
Design and Development of Parallel Biosensing System for
Personalized Chemotherapy Treatment
Ahmad Fairuzabadi Mohd Mansor
1
, Anis Nurashikin Nordin
1
, Kian Liang Goh
2
,
Soon Hin How
2
, Yumi Zuhanis Has-Yun Hashim
3
and Mardhiah Mohammad
4
1
Kulliyyah of Engineering, International Islamic University Malaysia (IIUM), Jalan Gombak, Kuala Lumpur, Malaysia
2
Kulliyyah of Medicine, IIUM Kuantan, Malaysia
3
International Institute for Halal Research and Training (INHART), IIUM Gombak, Malaysia
4
Kulliyyah of Allied Health Science, IIUM Kuantan, Malaysia
1 RESEARCH PROBLEM
Chemotherapy administration can sometimes inflict
negative side effects to the patient. The regimen or
cocktails of the drugs introduced into a patient’s body
has always needed careful consideration. Currently,
the combinations are determined by strength of the
regimen based on empirical technique; which is the
observation of the response exhibited by the patients.
However, an individual’s drug absorption rate is
influenced by many factors such as age, gender,
metabolism, disease state, organ function, drug-to-
drug interactions, genetics, and obesity.
Consequently, different patients can have different
body response towards the chemotherapy. Clinical
studies have proved that optimal treatment
effectiveness can be achieved only when the
chemotherapy treatment is individualized for each
patient (Zhang et al., 2013).
Current chemosensitivity assay such as MTT
Assay, ATP assay and molecular probes are tedious,
time consuming, labor intensive and expensive
(Kiilerich-Pedersen and Rozlosnik, 2012; Lazcka et
al., 2007). The tedious nature of these types of assays
prohibit individualized testing for patients before
chemotherapy. Therefore, there is a need for low-cost
point-of-care biosensors which can predict the
patient’s response towards different chemotherapy
regimens.
2 OUTLINE OF OBJECTIVES
Non-destructive monitoring of cell behaviors have
gained wide attention over the past decade. The
concept of Electrical Cell-Substrate Impedance
Sensing (ECIS) was pioneered by Giaever and Keese
in 1984 and evolved to be the most stable and
effective technique of measuring cultured cells on
microelectrodes with real-time impedance
monitoring (Cui et al., 2017; Hong et al., 2011).
In this research, the ECIS concept will be applied
to monitor adhesion, proliferation and death of cancer
cells in vitro due to exposure to chemotherapy drugs.
Chemosensitivity analysis will be performed using
the developed impedance biosensing system by
correlating the response of cell samples towards
several chemotherapy regimens. A comparison will
be made between tests conducted using biosensors
and the actual chemotherapy treatment prescribed to
patients.
3 STATE OF THE ART
Rapid and effective treatment of cancer is crucial to
improve patients’ quality of life and chance of
survival. Currently, cancer cell growth, apoptosis and
response to chemotherapeutic treatment involve
colorimetric assays, which require complex
laboratory equipment and extensive cell and drug
preparation. Measurements and cell preparation are
made at each endpoint, making the process labour-
intensive and high cost. As such personalized studies
on the efficacy of chemotherapeutical drugs on
patients are rarely done due to its high cost and
tedious process. Better disease-free survival rates
have been reported using neoadjuvant therapy where
treatment is given before surgery and is followed by
systemic chemotherapy (Ancona et al., 2001; Lowy
et al., 1999). In recent years, numerous efforts have
been made to develop better chemotherapeutic
regimens, resulting in improved outcomes and
prolonged survival (Sjoquist et al., 2011). To
facilitate this, there is great demand for rapid and real-
time techniques for studying cancerous cells
especially in terms of their reactions to drug and
toxins (Bramwell et al., 1997).
Mohd Mansor, A., Nordin, A., Goh, K., How, S., Hashim, Y. and Mohammad, M.
Design and Development of Parallel Biosensing System for Personalized Chemotherapy Treatment.
