Multitask Learning or Transfer Learning? Application to Cancer
Detection
Stephen Obonyo and Daniel Ruiru
Faculty of Information Technology, Strathmore University, Ole Sangale Link Road, Nairobi, Kenya
Keywords:
Multitask Learning, Transfer Learning, Deep Learning, Cancer Detection, Medical Imaging.
Abstract:
Multitask Learning (MTL) and Transfer Learning (TL) are two key Machine Learning (ML) approaches which
have been widely adopted to improve model’s performance. In Deep Learning (DL) context, these two learning
methods have contributed to competitive results in various areas of application even if the size of dataset is
relatively small. While MTL involves learning from a key task and other auxiliary tasks simultaneously and
sharing signals among them, TL focuses on the transfer of knowledge from already existing solution within
the same domain. In this paper, we present MTL and TL based models and their application to Invasive Ductal
Carcinoma (IDC) detection. During training, the key learning task in MTL was detection of IDC whereas
skin and brain tumor were auxiliary tasks. On the other hand, the TL-based model was trained on skin cancer
dataset and the learned representations transferred in order to detect IDC. The accuracy difference between
MTL-based model and TL-based model on IDC detection was 8.6% on validation set and 9.37% on training
set. On comparing the loss metric of the same models, a cross entropy of 0.18 and 0.08 was recorded on
validation set and training set respectively.
1 INTRODUCTION
In this study, we seek to investigate the effectiveness
of the application of Transfer Learning (TL) and Mul-
titask Learning (MTL) in Invasive Ductal Carcinoma
(IDC) cancer detection. Multitask Learning can be
defined as a learning method where a model learns by
not only focusing on a single task T but other aux-
iliary tasks T
0
, T
1
... T
k
as well. The signals from
the auxiliary tasks generally improves model’s per-
formance on key task (Ruder, 2017). This characteri-
zation can be attributed to the fact that MTL architec-
ture allows for sharing of informative representations
among the tasks involved. Further, MTL has been
shown to improve the ability of the model to gener-
alize well on unseen instances (Ruder, 2017).
In contrast, Transfer Learning (TL) focuses on the
improvement of a model’s performance via transfer of
knowledge from an already existing solution (source
task) often within the same domain. Given a problem
(target task), the aim of TL is to improve the perfor-
mance by combining source task knowledge represen-
tations and related data (Torrey and Shavlik, 2010).
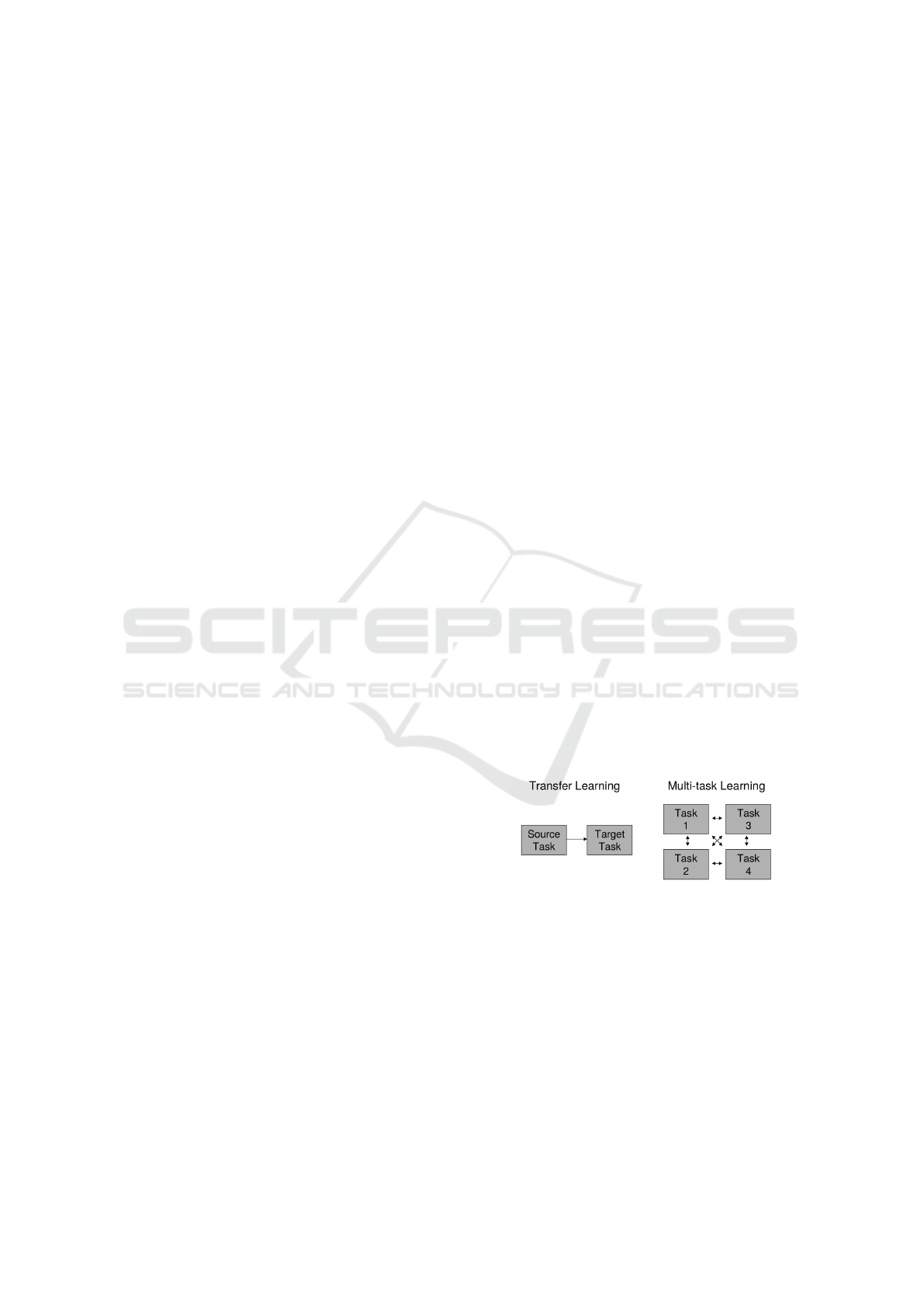
MTL and TL are somewhat related but the flow of
information between the two is restricted as shown in
Figure 1. In TL, information is unidirectional i.e. the
flow is from source (already learned representations)
to the target (new problem). On the other hand, in
MTL the information flow is unrestricted and infor-
mation can flow in any direction (among all the task
