
The Approach to the Detection of the Movement Precursor by
Electromyographic Signals
Semen Kurkin
1 a
, Vladimir Khorev
2 b
, Elena Pitsik
1 c
, Vladimir Maksimenko
1 d
and
Alexander Hramov
1 e
1
Neuroscience and Cognitive Technology Lab, Innopolis University, Innopolis, Russian Federation
2
Department of Dynamical Modeling and BioMedical Engineering, Saratov State University, Astrakhanskaya Street 83,
Saratov, Russian Federation
Keywords: EMG, Electromyogram, Movement Detection, Data Analysis, Pattern Recognition.
Abstract: We have developed a technique allowing automatic detection of the precursor of movement beginning based
on the analysis of electromyographic signals. Methods for determining the beginning of movement and the
moments of movement planning are of urgent need in neuroscience, and a separate problem is the use of
muscle electrical activity signals (electromyograms) to accurately determine the beginning of hand movement
due to the complexity, short duration and noise of the original signals. This issue is particularly significant
for experiments with simultaneous recording of electroencephalograms, when it is necessary to consider the
interaction between the structures of the brain. We have found out that in the case when the movement starts
on a certain sound signal, the moment of the movement beginning is detected with a some time delay.
1 INTRODUCTION
The development of effective methods for
determining the precursor of movement beginning
and the moments of movement planning is an urgent
problem of neuroscience and neurotechnology. In
particular, this task is closely related to the
development of human-machine interfaces. A
separate problem here is the use of muscle electrical
activity signals (electromyograms) for exact
detection of the of limb movement precursor. This
issue is particularly acute when conducting
experiments with simultaneous recording of
electroencephalograms, when the relationship
between the excitation of certain brain areas and
human motor activity is investigated.
Currently, the problem of studying the processes
occurring in human body related to the performance
of motor activity attracts a large scientific interest
(Wood et al., 2014; Hayashibe et al., 2015;
Maksimenko et al, 2018; Mondini et al., 2018). The
a
https://orcid.org/0000-0002-3438-5717
b
https://orcid.org/0000-0001-6613-8940
c
https://orcid.org/0000-0003-1850-2394
d
https://orcid.org/0000-0002-4632-6896
e
https://orcid.org/0000-0003-2787-2530
relevance of this research area is connected with the
possibility of applying the results in such areas as
rehabilitation, prosthetics, robotics and others.
However, the use of additional methods for the
analysis of motor activity, basically, involves
conducting an experiment according to a previously
developed plan, according to which movements are
performed on a special sound signal. In this case,
there is the problem of accurate determining the
moment of the start of movement (Reis, 2014).
To solve this problem, it seems promising to use
signals of electrical activity excited directly by
muscle fibers – electromyograms (EMG) (Rouillard
et al., 2015). The analysis of EMG signals, in turn, is
difficult due to the low amplitude of the potentials,
the strong nonstationarity, the presence of various
artifacts and the poor structuring of the initial data
(Basmajian, 1979; De Luka, 2010; Kastalskiy et al.,
2018).
Thus, there is now a need to develop new effective
methods for EMG signals analysis and for their
276
Kurkin, S., Khorev, V., Pitsik, E., Maksimenko, V. and Hramov, A.
The Approach to the Detection of the Movement Precursor by Electromyographic Signals.
DOI: 10.5220/0007916502760280
In Proceedings of the 16th International Conference on Informatics in Control, Automation and Robotics (ICINCO 2019), pages 276-280
ISBN: 978-989-758-380-3
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
application for a detailed study of human motor
activity.
2 METHODS
2.1 Data Preparation
In the course of the experiment, the registration of
non-invasive EMG signals from the elbow muscle
was carried out. The subject was in an upright
position. The subject had no pathologies of the central
nervous system.
The duration of the experiment was 150 minutes.
During the recording of signals, breathing was
arbitrary.