In Doctoral Consortium (BIOSTEC 2019), pages 15-19
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
15

Biosensors can be used to test the response of
tumours to different chemotherapy agents, similar to
how microorganisms are tested against different
antibiotics in vitro before the actual drugs are
employed in the patients. This enables an accurate
prediction as to which chemotherapy agent or
combination of agents that are likely to be successful
against the tumours without subjecting patients to
trial-and-error therapy as tumour behaviours are
unpredictable and differs from each individual
patient. This may also minimize the side effects of the
drugs as patients will not suffer unnecessarily from
ineffective treatment. Biosensors may also be used to
predict the aggressiveness of the tumour by
measuring the rate of tumour growth. This
information will be helpful to clinicians in deciding
which patients require more aggressive treatment to
prevent disease progression during treatment.
ECIS is the first impedance-based technique for
studies of quantifying cell behaviours. In ECIS, a
small gold electrode (250µm) is immersed in culture
medium at the bottom of the tissue culture wells.
Electrode surface is pre-coated with certain proteins
to enhance cell adhesion with the electrode. Two
electrodes, working and counter electrodes, exist in
the system. A relatively small circular gold electrode
behaves as a working electrode as compared to the
larger counter electrode at the bottom. In ECIS
biosensor systems, an AC signal with 1V amplitude
is applied through a 1MΩ series resistor at 4 kHz
frequency. The voltage across the electrodes is
measured using an amplifier (Luong et al., 2001).
Various cellular study using ECIS has been reported
such as in monitoring growth, proliferation and
differentiation of cells, cell migration and
cytotoxicity (Cui et al., 2017; Anh-Nguyen et al.,
2016; Sun et al., 2013; Mansor and Nordin, 2018)
4 METHODOLOGY
There are several aspects that need to be considered
when developing a biosensing system to predict the
outcome of chemotherapy. ECIS is the gold standard
for monitoring cellular interaction towards drugs
exposure using impedance biosensing technique. This
technique, however, requires expensive equipment
and electrodes to be implemented in high throughput
testing (HTT). By leveraging the cheap mass
fabrication costs of printed circuit board (PCB) in the
electronics industry, we propose as an alternative
Lab-on-Chip the Lab-on-PCB as a single use,
disposable biosensor. Optimization of electrode
configuration will be done analytically and
experimentally to find the best design. A portable and
wireless impedance data acquisition system will also
be developed by embedding commercialized
AD5933 Integrated Circuit (IC) impedance converter
IC with microcontroller. Finally, chemosensitivity
analysis will be performed using the developed
impedance biosensing system by correlating the
response of cell samples toward several
chemotherapy regimen tested using biosensor and the
actual chemotherapy outcome of the patients.
4.1 Design of Electrodes
The extensive research efforts in lab-on-a-chip (LoC)
in biomedical field have shown the advantages and
feasibility of the devices in real-life application.
However, despite the said advantages, LoC has less
commercialization potential due to expensive setup
for mass-manufacturing (Moschou and Tserepi,
2017). Recently technology of lab-on-PCB (lab-on-
printed circuit board) has re-emerged as a potential
alternative to LoC in biomedical field. PCB is widely
used in the electronics industry, thus established
manufacturing companies for fabrication process can
easily be found. The biosensor proposed is a thin film
nickel-gold finishing plated on copper electrodes
through electroplating. Previous study found that the
gold-plated PCB was biocompatible with human
K562 cells (Mazzuferi et al., 2010).
Optimization of electrodes will be done to
determine the highest electric field generated on the
electrode surface. This proposed design will also be
compared with conventional Interdigitated Electrodes
(IDEs) configuration to come out with the best
design. Width (w), spacing (s) and length (l) will be
varied and simulated using COMSOL Multiphysics
to find the optimum design. The design of the IDEs is
optimized to maximize sensitivity towards changes in
the cells using ECIS technique. the IDEs geometry
will be optimized such that both the cut-off frequency
of the interfacial impedance and the solution
resistance are minimized. This allows the highest
electric field to be generated by the IDEs.
4.2 Design of Data Acquisition System
Although many researches have been conducted
using the impedance monitoring technique, most
work rely on impedance spectroscopy measurement
using traditional impedance measurement
instruments such as HP 4284A precision LCR meter
(Zou et al., 2007), HP 4194A Impedance/Gain-Phase
Analyzer (Webster et al., 2009), Agilent 4294A
Impedance Analyzer (Price et al., 2009). However,
DCBIOSTEC 2019 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
16

these traditional instruments are mostly bulky,
expensive and are difficult to be used in a portable
environment.