related models). This behaviour is captured by Figure
1.
Figure 1: Multitask Learning vs Transfer learning. Source
(Torrey and Shavlik, 2010).
This research compares the application of both
MTL and TL to cancer detection. The tasks which
were involved include detection of i) Invasive Duc-
tal Carcinoma (a common sub-type of breast cancer),
ii) skin cancer and iii) brain tumour. Based on these
tasks both MTL-based and TL-based models were de-
veloped and their performance compared.
The key learning task for MTL-based model was
IDC detection. Skin and brain cancer detection were
auxiliary tasks. The TL-based model was developed
by first training the base network to detect the skin
548
Obonyo, S. and Ruiru, D.
Multitask Learning or Transfer Learning? Application to Cancer Detection.
DOI: 10.5220/0008495805480555
In Proceedings of the 11th International Joint Conference on Computational Intelligence (IJCCI 2019), pages 548-555
ISBN: 978-989-758-384-1
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

cancer. The learned representations from the model
were transferred and used to detect IDC. The outcome
of these two learning methodologies were presented
and discussed on the results and discussion sections
of this document.
Cancer research is likely to benefit from both
MTL and TL learning approaches since they hold
the potential towards developing robust models, even
when relatively smaller datasets are available. This
has translated to a development of stable models with
an ability to generalize well on unseen examples.
2 RELATED WORK
2.1 Machine Learning in Cancer
Medical Imaging
Cancer is by far the most adaptive and self-sustaining
conditions currently known within the medical field.
It dynamically interacts with its micro-environments
to constantly thwart the efforts of practitioners, re-
searchers and most importantly the affected patients.
Various complexities associated with the disease
results in endless dilemmas at the different stages of
its management, including the need for reliable early
detection (Bi et al., 2019). Moreover, there are the
problems associated with the accurate identification
of preneoplastic and neoplastic lesions; the tracking
of tumors; resistance to treatment; and the regulation
of infiltrative tumor margins in surgical procedures
etc. However, technological advances particularly in
medical imaging and the identification of bio-markers
hold great promise in addressing these challenges.
Machine Learning and its sub-branches have the
ability to automate image analysis and this could
potentially bridge the gap between cancer medical
imaging and the accurate interpretation of conditions
(Klang, 2018). Currently, there is disparity in manual
cancer diagnosis. This hinders the treatment and the
probable recovery of patients. These conventional or
manual method of image evaluation rely heavily on
the qualitative features of tumor cells such as density,
pattern, cellular composition and anatomic relation-
ship, among others. These features are hard to accu-
rately determine due to the varying image dimensions.