The subject was instructed to perform on the
sound signal the following actions: (1) flexion and (2)
subsequent extension of the hand with intermediate
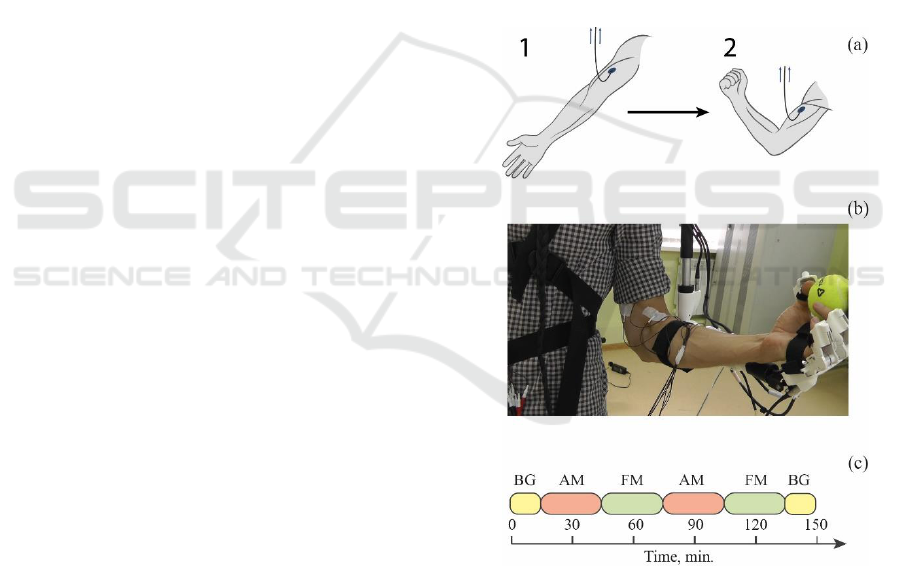
fixation in the upper position (see Fig. 1a).
Registration of EMG signals was carried out using a
multichannel electroencephalograph-analyzer
“Encephalan-131-03”, model 10 (Taganrog, Russian
Federation) with a set of standard sensors. Signals
were recorded at a sampling frequency of 250 Hz with
a 12-bit resolution.
For additional control and registration of motor
activity, a copy-type setting device was used, which
is a lever construction made of plastic and light
alloys, made similar to the human skeletal scheme
with the coincidence of the position of the mobility
axes and joints (exoskeleton). The lever mechanism
was identical to the kinematic scheme of a human
hand and contained an analogue of the forearm
connected to the shoulder with a rotational pair with
one degree of freedom, which allows to obtain data
on the flexion of the elbow joint simultaneously with
EMG recording.
2.2 Experimental Setup
The structure of the experiment is shown in Fig. 1c.
In total, the experiment consisted of six sessions and
included pre-registration of background activity
without subject performing special instructions (BG,
15 minutes), two half-hour sessions with flexion of
the hand on the sound signal (AM), two sessions with
arbitrary flexion of the hand (FM), the final
registration of the background activity without the
subject performing special instructions for 15
minutes. The beginning of each session was preceded
by an automatic audiovisual warning of the subject
about its occurrence. For sessions with flexion of the
hand on the sound signal 50 movement repetitions
were planned. Sound stimuli were given at arbitrary
moments of time but provided for at least 10 seconds
of rest between every two movements. For the session
with an arbitrary flexion of the hand, no sound stimuli
were given, however, the subject was instructed to be
at rest also for at least 10 seconds after each period of
motor activity. The experiment was conducted in the
first half of the day in a specially equipped laboratory,
where the volunteer was in comfortable environment,
eliminating the presence of distracting factors, such as
background noise and bright light.
2.3 Methods
To detect the precursor of the hand movement, the
EMG signal was numerically filtered in the frequency
band 1–10 Hz, then smoothed by a sliding window of
2 s in length, after which the derivative of the signal
was found along the smoothed series.
Figure 1: (a) A schematic representation of the movement of
the subject's hand with a connected sensor for measuring the
EMG signal during the experiment; 1 corresponds to the
extension of the hand, and 2 – to the flexion. (b) Photograph
of the subject's arm with sensors for measuring EMG signals
and exoskeleton. (c) The structure of the experiment that
contains the following sessions: BG, AM and FM denote a
single period of background activity, audio stimulated
movement and free movement, respectively. Each session
was preceded by the video message with instructions.