Intensive research has been growing to construct
a miniaturized module to replace these
instrumentations for point of care setting. The first
commercially available impedance network analyzer
implemented as a single integrated circuit (IC) was
designed and introduced by Analog Device Inc that
combines a frequency generator with a 12-bit,
1MSPS (sampling per second), analog-to-digital
converter (ADC). In this research, AD5933 will be
embedded with microcontroller unit (MCU) to make
a portable wireless data acquisition system for
impedance monitoring of the cells.
4.3 Biological Test
4.3.1 A549 Cell Lines Test
The first biological study using A549 lung cell lines
will be conducted to test the performance and
biocompatibility of the sensor. This is to come out
with the optimal electrode configuration and to ensure
the sensors are non-toxic and suitable for monitoring
cellular activities in vitro.
Electrode surface will be coated with extracellular
matrix (ECM) coating such as collagen type I to
promote cellular adhesion on to the surface of the
electrodes.
4.3.2 Chemosensitivity Test
Once the design is fixed, the chemosensitivity test
will be performed using primary lung cancer cells.
Samples will be taken from biopsy of the patients and
will be cultured in the lab until it reached suitable
passage for biosensor testing. Targeted samples are
between 10-30 different samples, as an essential
standard for pilot study of clinical device.
Chemosensitivity response of conventional
chemotherapy drugs used in treating lung cancer will
be tested against the cultured cells on the sensor, to
predict the response of each drugs on the cells.
Correlation between the sensors’ results with the
actual chemotherapy response of the patients will be
made to analyse the significance of the results
predicted by the sensors.
5 EXPECTED OUTCOME
The expected outcome of this project would be a
chemosensitivity device that is able to predict the
response of chemosensitivity based on in vitro cell
culture using the ECIS techniques. The general
system architecture is shown in Figure 1 below.
Figure 1: Overall system architecture for personalized
chemotherapy response.
There are three main parts in the system. The first
part is the impedance biosensor, which will be
fabricated using gold-plated PCB. The electrodes will
be applied with low alternating voltage at 10kHz
frequency for monitoring the cellular behaviour based
on cellular adhesion on the electrodes.
The second part is the portable impedance
measurement system, which mainly consist of
AD5933 impedance analyser IC which will be
controlled by MCU together with the Analog Front
End (AFE) as the signal conditioning circuit for
controlling current exposure to the cells on the
electrodes.
The last part is the wireless data acquisition
system, which is also controlled by the MCU
connected with the network. All raw data measured
by the system will be transferred and stored in a cloud
server and can be remotely access from other
location.
The system will be embedded in a small device
packaging to make it suitable to be placed inside the
incubator during testing. Cellular behaviour of the
cells can continuously be monitored for several days
Design and Development of Parallel Biosensing System for Personalized Chemotherapy Treatment
17
to show the response of chemotherapy drugs on the
cancer cells.