In comparison, there exist radiomics which use
quantitative features to analyze radiographic images.
Broadly, radiomics uses the quantitative features of
size, shape and textual patterns to describe medical
images, which are better representations of tasks in
ML (Bi et al., 2019). These types of descriptors
could thus facilitate the role of Artificial Intelligence
in medicine as the field has made great strides in au-
tomating the quantification of medical patterns. Deep
learning in particular has the most promise having
developed various models for learning and match-
ing features in different problems. The ability of the
implemented algorithms even surpasses those of hu-
man expert which further defines their significance in
task-specific functions as they can be specialized as
needed. Moreover, they are able to overcome the bar-
riers of large data sets including the ability to with-
stand noise in foundation truth tables. In all, the ca-
pabilities of deep learning could provide exceptional
insights into both qualitative and quantitative analy-
sis further helping medical evaluations. This facili-
tation could for instance be achieved by the precise
delineation of tumors, parallel tracking of lesions and
the cross-referencing of tumors in related fields. Ul-
timately, deep learning methods promise the greatest
generalization capabilities through the transfer of in-
sights across various medical domains. It is these ben-
efits among others that could provide the health care
industry with the necessary tools for future break-
throughs.
2.2 Transfer Learning
The idea of generalizing models is an important ele-
ment of transfer learning which in recent times has in-
creased the popularity of the technique. Like in many
other domains, transfer learning in medical imaging
aims to transfer information from a particular clas-
sification problem(s) (source) to another (the target),
hence improve the performance of the final classifier.
In cancer treatments, this facilitation is important as
the field has minimal data owing to the expenses in-
volved and the few breakthroughs seen so far.
2.2.1 Transfer Learning Approaches
Despite the progress made in transfer learning there
exist a lot of inconsistencies in the classification of
its sub-branches. Traditionally, the categorization has
been done using three main groups which are based
on the similarities between domains and the availabil-
ity of labeled/unlabeled data. Inductive, transductive
and unsupervised transfer learning have thus been the
three main categories. However, advances in deep
learning have increased the scope of TL and today
have led to a more flexible taxonomy having two main
categories (Asgarian, 2019). This new taxonomy is
based on the similarity of domains and has two major
groupings: Homogenous transfer learning and het-
erogeneous transfer learning.
Multitask Learning or Transfer Learning? Application to Cancer Detection
549

2.2.2 Homogeneous and Heterogeneous
In homogenous TL, both the source and target tasks
have the same feature and label space. As such, the
aim is usually to bridge the gap of data distribution
that exist in the two instances (source and target). The
somewhat reverse outcome is exhibited by heteroge-
neous TL as the source and target tasks have different
feature spaces (non-overlapping). However, for the
label space, a unique set up exists where the source
and target domains can either share or have different
data labels. From these broad classifications of TL,
the solutions offered by the technique can be summa-
rized into five different classes as outlined below.
1. Instance-based Approaches: Tries to re-weight
samples in source tasks to correct marginal dis-
tribution differences. These re-weight instances
are then directly applied in target domains during
training
2. Feature-based Approaches: Are applied in both
homogeneous and heterogeneous problems where
in the latter setup they aim to reduce the differ-
ences in feature spaces. In homogeneous prob-
lems they are then used to correct the marginal
and conditional distributions
3. Parameter-based Approaches: These approaches
transfer knowledge using the shared parameters of
the domains involved
4. Hybrid-based Approaches: These techniques
transfer knowledge through both the instances and
parameters shared by the tasks involved
5. Relational-based Approaches: These final ap-
proaches use the common relationships between
the source and target domains to transfer knowl-
edge (Asgarian, 2019).
2.2.3 Empirical Results
Motivated by the current trends in deep learning, ma-
chine learning researchers have moved to develop al-
gorithms that automatically classify cancer medical
images. In particular, there has been a great empha-
sis on the transfer of features from pre-trained models
due to the limited datasets (training corpus) found in
the cancer domain. This borrowing of factors, bet-
ter known as transfer learning has yielded better clas-
sification results and even helped generalize models.
Take the example of transfer learning in the early gas-
tric cancer classification as done by Liu et al. (2018).
Using Magnifying Narrow-Band Imaging Images (M-
NBI), this group of researchers were able to attain
higher prediction accuracy with TL as compared to
traditional ML methods. On average, a 96 percent
accuracy was achieved, a value that on occasion im-
proved by either fine tuning the final layers or all the
layers of the applied model (Convolutional neural net-
works (CNN)). To further test the credibility of the
result, different variations of CNN were used namely;
VGG-16, InceptionResNet-v2 and Inception-v3. Ul-
timately, the research conducted found that the per-
formance gain increased as the convolutional layers
were fine-tuned with natural data (Liu et al., 2018).