Comparing the original EMG signal and the derived
The Approach to the Detection of the Movement Precursor by Electromyographic Signals
277
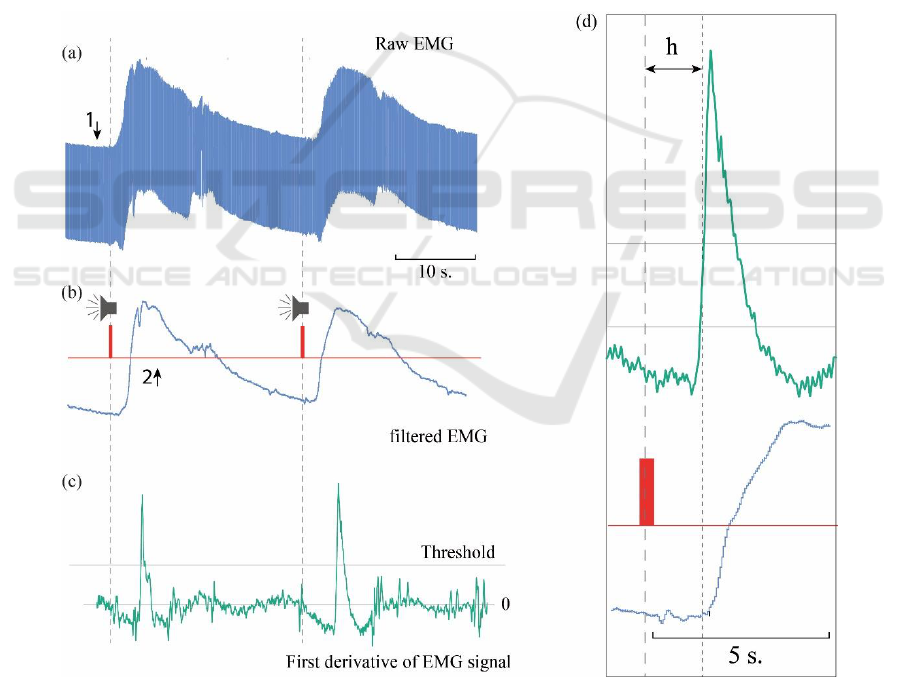
derivative, it was found that at the time points
corresponding to the beginning of the movement, value
of the derivative exceeds the threshold value.
Thus, comparing the received signal with the
threshold value at each moment of time, the moments
corresponding to the beginnings of movement
(precursors of the movement) were determined.
Figure 2 shows a fragment of typical raw EMG signal
recorded from an elbow muscle (Fig. 2a), smoothed
time series of the EMG (Fig. 2b), and its derivative
(Fig. 2c). The grey line corresponds to the threshold
value of the derivative used for automatic detection
of the moment of movement beginning.
The red risks mark the moments of the sound signals
corresponding to the commands. The Fig. 2 shows
that a sharp increase in the amplitude of the registered
signal corresponds to the moments of the beginning
of the movements.
3 RESULTS
On the basis of the conducted research, the optimal
threshold value of the derivative of the EMG signal
was determined, which ensures the best ratio of the
sensitivity and the percentage of false conclusions of
the algorithm for determining the moments of the
movement beginning. This value is equal to 0.5 of the
maximum value of the series derivative.
Fig. 3 shows the dependencies of true positives (TP)
and false positives (FP) of the algorithm for
determining the movement beginning on the
threshold value R. The threshold value varied in the
range from zero to the maximum value of the series
with a step of 0.05. It is clearly seen from Fig. 3 that
the maximum difference between TP and FP (the best
accuracy of the algorithm) is observed at the value of
Figure 2: (a) Fragment of the original (raw) experimental EMG signal; (b) smoothed and filtered EMG signal (blue curve);
(c) its derivative (green curve). The moments of the sound signals are marked in red, the line of the threshold value used to
determine the moments of the beginning of the movements is marked with the grey line (threshold). (d) Enlarged fragment
of the filtered signal and its derivative, which demonstrates the delay h between the moment of presentation of the sound
signal and the moment of beginning of the movement.
