
Polyp Detection in Gastrointestinal Images using Faster Regional
Convolutional Neural Network
Lourdes Duran-Lopez, Francisco Luna-Perejon, Isabel Amaya-Rodriguez, Javier Civit-Masot,
Anton Civit-Balcells, Saturnino Vicente-Diaz and Alejandro Linares-Barranco
Robotics and Computer Technology Lab., University of Seville, Av. Reina Mercedes s/n, Seville, Spain
Keywords:
Polyp, Colonoscopy, Deep Learning, Image Analysis, Faster Regional Convolutional Neural Network.
Abstract:
Colorectal cancer is the third most frequently diagnosed malignancy in the world. To prevent this disease,
polyps, the principal precursor, are removed during a colonoscopy. Automatic detection of polyps in this
technique could play an important role to assist doctors for achieving an accurate diagnosis. In this work,
we apply a state-of-the-art Deep Learning algorithm called Faster Regional Convolutional Neural Network to
each colonoscopy frame in order to detect the presence of polyps. The proposed detection system contains
two main stages: (1) processing of the colonoscopy frames for training and testing datasets generation, where
artifacts are extracted and the number of images in the dataset is augmented; and (2) the Neural Network
model, which performs feature extraction over the frames in order to detect polyps within the frames. After
training the algorithm under different conditions, our result shows that the proposed system detection has a
precision of 80.31%, a recall of 75.37%, an accuracy of 71.99% and a specificity of 65.70%.
1 INTRODUCTION
Colorectal cancer (CRC) is the third most frequently
diagnosed malignancy and the fourth leading cause
of cancer-related deaths in the world. Its burden is
expected to increase by 60% to more than 2.2 million
new cases and 1.1 million deaths from cancer by 2030
(Arnold et al., 2017). Certain types of polyps that
grow in the colon are the precursors of more than 95%
of cancer cases in this organ. Thus, the removal of
polyps is usually practiced as a safety measure.
Polyps are detected and extracted during a colo-
noscopy, which is a medical analysis in which the
colonoscopist examines the meter and a half length
tube of the large intestine with the use of an colo-
noscope. This technique is a commonly used primary
method to detect polyps (Lieberman et al., 2012). Ho-
wever, by using this technique, the polyp detection is
not always effective (Corley et al., 2014), as there are
some factors that make the correct evaluation diffi-
cult. These factors may be related to the experience of
the physician, the conditions of the patient, the qua-
lity of the instruments, among others. Highly expe-
rienced colonoscopists can achieve an accuracy in the
detection that is greater than 90%, while colonosco-
pists with less experience cannot perform better than
an 80% in the recognition rate (Hewett et al., 2012)
(Ignjatovic et al., 2009). On the other hand, there may
be other external factors, such as the ambiguity in the
characteristics of the polyp. Polyps could be difficult
and confusing to diagnose depending on the characte-
ristics shown when performing the colonoscopy (He-
wett et al., 2012). An automated computerized diag-
nosis could provide a cross validation mechanism in
order to assist colonoscopists to make a decision in
this task and, thus, reducing the possibility of com-
mitting mistakes in the diagnosis and improving the
quality of life of the affected patients. This technique
could also be used for training purpose of new colo-
noscopists at the university or medical centers.
In the past few years, several researches investiga-
ted the problem of detecting polyps in colonoscopies
(Bernal et al., 2017), (Zhang et al., 2017), (Ribeiro
et al., 2016), (Axyonov et al., 2016), (Park and Sar-
gent, 2016). Many of them used conventional Con-
volutional Neural Networks (CNNs) to classify fra-
mes obtained from videos, indicating the presence of
a polyp. However, none of these solutions are able to
locate the polyp inside the image, which could be very
useful when making a colonoscopy to aid the experts
in their task.
In this paper, we report our study of an automa-
ted computerized system for detecting polyps in co-
lonoscopy videos using a Deep Learning based al-
626
Duran-Lopez, L., Luna-Perejon, F., Amaya-Rodriguez, I., Civit-Masot, J., Civit-Balcells, A., Vicente-Diaz, S. and Linares-Barranco, A.