Furthermore, the amount of the input data (images)
influenced the final result of the deep learning models.
Since, the field in question has limited data (cancer
and more specifically M-NBI), transfer learning pro-
vided the means to meet this functional requirements
and hence improved the models’ performance.
2.2.4 Theoretical Framework
TL is common in deep learning owing to the amount
of data needed to train models. Deep learning models
require lots of data to make any meaningful predic-
tions which often is not available. TL therefore works
because it enables networks to use features learned
in previous tasks by mixing and matching their func-
tions into new as well as meaningful combinations. It
is the new collaboration that helps improve the classi-
fication of a model. This outcome is observed in both
theory and practice as models converge faster and are
more accurate with TL as compared to when they
are randomly initialized. Therefore, TL not only im-
proves prediction results but also helps to train models
faster.
Its mathematical representation highlights its the-
oretical background. Defining a domain D as a two el-
ement tuple consisting of a feature space x and prob-
ability P(X) (while space x = a sample data point),
then Domain D can be defined as
D = x,P(X). (1)
Note: In probability
P(X), X = x
1
,x
2
,. . . . . . . . . . . . , x
n
(2)
i.e.
x
i
εX (3)
Additionally, if X
i
is a specific vector. A task
T can be accurately defined by corresponding tuples
of y as label space and n as the objective function.
Therefore, for the given domain (D), Task T can be
represented as
T = y,P(Y |X) = y, nY = y
1
,. . . . . . .,y
n
,y
i
εY (4)
NCTA 2019 - 11th International Conference on Neural Computation Theory and Applications
550

2.3 Multitask Learning
Deep learning models use a combination of many hid-
den layers and parameters in their learning process to
give results. As such, they require lots of data. Can-
cer like many other medical fields does not meet this
data requirement more so, because it applies manual
labor to label data instances (Zhang and Yang, 2017).
It is therefore, a perfect case for applying multitask
learning (MTL) where useful information from mul-
tiple relevant tasks are used to alleviate the problem of
data sparsity. MTL has been a promising field in ma-
chine learning since its initial formulation by Caruana
(1997). Broadly, the goal of MTL is to leverage use-
ful data/information found in multiple learning tasks
to get more accurate learners. Of course, this ob-
jective assumes that the tasks (or their subsets) are
related. Empirically and theoretically, jointly learn-
ing various tasks has been found to give better per-
formances than when learning is done independently.
Moreover, based on the tasks, MTL can take differ-
ent setup which outlines its effective classification as:
MTL supervised learning, MTL unsupervised learn-
ing, MTL semi-supervised learning and MTL online
learning among others.
MTL helps to promote the notion that machines
can mimic human learning activities as people trans-
fer knowledge from different tasks to further others.
For instance, the skills of long jump and, running
track and field can facilitate each other, hence im-
prove the performance of an athlete. Thus, MTL is
simply an inductive transfer mechanism that aims to
improve the generalization of machine learning mod-
els (Caruana, 1997). A concept (generalization) that
it fulfills by leveraging domain-specific data from re-
lated activities through parallel training. Therefore,
the training power of the additional tasks acts as an in-
ductive bias. In this case, an inductive bias hails from
its general definition which is anything that influences
an inductive learner to prefer certain hypotheses as
compared to others.
2.3.1 Empirical Studies
Most of observation studies of MTL have focused
on feature selection problems where some attributes
in multi-source data have been used in classification
of regression experiments. In most cases, the fea-
tures in question have been related even though they
are derived from different data sources. Based on
these underlying relations, it has been found to be
easier to jointly select the necessary attributes (fea-
tures) from various sources using joint selection reg-
ularizers. These regularizers, which are simply select
constraints, have been found to improve the perfor-
mance of classification models as compared to other
conventional techniques that evaluate features indi-
vidually based on their data sources. Examples of
regularizers commonly introduced include joint spar-
sity, graph sparse coding, graph self-representation
and low rank. It is the inclusion of these elements that
has helped MTL deal with complex worldly problems
such as the diagnosis of neurodegenerative diseases
(Bib, 2019). Using structural Magnetic Resonance
Imaging (sMRI), researchers have been able to pre-
dict the values of various types of clinical scores in
these conditions, including their specific subject di-
agnostic labels. An example of this success is high-
lighted by the study of Alzheimer disease (AD) where
clinical scores such as Mini-Mental State Examina-
tion (MMSE) and Dementia Rating Scale (DRS) have
been used to grade the healthiness (functionality) of
the brain.
