2018]
Farragher, J. F., 2015. Translational research in kidney
transplantation and the role of patient engagement. Can
J Kidney Health Dis. 2. 42
Frank, J.R., Snell, L.S., Cate, O.T. 2010. Competency-
based medical education: theory to practice. Med
Teach. 32(8). 638-45.
Gehr, S. and Garner, C., 2016. Rescuing the Lost in
Translation. Cell. 165. 765-70
Kolb, D. 1984. Experiential Learning: experience as the
source of learning and development. Englewood Cliffs,
NJ: Prentice Hall. p. 21
Marcus, H. J et al., 2016. Regulatory approval of new
medical devices: cross sectional study. BMJ. 353.
I2587
Murphy, L. and Edwards, P., 2003. Bridging the valley of
death: Transitioning from public to private sector
financing. National Renewable Energy Laboratory.
Plattner, H., Meinel, C., Leifer, L J. 2011. Design thinking:
understand, improve, apply. Understanding innovation.
Berlin; Heidelberg: Springer-Verlag. pp. xiv–xvi.
ClinMed 2019 - Special Session on Designing Future Health Innovations as Needed
610