Polyp Detection in Gastrointestinal Images using Faster Regional Convolutional Neural Network.
DOI: 10.5220/0007698406260631
In Proceedings of the 14th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2019), pages 626-631
ISBN: 978-989-758-354-4
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

gorithm called Regional Convolutional Neural Net-
works (R-CNN). These methods try to determine the
presence of an object and also locate its position in-
side the image. One of the most recent algorithms
based on the R-CNN approach are called Faster Regi-
onal Convolutional Neural Network (Faster R-CNN),
which were developed in 2015 (Ren et al., 2015). Fas-
ter R-CNNs were designed with the purpose of redu-
cing its predecessors computation time. In ILSVRC
and COCO 2015 competitions, Faster R-CNN was the
foundations of the 1st-place winning entries in several
tracks.
Faster R-CNNs have been used for different pur-
poses: face detection (Jiang and Learned-Miller,
2017), driver’s cell-phone usage and hands on steer-
ing wheel detection (Hoang Ngan Le et al., 2016) are
some application examples of this algorithm, which
has proven to show good results.
In this paper, the authors present an application
of Faster R-CNNs in order to build an automated re-
cognition system to detect the presence of polyps in
colonoscopy images.
The rest of the paper is structured as follows:
section 2 presents the methods that are used in the
approach that has been carried out in this paper. First,
the dataset used is explained from its acquisition to
its preprocessing and augmentation. Then, the Fas-
ter R-CNN algorithm is presented, along with the ar-
chitecture, design, training and testing of the neural
network that has been used in this work, which is ba-
sed on those algorithms. Also, to evaluate the system,
some tests to prove its performance and robustness are
performed. Then, Section 3 presents the classification
results, and Section 4 a discussion over the obtained
values. Finally, the conclusions of this work are pre-
sented in Section 5.
2 MATERIALS AND METHODS
In this section the main materials and methods used
in this work are presented, distinguishing between the
dataset and the neural network. For the dataset, the
applied stages are: image acquisition, pre-processing
and data augmentation. For the neural network sub-
section, the architecture of the Faster R-CNN, the trai-
ning and testing steps for polyp detection in colo-
noscopy images and the performance evaluation are
presented.
A general view of our study can be seen in the
block diagram that is shown in (Fig. 1).
Figure 1: Block diagram of the implemented approach.
2.1 Dataset
2.1.1 Image Acquisition
We used the database provided by Endoscopic Vi-
sion’s sub-challenge (MICCAI 2018
1
) called Gas-
trointestinal Image ANAlysis (GIANA
2
) (Angermann
et al., 2017) (Bernal et al., 2018), which is composed
of 18 videos collected from colonoscopies from diffe-
rent patients. These videos are already provided as a
segmented set of frames. The dataset contains a total
of 11954 images with their corresponding masks in-
dicating where the polyp is located (only in the case
that there was one in that specific image) (Fig. 2). All
images have a resolution of 384x288 pixels.
2.1.2 Artifact Extraction
In order to enhance the performance of the system, we
applied a pre-processing step to the images to make
the network be able to extract descriptors more ap-
propriately and, therefore, improve the results of the
detection (Fig. 3). For this, we extracted the black ed-
ges of the endoscopy frames to remove the unwanted
areas, reducing the images to a size of 284x265.