As specified by MTL principles, the classification
in this instance is based on the prediction of a target
output. Because the target outputs, such as diagnos-
tic labels and clinical score, are related then one gets
better results unlike when each task is learned inde-
pendently. It is this ’similar’ approach that has led
to the recent success of self-driving automation sys-
tems. In this case, images from cameras attached to
subjects are used to detect objects (road signs, traffic
lights etc.) which are then fed into neural networks to
train a model for autonomous driving. A more robust
system is acquired because the model gets to learn
multiple objects simultaneously.
2.3.2 Multitask Learning Approaches
From the discussion above, MTL is simply a type
of inductive transfer which improves algorithms by
adding an inductive bias. This bias helps a model dis-
criminate some attributes and thus, prefer some hy-
potheses over others. `
1
regularization is the most
common type of inductive bias known in ML and is
often used to get preferences for various sparse so-
lutions. In contrast, MTL attains its inductive bias
through auxiliary tasks which through their contri-
butions models certain hypotheses inclinations. To
achieve its goals, MTL commonly employs two con-
trasting ways in deep neural networks. They are; hard
and soft parameter sharing (Ruder, 2017) The shared
element comes from segmentation (sharing) of hidden
layers.
1. Hard Parameter Sharing: Its application in neu-
ral networks goes back to Caruana (1997). It
shares the hidden layers between all tasks in-
volved but also maintains a few task-specific out-
put layers. Because of its efficiency and simplicity
Multitask Learning or Transfer Learning? Application to Cancer Detection
551

it is the most common approach of MTL. It also
reduces the risks of over-fitting, a result that stems
from the ability to develop a model that represents
all tasks and not just the original concept (task).
2. Soft Parameter Sharing: On the other hand, this
approach sees each task having its own model plus
their own set of parameters. To encourage a sim-
ilarity between the distinct parameters, the dis-
tance between them in the overall model is regu-
larized using a bias function say `
2
. This applica-
tion of inductive bias functions, explains the huge
inspiration that regularization techniques have had
on the constraints of soft parameter sharing, an
outcome that still stands today.
2.3.3 Empirical Results
As highlighted before, MTL helps with the simulta-
neous solving of multiple tasks by optimizing several
loss functions instead of one. It is this application that
has seen it applied in several fields such as cancer di-
agnostics. Khosravan and Bagci (2018) specifically
applied the technique in lung cancer and eventually
were able to overcome their limitations of labeled data
for task segmentation.
Having the highest mortality rate among cancer
affiliated deaths, lung cancer has invoked a lot of re-
search in attempts to yield conclusive results. This
interest has produced many systems and models but
they all seem to suffer from the same problem of
false positive results. Additionally, the limitations of
data segmentation (initial step of data quantification)
lower the performance of the developed model. Khos-
ravan and Bagci (2018) improved on this available
models by incorporating MTL into their 3D encoder-
decoder CNN structure. In doing so, they shared un-
derlying features of tasks and trained single models
using shared features that are essential in lung can-
cer screening. Eventually, the finding of their study
saw the importance of MTL in semi-supervised learn-
ing where improved results are obtained even with-
out large data sets. Essentially, minimal labeled data
is needed when features are shared between tasks as
they get to learn from one another. Moreover, the fi-
nal model was easily generalized not accounting for
the reduced false positive result.
2.3.4 Theoretical Framework
Most learning algorithms will perform poorly when
faced with tasks having minimal data labels as well
as high dimensional space. This is a familiar outcome
in medical image analysis as seen before. MTL works
in such instances by sharing attributes and features be-
tween tasks. Caruana (1997) best summarized the im-
portance of MTL by highlighting it as technique that
simultaneously learns tasks (parallel learning) while
sharing low dimensional representations (Bib, 2019).
Thus, a common assumption that is held by MTL is
that tasks or their subsets, associate with each other
and share information. This collaboration facilitates
a joint learning process that also compares functions
eventually producing optimal independent models.
This idea of jointly learning problems can be for-
mulated as shown below. Taking N as the number of
supervised tasks. The training set for each and every
task can be denoted as T
n
= (x
i
n, y
i
n). Where I = 1: k
n
(kn being the number of training samples for all the
tasks).
Because x
i
n is an element in the set of X(n) i.e.
x
i
n part of X(n) and, y
i
n part of Y (n). Then the overall
problem of multitask learning can be summarized to
an optimization problem as defined below:
min
w
N
∑
n=1
L(Y
(n)
, f (X
(n)
+ λ k f k) (5)
L is the loss function that measures the pre-task pre-
diction error, while f is the actual multitask model. W
on the other hand is the algorithm’s parameter set.