ICINCO 2019 - 16th International Conference on Informatics in Control, Automation and Robotics
278
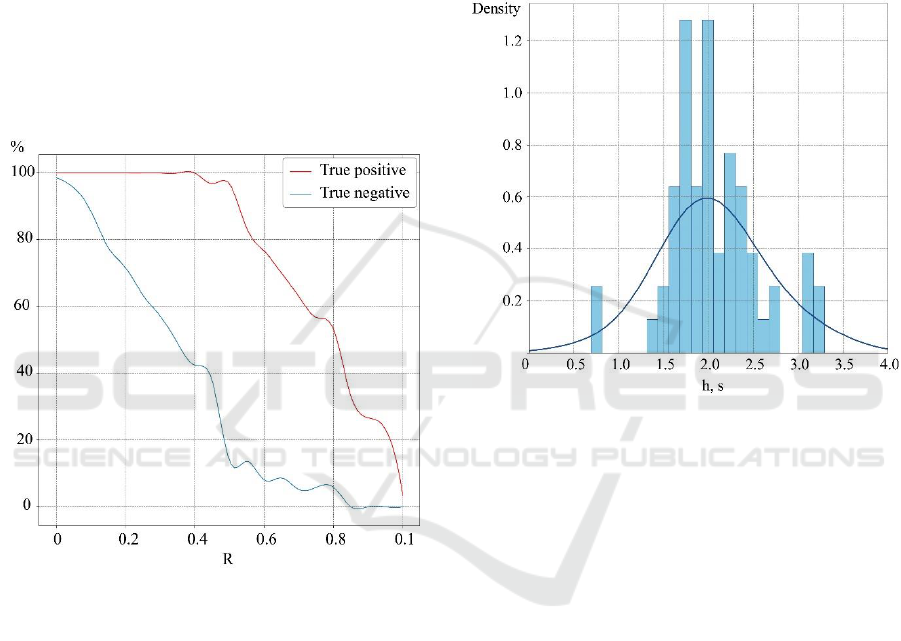
R equal to 0.5 of the maximum value of the series.
Note, that this value of R was then used to calculate
the distribution of delays h between the time of sound
signal presentation and the beginning of the
movement. The advantage of this approach is its
simplicity and speed, compared with more accurate
and complex methods that require individual training
of subjects.
Indeed, it can be seen from Fig. 2 that there is a time
delay h between the moment when the sound signal is
presented, and the detected time moment (precursor)
of movement beginning. The analysis of the
characteristic time delay h is shown in Fig. 4 in the
form of the distribution obtained in one typical
experimental session. The blue curve in the figure is
Gaussian kernel density estimate.
Figure 3: Dependencies of true positives (TP, red curve)
and false positives (FP, blue curve) of determining the
movement beginning on the threshold value R. The
dependencies are given in percent.
It can be seen that the mode of the distribution
corresponds to the time 1.6–1.8 s. The narrowness of
the distribution obtained suggests that the preparation
time for movement can be estimated and then
considered in the experiment without presenting a
sound signal. The causes of the time delay detected in
the work may be related to the processes of the
stimulus processing and movement planning. In this
context, the use of EMG signals provides great
potential for identifying the various phases associated
with the implementation of human motor activity.
The modern concept of the mechanism of conditional
connection closure (Hazy et al., 2009) assumes that
the association of excitation foci corresponding to the
conditioned and unconditioned stimuli can occur both
at the level of the cortex and at the level of the
subcortex. With continued flowing along specific
paths to a certain limited cortical focus of afferent
impulses, gradually generalized excitation is
concentrated in this focus. Then it gives a significant
part of its influence on the construction of movement
to the underlying foci of excitation, which have the
advantage that afferent proprioceptive impulses
continue to flow to them.
Figure 4: The distribution of time delays between the
moment of presentation of the sound signal and the detected
time moment (precursor) of movement beginning. The
distribution is based on the results of one experimental
session consisting of a series of repetitions of the
movement. The results were obtained for the optimal
threshold value R for the derivative of the smoothed EMG
signal. The blue curve is Gaussian kernel density estimate.
Recent studies indicate the presence of delays in the
activation of sensorimotor processing in the human
brain associated with the phases of formation,
recognition of the stimulus, categorization of
response, decision making and reaction of afferent
neurons that have times comparable to those obtained
in this work, although they take less values due to the
specifics of the experiment (Melnik et al., 2017;
Asakawa et al., 2014).
It should be noted, that the distribution in Fig. 4 is
rather well approximated by the Gaussian-like
distribution. The time it takes for the pulse to travel
from the brain to the muscle and the reaction time of
the muscle are approximately constant for all
repetitions of movement. Consequently, the noise
component, which determines the form of distribution
of the time delay h (Fig. 4), is a consequence of the
processes occurring in the brain, when processing the
The Approach to the Detection of the Movement Precursor by Electromyographic Signals
279

stimulus and the generating the control signal. Indeed,
the effect of “brain noise” was discovered in
(Pisarchik et al., 2019; Runnova et al., 2016). Thus, it
can be assumed that the initial state of the brain before
each act of movement is different and is determined
by the processes in the brain at this moment, which
determines the noise nature of the distribution.