1
https://www.miccai2018.org/en/
2
https://giana.grand-challenge.org/
Polyp Detection in Gastrointestinal Images using Faster Regional Convolutional Neural Network
627
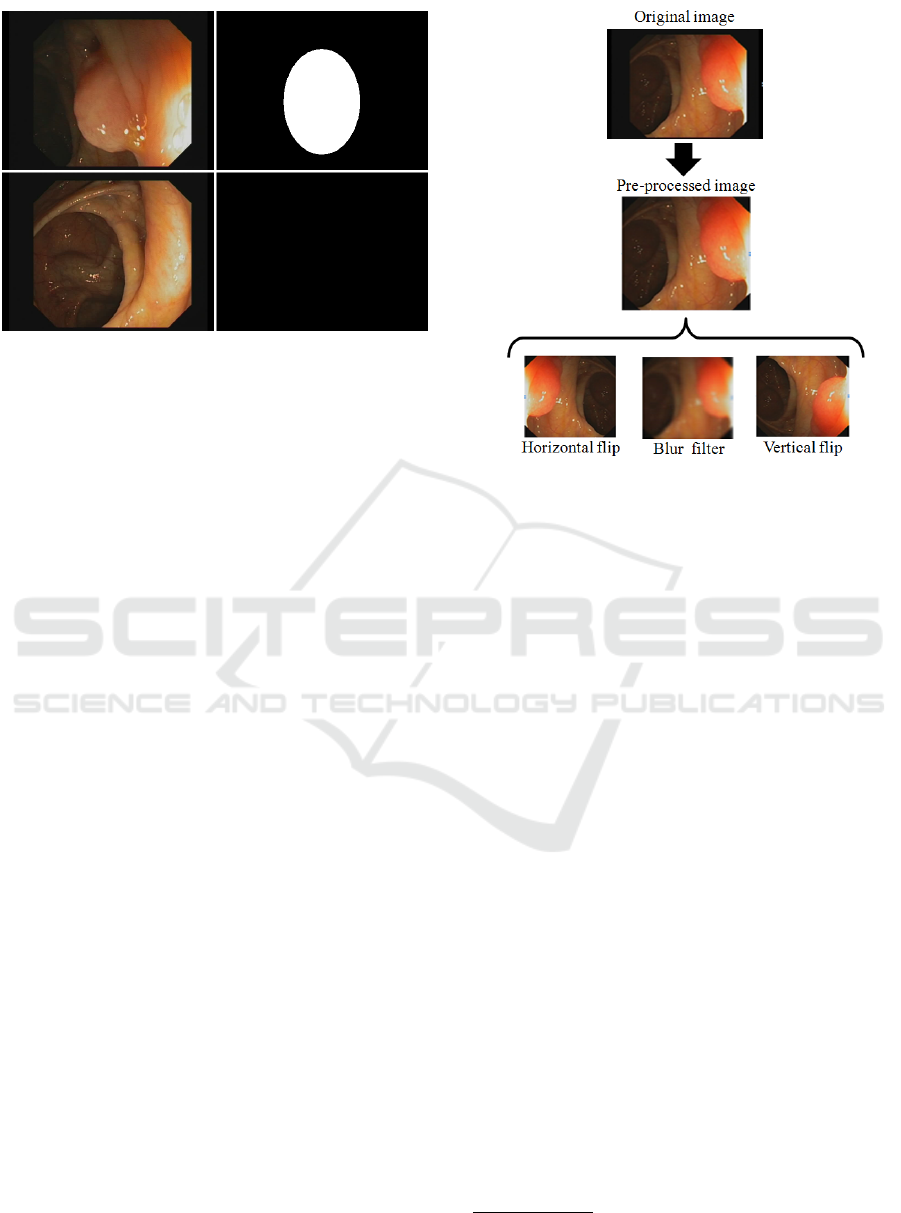
Figure 2: Samples of the database. Left: frames captu-
red from the colonoscopy video recordings. Right: binary
masks indicating if there is a polyp (marked as a white area)
and its location inside the frame.
2.1.3 Data Augmentation
Deep learning models perform better when training
with large datasets that include more variability in the
samples. The most popular method to make data-
sets bigger is called data augmentation, which helps
prevent overfitting when training on very little data
(Wong et al., 2016). The simplest way to perform
data augmentation is to add noise or apply geometric
transformations to data to simulate other conditions
of camera recordings with the same objects (polyps in
our case). Hence, all the transformations would boost
the models to learn better.
The dataset was augmented using a series of
transformations so that the model would never train
twice the exact same image. For each original pre-
processed image, an horizontal flip, a vertical flip and
a blur filter have been applied. Thus, we obtain three
new images from each original sample (Fig. 3). After
this data augmentation step, we obtain a dataset that
consists of 47.816 images in total.
2.2 Faster R-CNN
2.2.1 Arquitecture
As it was mentioned in the introduction, a Faster
RCNN was used in this work for the polyp detection
task. This algorithm is divided in two modules (Fig.