3 DATASET & PREPROCESSING
3.1 Dataset
Different types of datasets were used in this study.
The First dataset was Invasive Ductal Carcinoma
(IDC). It was composed of 198,738 negative samples
and 78,786 positive samples. IDC is one of the most
prevalent of breast cancer sub-type.
The second dataset was skin cancer with a total of
3297 training samples. Out of this 1497 were benign
while the remaining 1800 were malignant. The third
dataset, brain MRI, was relatively smaller in compari-
son to the first and the second one. It was composed of
155 brain MRI images that are tumorous and 98 brain
MRI images that are non-tumorous. All the datasets
used in this study were obtained from various compe-
titions listed on Kaggle.
The skin, brain tumor and IDC datasets were
used in building MTL-based and TL-based models.
Succinctly, the key learning task for the MTL-based
model was detection of IDC and the corresponding
dataset was used. The same applied to the auxiliary
tasks: detection of skin and brain cancer.
Skin dataset was again used in building the TL-
based model. It was used to train the base model.
The learned representations from the base model were
NCTA 2019 - 11th International Conference on Neural Computation Theory and Applications
552
transferred and used to train a new model to detect
IDC.
3.2 Preprocessing
All the images in this study were resized to 32x32x3.
This was based on the fact that most of the tumor cells
usually occupies a very small segment given a sample
image. Apart from resizing the images, other image
preprocessing techniques applied during training in-
cluded: i) Random cropping with a padding (4), ii)
Random Horizontal Flip, iii) Random Rotation and
iv) Normalization with a mean and standard deviation
of 0.5.
4 MODEL ARCHITECTURE &
TRAINING
Two sets of models were built in this study; Multitask
Learning (MTL) and Transfer Learning (TL) based
models. These two models had a similar goal: IDC
detection.
The MTL-based model was inspired by the clas-
sical research done by Caruana (1997). The model
was built with IDC detection as the main task and
skin and brain cancer as auxiliary tasks. TL-based
model on the other hand, was built by first training
a model to detect skin cancer then the learned repre-
sentations were transferred to a new model which was
further trained (with additional layers) to detect IDC.
MTL and TL-based model’s base architecture were