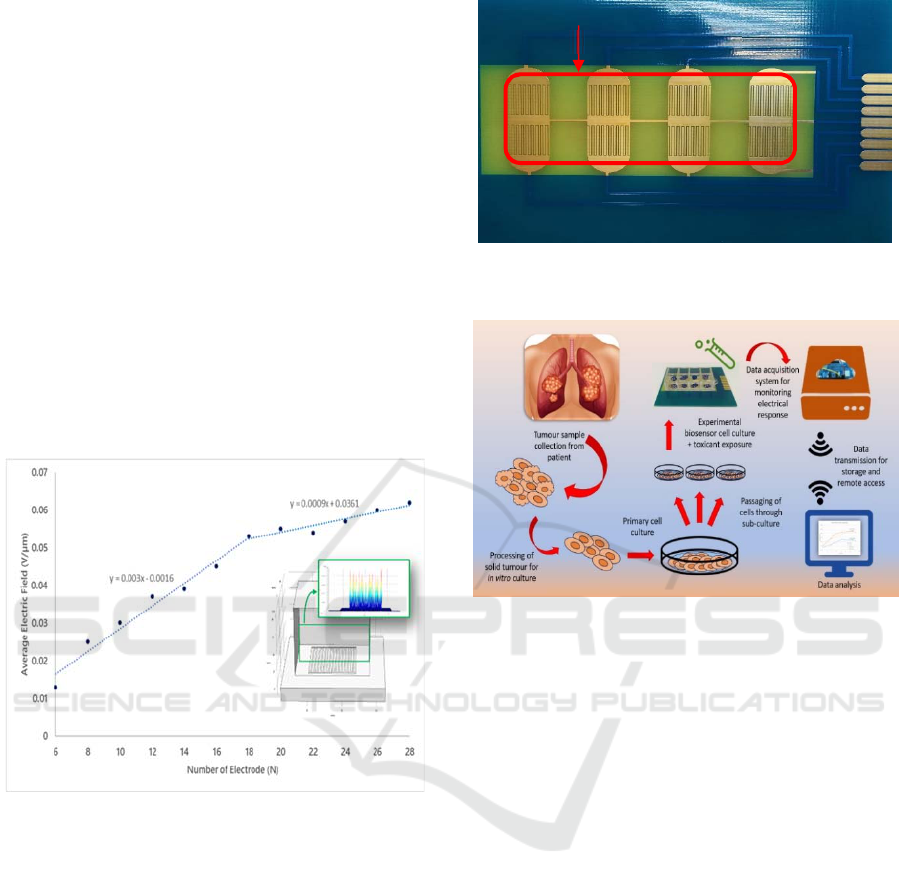
6 STAGE OF THE RESEARCH
This research has arrived at the stage of development
of data acquisition system and sensor validation.
Electrode design was analytically optimized to obtain
the best configuration for the sensor. Simulation
using COMSOL was done such as shown in Figure 2.
Optimization of electrode configuration was done in
term of finding lower cut-off frequency and
minimizing bulk resistance by varying width of
electrodes (W), spacing between electrodes (S) and
total number of electrodes (N). After simulation, the
design was sent out for fabrication using gold coated
PCB technique. Prototype sensor is shown in Figure
3.
Figure 2: COMSOL Simulation of optimizing the
parameter for the electrode configuration. Simulation
suggested N=18 is the optimal number of electrodes for
having saturated average electric field on electrode’s
surface.
As of the current stage, sensors are tested with
A549 lung cancer cell lines to optimize cell seeding,
coating concentration and to check for toxicity of the
biomaterial. Next step involves development of
wireless data acquisition system and establishment of
primary lung cancer cell culture protocol before
chemosensitivity testing using biosensor. Figure 4
shows the flows of expected outcome of the research.
Figure 3: Fabricated sensor with gold coated surface
finishing PCB.
Figure 4: Flows of expected outcome of the research.
REFERENCES
Anh-Nguyen, T., Tiberius, B., Pliquett, U. and Urban, G.A.,
2016. An impedance biosensor for monitoring cancer
cell attachment, spreading and drug-induced apoptosis.
Sensors and Actuators A: Physical, 241, pp.231-237.
Ancona, E., Ruol, A., Santi, S., Merigliano, S., Chiarion
Sileni, V., Koussis, H., Zaninotto, G., Bonavina, L. and
Peracchia, A., 2001. Only pathologic complete
response to neoadjuvant chemotherapy improves
significantly the long-term survival of patients with
resectable esophageal squamous cell carcinoma: final
report of a randomized, controlled trial of preoperative
chemotherapy versus surgery alone. Cancer:
Interdisciplinary International Journal of the American
Cancer Society, 91(11), pp.2165-2174.
Bramwell, V.H., Burgers, M.V., Souhami, R.L., Taminiau,
A.H., Van Der Eijken, J.W., Craft, A.W., Malcolm,
A.J., Uscinska, B., Kirkpatrick, A.L., Machin, D. and
Van Glabbeke, M.M., 1997. A randomized comparison
of two short intensive chemotherapy regimens in
children and young adults with osteosarcoma: results in
patients with metastases: a study of the European
Osteosarcoma Intergroup. Sarcoma, 1(3-4), pp.155-
160.