4):
– First of all, a deep Fully Convolutional Network
(FCN) (Ren et al., 2015) receives the images from
the dataset that was presented in this section as in-
put. Then, it extracts feature maps or descriptive cha-
racteristics and analyzes them to propose regions of
Figure 3: Processing applied to the original images. First,
black edges are removed in a pre-processing step. Then
data augmentation is applied, generating three different new
images.
interest. The novel step that this architecture intro-
duces is the way to determine the regions of interest,
by using a neural network that takes advantage of the
mathematical operations made in the convolution lay-
ers. In our architecture we have used the ResNet50
model (He et al., 2016) as FCN. ResNet models try to
solve the saturation of the accuracy caused by increa-
sing the network depth.
– Secondly, the proposed regions are the input
of the second module, called Fast RCNN detector,
composed by two fully-connected layers, a regression
layer and a classification layer (Ren et al., 2015).
2.2.2 Training and Testing
In order to reduce the number of false positives, a
technique called hard-negative mining was used (Fel-
zenszwalb et al., 2010). It consists on adding negative
samples, which means, including examples of images
that do not contain polyps in the training step, labe-
ling them as background.
85% of the augmented dataset (15 videos) was
used to train the network, while the remaining 15%
(3 videos) for evaluating its accuracy and robustness
in the detection. These training and testing dataset
are obtained in such way that different patients are in-
volve in each dataset.
In this work, both TensorFlow
3
and Keras
4
, which
3
https://www.tensorflow.org/?hl=es
4
https://keras.io/
GIANA 2019 - Special Session on GastroIntestinal Image Analysis
628
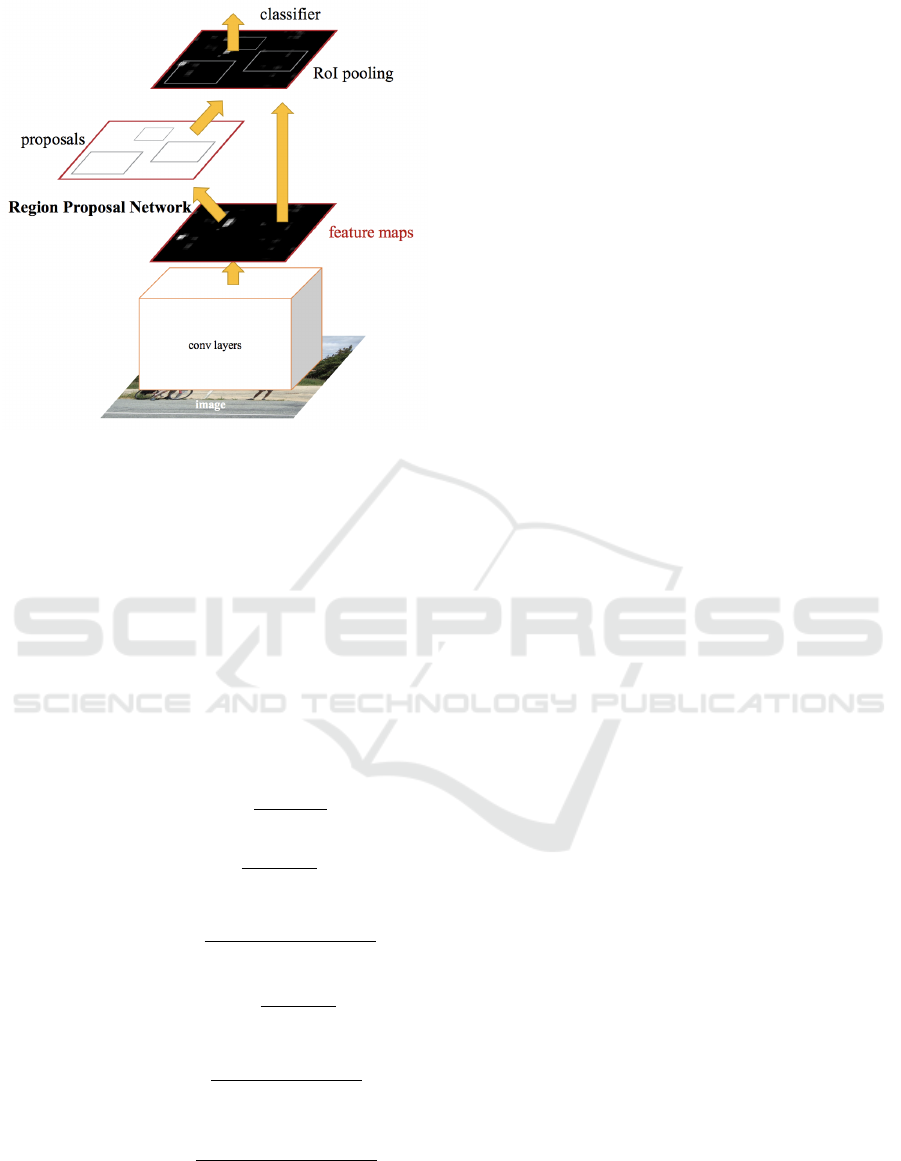
Figure 4: Faster R-CNN architecture. Image taken from
(Ren et al., 2015).