4 CONCLUSIONS
In the work, a method was proposed that allows to
automatically determine the precursor of the
movement beginning, based on the analysis of EMG
signals. It was found out that in the case when the
motion begins on the sound signal, the moment of the
start of motion is detected some time after the signal,
the distribution of which is approximated fairly well
by the Gaussian-like distribution. Possible causes and
background of the obtained results are discussed. The
obtained results can be used to isolate the phases of
“movement planning” and contribute to solving a
number of applied problems associated with
improving the quality of life of people and with
development of human-machine interfaces. The
proposed technique has the potential for application
in human-machine interfaces.
ACKNOWLEDGEMENTS
This work has been supported by Russian Science
Foundation (Grant 17-72-30003).
REFERENCES
Wood, G., Kober, S., Witte, M., Neuper, C., 2014. On the
need to better specify the concept of “control” in
brain-computer-interfaces/neurofeedback research.
Front Syst Neurosci. Vol. 8, pp. 171.
https://doi.org/10.3389/fnsys.2014.00171.
Hayashibe, M., Guiraud, D., Pons, J., Farina, D., 2015
Editorial: biosignal processing and computational
methods to enhance sensory motor neuroprosthetics.
Front Syst Neurosci. Vol. 9, pp. 434.
Maksimenko V.A., Pavlov A., Runnova A.E., Nedaivozov
V., Grubov V., Koronovskii A., Pchelintseva S.V.,
Pitsik E., Pisarchik A.N., 2018. Hramov A.E. Nonlinear
analysis of brain activity, associated with motor action
and motor imaginary in untrained subjects. Nonlinear
Dynamics. Vol. 91(4) pp. 2803-2817 DOI:
10.1007/s11071-018-4047-y.
Mondini, V., Mangia, A., Cappella, A., 2018. Single-
session tDCS over the dominant hemisphere affects
contralateral spectral EEG power, but does not enhance
neurofeedback-guided event-related desynchronization
of the non-dominant hemisphere's sensorimotor
rhythm, PLoS One. Vol. 13(3), e0193004.
Reis, P., Hebenstreit, F., Gabsteiger, F., von Tscharner, V.,
Lochmann, M., 2014. Methodological aspects of EEG
and body dynamics measurements during motion.
Frontiers in Human Neuroscience. Vol. 8, pp. 156.
Rouillard, J., Duprèsa, A., Cabestainga, F., Leclercqb, S.,
Bekaerta, M., Piaua, C., Vannobela, J., Lecocq, C.,
2015. Hybrid BCI coupling EEG and EMG for severe
motor disabilities, Procedia Manufacturing. Vol. 3, pp.
29–36.
Basmajian, J., 1979. Muscle alive, their functions are
revealed by electromyography. Williams and Wilkins,
4th edition.
De Luca, C., 2010. Filtering the surface EMG signal:
Movement artifact and baseline noise contamination,
Journal of Biomechanics. Vol. 43, pp. 1573–1579.
Kastalskiy, I., Mironov, V., Lobov, S., Krilova, N.,
Pimashkin, A., Kazantsev, V., 2018. Neuromuscular
Interface for Robotic Devices Control. Computational
and Mathematical Methods in Medicine. 8948145.
Hazy, T., Frank, M., O’Reilly, R., 2009. Neural
Mechanisms Supporting Acquired Phasic Dopamine
Responses in Learning: An Integrative Synthesis.
Neuroscience and Biobehavioral Reviews. Vol. 34(5),
pp. 701–720.
Melnik, A., Hairston, W., Ferris, D., König, P., 2017. EEG
correlates of sensorimotor processing: independent
components involved in sensory and motor processing.
Scientific Reports. Vol. 7, pp. 4461.
Asakawa, T., Muramatsu, A., Hayashi, T., Urata, T., Taya
M., Mizuno-Matsumoto Y., 2014. Comparison of EEG
propagation speeds under emotional stimuli on
smartphone between the different anxiety states.
Frontiers in Human Neuroscience. vol. 8, pp. 1006.
Pisarchik, A.N., Chholak, P., Hramov, A.E., 2019. Brain
noise estimation from MEG response to flickering
visual stimulation. Chaos, Solitons & Fractals: X.
Vol. 1, pp. 100005. DOI: 10.1016/j.csfx.2019.100005
Runnova, A.E., Hramov, A.E., Grubov, V.V., Koronovskii,
A.A., Kurovskaya, M.K., Pisarchik, A.N., 2016.
Theoretical background and experimental
measurements of human brain noise intensity in
perception of ambiguous images. Chaos, Solitons and
Fractals. Vol. 93, pp. 201-206.
ICINCO 2019 - 16th International Conference on Informatics in Control, Automation and Robotics
280