Cui, Y., An, Y., Jin, T., Zhang, F. and He, P., 2017. Real-
time monitoring of skin wound healing on nano-
Sensin
g
Area
DCBIOSTEC 2019 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
18

grooves topography using electric cell-substrate
impedance sensing (ECIS). Sensors and Actuators B:
Chemical, 250, pp.461-468.
Hong, J., Kandasamy, K., Marimuthu, M., Choi, C.S. and
Kim, S., 2011. Electrical cell-substrate impedance
sensing as a non-invasive tool for cancer cell study.
Analyst, 136(2), pp.237-245.
Kiilerich-Pedersen, K. and Rozlosnik, N., 2012. Cell-based
biosensors: electrical sensing in microfluidic devices.
Diagnostics, 2(4), pp.83-96.
Lazcka, O., Del Campo, F.J. and Munoz, F.X., 2007.
Pathogen detection: a perspective of traditional
methods and biosensors. Biosensors and bioelectronics,
22(7), pp.1205-1217.
Lowy, A.M., Mansfield, P.F., Leach, S.D., Pazdur, R.,
Dumas, P. and Ajani, J.A., 1999. Response to
neoadjuvant chemotherapy best predicts survival after
curative resection of gastric cancer. Annals of surgery,
229(3), p.303.
Luong, J.H., Habibi-Rezaei, M., Meghrous, J., Xiao, C.,
Male, K.B. and Kamen, A., 2001. Monitoring motility,
spreading, and mortality of adherent insect cells using
an impedance sensor. Analytical chemistry, 73(8),
pp.1844-1848.
Mansor, A.F.M. and Nordin, A.N., 2018, September.
Theoretical Modelling of Interdigitated Electrode
Sensor for Mammalian Cell Characterization. In 2018
7th International Conference on Computer and
Communication Engineering (ICCCE) (pp. 62-67).
IEEE.
Mazzuferi, M., Bovolenta, R., Bocchi, M., Braun, T.,
Bauer, J., Jung, E., Iafelice, B., Guerrieri, R., Destro, F.,
Borgatti, M. and Bianchi, N., 2010. The
biocompatibility of materials used in printed circuit
board technologies with respect to primary neuronal
and K562 cells. Biomaterials, 31(6), pp.1045-1054.
Moschou, D. and Tserepi, A., 2017. The lab-on-PCB
approach: tackling the μTAS commercial upscaling
bottleneck. Lab on a Chip, 17(8), pp.1388-1405.
Price, D.T., Rahman, A.R.A. and Bhansali, S., 2009.
Design rule for optimization of microelectrodes used in
electric cell-substrate impedance sensing (ECIS).
Biosensors and Bioelectronics, 24(7), pp.2071-2076.
Sjoquist, K.M., Burmeister, B.H., Smithers, B.M.,
Zalcberg, J.R., Simes, R.J., Barbour, A., Gebski, V. and
Australasian Gastro-Intestinal Trials Group, 2011.
Survival after neoadjuvant chemotherapy or
chemoradiotherapy for resectable oesophageal
carcinoma: an updated meta-analysis. The lancet
oncology, 12(7), pp.681-692.
Sun, X., Ji, J., Jiang, D., Li, X., Zhang, Y., Li, Z. and Wu,
Y., 2013. Development of a novel electrochemical
sensor using pheochromocytoma cells and its
assessment of acrylamide cytotoxicity. Biosensors and
Bioelectronics, 44, pp.122-126.
Webster, M.S., Timoshkin, I.V., MacGregor, S.J. and
Mattey, M., 2009. Computer aided modelling of an
interdigitated microelectrode array impedance
biosensor for the detection of bacteria. IEEE
Transactions on Dielectrics and Electrical Insulation,
16(5).
Zhang, B., Wang, R., Wang, Y. and Li, Y., 2013,
November. A portable impedance biosensor for
detection of multiple avian influenza viruses. In
Sensors, 2013 IEEE (pp. 1-4). IEEE.
Zou, Z., Kai, J., Rust, M.J., Han, J. and Ahn, C.H., 2007.
Functionalized nano interdigitated electrodes arrays on
polymer with integrated microfluidics for direct bio-
affinity sensing using impedimetric measurement.
Sensors and Actuators A: Physical, 136(2), pp.518-526.
Design and Development of Parallel Biosensing System for Personalized Chemotherapy Treatment
19