are two widely known Deep Learning frameworks,
are used to design, train and test the network.
2.2.3 Performance Evaluation Measures
To quantitatively present the capabilities of the pro-
posed computerized-aided diagnosis method, perfor-
mance metrics were used to show how the implemen-
ted technique was able to detect and classify the po-
lyps. These are precision (Equation 1), recall (Equa-
tion 2), accuracy (Equation 3), specificity (Equation
4), F1-score (Equation 5) and F2-score (Equation 6).
Precision = 100 ×
T P
T P + FP
(1)
Recall = 100 ×
T P
T P + FN
(2)
Accuracy = 100 ×
T P + T N
T P + T N + FP + FN
(3)
Speci f icity = 100 ×
T N
T N + FP
(4)
F1 − score = 2 ×
Precision × Recall
Precision + Recall
(5)
F2 − score = 5 ×
Precision × Recall
4 × Precision + Recall
(6)
where, TP and FP denote true positive cases (the sy-
stem detects a polyp in a frame that contains a polyp)
and false positive cases (the system detects a polyp in
a frame that does not have a polyp), respectively. TN
and FN denote true negative cases (the system does
not detect a polyp in a frame that does not have a
polyp) and false negative cases (the system does not
detect a polyp in a frame that contains a polyp), re-
spectively.
These metrics was applied to the classification
layer of this method. Since this layer returns as out-
put a confidence value, a minimal threshold was es-
tablished to apply these metrics. On this way, if the
confidence value exceeds the threshold then the polyp
is detected.
3 RESULTS
Different experiments were carried out to determine
if polyps were detected correctly or not. Tests were
performed every 50 epochs, selecting different confi-
dence thresholds in order to obtain the best results.
Polyps detection performance is reported in Table
1. The results show the robustness of the propo-
sed Faster R-CNN architecture on detecting the polyp
position in colonoscopy images with a precision of
80.31%, a recall of 75.37%, an accuracy of 71.99%
and a specificity of 65.70%. The minimal threshold
was established at 0.80.
In Fig. 5 the results of our recognition system can
be seen by showing the precision when detecting po-
lyps inside samples from the dataset, and their corre-
sponding mask images (as a ground truth) indicating
where the polyps are located.
4 DISCUSSION
In this work, we have performed a polyp detection
system using Faster R-CNN. This kind of networks
have had a very high impact in recognition systems
in recent years thanks to the fact that they are able
to both classify objects or items inside images and
also provide the specific location in which that item
is. The application of this kind of networks in medi-
cal image analysis is becoming more and more usual.
In this case, this work presents, what we believe it is,
the first application of Faster R-CNNs to polyp de-
tection in videos obtained from colonoscopies. As it
can be seen, we have focused on the polyp detection
task but, as we mentioned earlier, Faster R-CNNs can
also provide the location of polyps inside images. The
results obtained show that there is still room to im-
prove and we will continue to pursue the goal of im-
plementing an automatic polyp detection system that
Polyp Detection in Gastrointestinal Images using Faster Regional Convolutional Neural Network
629

Table 1: Polyps detection performance. TP: True Positive, FP: False Positive, TN: True Negative, FN: False Negative.