designed based on ResNext network model.
ResNext, a deep neural network architecture, was
inspired by another network model called ResNet.
The ResNet network design is a deep learning model
which overcame the challenges associated with train-
ing very deep neural networks. Based on its design,
the output of successive layers is concatenated with
the original input then fed to the next layer and the
same process repeated while going deeper into the
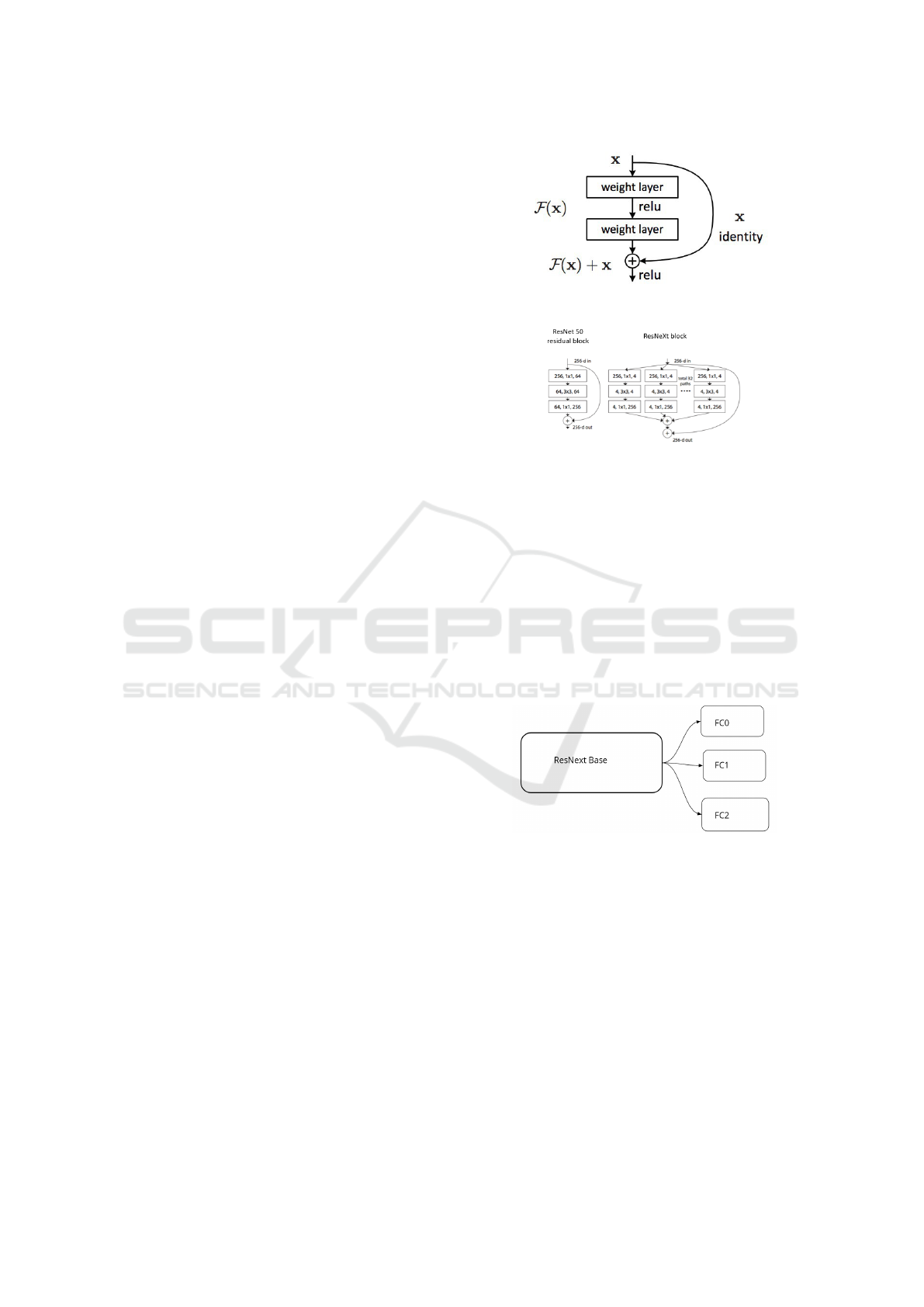
network (He et al., 2016). This design behaviour
proved effective in training a neural network with over
a hundred and even a thousand layers. Figure 2 shows
a ResNet block.
ResNeXt leverages on a split-transform-merge
strategy where branched paths are used within cells.
Instead of performing convolutions on the full input
feature map, the input block is projected over a se-
ries of lower dimensional representations which sep-
arately apply a few convolutional filters before merg-
ing them into the final result (Xie et al., 2017). Fig-
ure 3 captures the key difference between ResNet and
ResNext Architecture.
Figure 2: ResNet Block Source He et al. (2016).
Figure 3: ResNet Vs ResNext Source Xie et al. (2017).
4.1 MTL-based Model
MTL-based model was designed to learn to detect
IDC (key task), brain tumour and skin cancer (auxil-
iary tasks). The aforementioned tasks shared common
base representations (parameters) with each having a
unique last layer composed of a Fully Connected (FC)
Layer. The MTL parameter sharing technique used in
this context was hard parameter.
Figure 4 is a diagrammatic illustration of the
MTL-based model. It is composed of two major parts:
i) the base and ii) head. The base sub-section is com-
Figure 4: MTL-based Model.
posed ResNext architecture (depth: 29, cardinality: 8,
base width: 64 , widening factor: 4) shared by all the
tasks. The head part on the other hand is composed
of three different fully connected (FC) layers: FC0,
FC1 and FC2, each representing IDC, skin, brain de-
tection model respectively. All the FC layers shares
a common base while focusing on different tasks. In
essence the learning signals from skin, IDC and brain
affects the base weights as the head focuses on a more
specific tasks. The model design was influenced by
the question, Can learning from different tasks im-
prove model’s performance? which is theoretically
and empirically underlined by Caruana (1997).