TP FP TN FN Precision Recall Accuracy Specificity F1 F2
3533 866 1659 1154 80.31 75.37 71.99 65.70 77.76 76.30
Figure 5: Polyp detection results. Left: polyps detected by
Faster R-CNN. Confidence values are represented in blue.
Right: their corresponding ground truth. A and B show the
performance in case a polyp appears, while C shows the
performance in case there is no polyp.
could be used in hospitals as an aid for colonosco-
pists in their task. This future work will include the
polyp location task on top of the already performed
detection system by adding a new analysis layer and
a set of metrics that would improve the overall recog-
nition system.
For now, we have achieved first results using this
aforementioned Deep Learning technique. Desig-
ning a new and more appropriate CNN model instead
of using the well-known ResNet50, along with fine-
tuning the hyperparameters of it could improve the
classification results not only in terms of accuracy but
also in terms of specificity and sensitivity. Also, ot-
her methods for pre-processing the data and increa-
sing the variability of the dataset will make the recog-
nition system more robust and plausible to use in a
real case scenario. These two approaches will be stu-
died in future works in which we will try to improve
state-of-the-art solutions and also the accuracy achie-
ved by colonoscopist in order to build a system that
could work together with them and aid them in their
recognition task.
5 CONCLUSIONS
In this paper, we present the use of Faster R-CNN as
a mechanism for a computarized-aided diagnosis sy-
stem that automatically detects polyps in colonosco-
pies in order to assist doctors in this task. The images
were obtained from GIANA Endoscopic Vision’s sub-
challenge to create the dataset. These samples from
the dataset were first pre-processed, removing the un-
wanted areas and, after that, increased in number by
using data augmentation techniques. 85% of dataset
was used to train the network, which, on a first step,
extracts features over the colonoscopy frames; then,
it proposes regions of interests where polyps could
be located; and finally, it classifies the regions most
likely to contain a polyp. The remaining 15% of data-
set was used to test the network in order to evaluate its
performance. The results show that the proposed re-
cognition system has a precision of 80.31%, a recall
of 75.37%, an accuracy of 71.99% and a specificity
of 65.70%. The system is able to detect the presence
of polyps in the images studied in this paper and the
results prove that the system could be used as an aid
for doctors in this task. In future works, the authors
will study and evaluate different custom CNN models
in order to improve the performance, classification re-
sults and also the polyp location task, along with new
pre-processing algorithms to improve the variability
of the dataset and the feature extraction step.
ACKNOWLEDGEMENTS
This work was supported by the Spanish grant (with
support from the European Regional Development
Fund) COFNET (TEC2016-77785-P).
REFERENCES
Angermann, Q., Bernal, J., S
´
anchez-Montes, C., Ham-
mami, M., Fern
´
andez-Esparrach, G., Dray, X., Ro-
main, O., S
´
anchez, F. J., and Histace, A. (2017). To-
wards real-time polyp detection in colonoscopy vi-
deos: Adapting still frame-based methodologies for
video sequences analysis. In Computer Assisted and
Robotic Endoscopy and Clinical Image-Based Proce-
dures, pages 29–41. Springer.
Arnold, M., Sierra, M. S., Laversanne, M., Soerjomataram,
I., Jemal, A., and Bray, F. (2017). Global patterns
GIANA 2019 - Special Session on GastroIntestinal Image Analysis
630

and trends in colorectal cancer incidence and morta-
lity. Gut, 66(4):683–691.
Axyonov, S., Liang, J., Kostin, K., Zamyatin, A. V., et al.
(2016). Advanced pattern recognition and deep lear-
ning for colon polyp detection.
Bernal, J., Histace, A., Masana, M., Angermann, Q.,
S
´
anchez-Montes, C., Rodriguez, C., Hammami, M.,
Garcia-Rodriguez, A., C
´
ordova, H., Romain, O., et al.
(2018). Polyp detection benchmark in colonoscopy vi-
deos using gtcreator: A novel fully configurable tool
for easy and fast annotation of image databases. In
Proceedings of 32nd CARS conference.
Bernal, J., Tajkbaksh, N., S
´
anchez, F. J., Matuszewski, B. J.,
Chen, H., Yu, L., Angermann, Q., Romain, O., Ru-
stad, B., Balasingham, I., et al. (2017). Comparative
validation of polyp detection methods in video colo-
noscopy: results from the miccai 2015 endoscopic vi-
sion challenge. IEEE transactions on medical ima-
ging, 36(6):1231–1249.
Corley, D. A., Jensen, C. D., Marks, A. R., Zhao, W. K.,
Lee, J. K., Doubeni, C. A., Zauber, A. G., de Boer,
J., Fireman, B. H., Schottinger, J. E., et al. (2014).
Adenoma detection rate and risk of colorectal can-
cer and death. New england journal of medicine,
370(14):1298–1306.
Felzenszwalb, P. F., Girshick, R. B., McAllester, D., and
Ramanan, D. (2010). Object detection with discri-
minatively trained part-based models. IEEE tran-
sactions on pattern analysis and machine intelligence,
32(9):1627–1645.
He, K., Zhang, X., Ren, S., and Sun, J. (2016). Deep resi-
dual learning for image recognition. In Proceedings of
the IEEE conference on computer vision and pattern
recognition, pages 770–778.
Hewett, D. G., Kaltenbach, T., Sano, Y., Tanaka, S., Saun-
ders, B. P., Ponchon, T., Soetikno, R., and Rex,
D. K. (2012). Validation of a simple classification sy-
stem for endoscopic diagnosis of small colorectal po-
lyps using narrow-band imaging. Gastroenterology,
143(3):599–607.
Hoang Ngan Le, T., Zheng, Y., Zhu, C., Luu, K., and Savvi-
des, M. (2016). Multiple scale faster-rcnn approach to
driver’s cell-phone usage and hands on steering wheel
detection. In Proceedings of the IEEE Conference on
Computer Vision and Pattern Recognition Workshops,
pages 46–53.
Ignjatovic, A., East, J. E., Suzuki, N., Vance, M., Guent-
her, T., and Saunders, B. P. (2009). Optical diagno-
sis of small colorectal polyps at routine colonoscopy
(detect inspect characterise resect and discard; discard
trial): a prospective cohort study. The lancet oncology,
10(12):1171–1178.
Jiang, H. and Learned-Miller, E. (2017). Face detection
with the faster r-cnn. In Automatic Face & Gesture
Recognition (FG 2017), 2017 12th IEEE International
Conference on, pages 650–657. IEEE.
Lieberman, D. A., Rex, D. K., Winawer, S. J., Giardiello,
F. M., Johnson, D. A., and Levin, T. R. (2012). Gui-
delines for colonoscopy surveillance after screening
and polypectomy: a consensus update by the us multi-
society task force on colorectal cancer. Gastroentero-
logy, 143(3):844–857.
Park, S. Y. and Sargent, D. (2016). Colonoscopic polyp
detection using convolutional neural networks. In
Medical Imaging 2016: Computer-Aided Diagnosis,
volume 9785, page 978528. International Society for
Optics and Photonics.
Ren, S., He, K., Girshick, R., and Sun, J. (2015). Faster
r-cnn: Towards real-time object detection with region
proposal networks. In Advances in neural information
processing systems, pages 91–99.
Ribeiro, E., Uhl, A., Wimmer, G., and H
¨
afner, M. (2016).
Exploring deep learning and transfer learning for co-
lonic polyp classification. Computational and mathe-
matical methods in medicine, 2016.
Wong, S. C., Gatt, A., Stamatescu, V., and McDonnell,
M. D. (2016). Understanding data augmentation
for classification: when to warp? arXiv preprint
arXiv:1609.08764.
Zhang, R., Zheng, Y., Mak, T. W. C., Yu, R., Wong, S. H.,
Lau, J. Y., and Poon, C. C. (2017). Automatic de-
tection and classification of colorectal polyps by trans-
ferring low-level cnn features from nonmedical dom-
ain. IEEE J. Biomedical and Health Informatics,
21(1):41–47.
Polyp Detection in Gastrointestinal Images using Faster Regional Convolutional Neural Network
631