Multitask Learning or Transfer Learning? Application to Cancer Detection
553
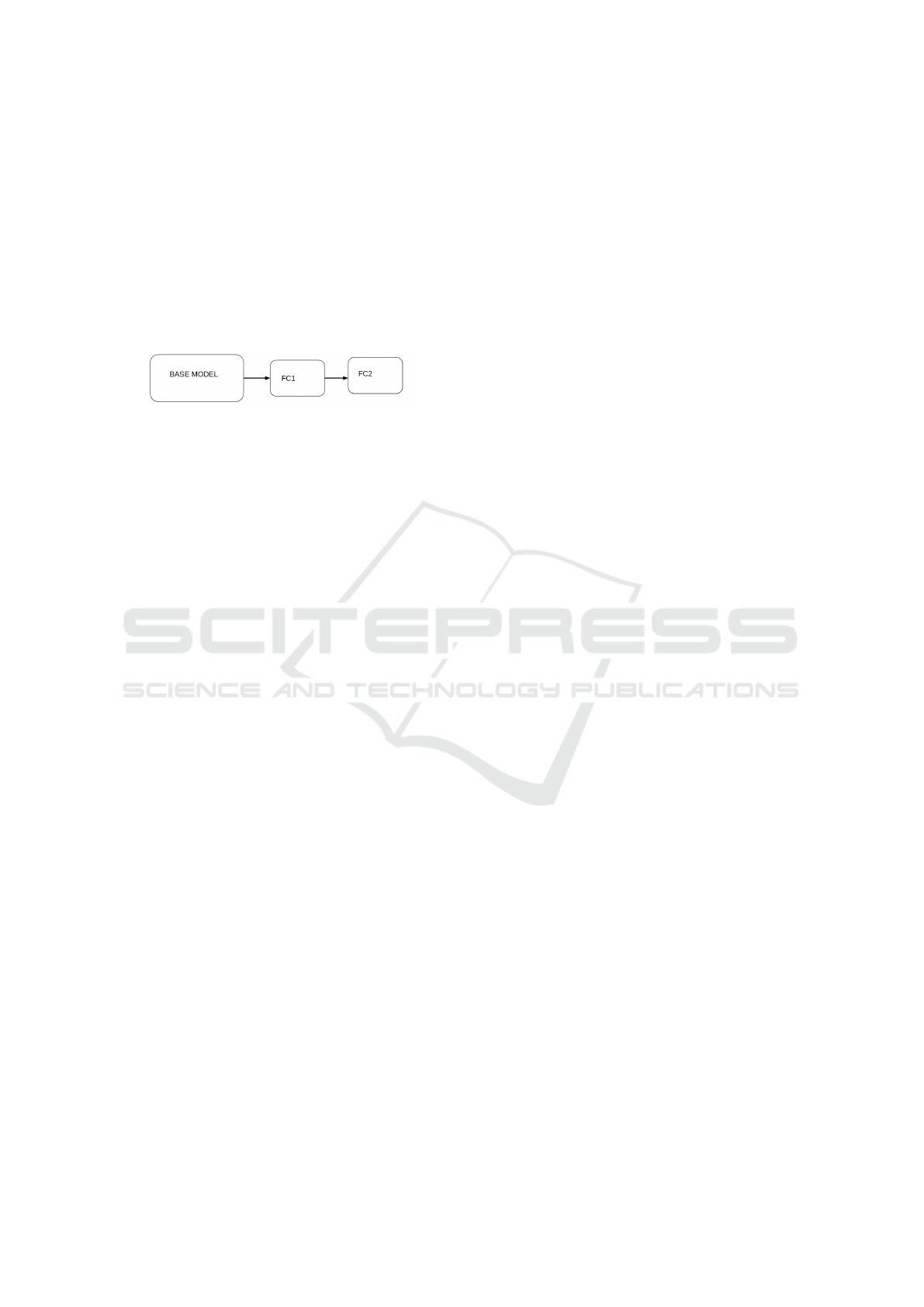
4.2 TL-based Model
The second model which was designed in this study
is TL-based model. In contrast to the first model
(MTL-based), it was first trained to detect skin cancer.
The learned representations were then transferred and
used to train a new model-with additional layers. The
new model was fine-tuned to detect IDC by adding the
following layers: i) two FC’s, ii) RELU , iii) Dropout
and iv) Batch Norm. Figure 5 shows the high level
architecture of the TL-based model. Similar to the
Figure 5: TL-based Model.
MTL-based model architecture, the TL’s base model
(where knowledge is transferred from) is made up of
ResNext architecture as well. The key difference is
that MTL-based model is focused on multiple tasks
while TL-based model simply reuses to source knowl-
edge to enhance the new model’s performance.
4.3 Training
The models were trained for 40 epochs. Their learn-
ing rates were varied over time (significantly lowered
as the loss gradually decreases) on the range between
0.0001 - 0.01. All the experiments were carried out
using PyTorch version 1.0 hosted on AWS’s p2.xlarge
GPU machine. Vanilla Stochastic Gradient Descent
with momentum of 0.9 was used as the optimization
algorithm for the two models.
5 RESULTS
MTL-based model was trained to detect brain tu-
mor, skin and IDC. The model’s key task was detec-
tion of IDC whereas skin and brain tumor detection
were auxiliary tasks. Skin, brain and IDC sub-models
shared common base but different last layers as de-
picted in Figure 4. Table 1 shows the result summary
of the MTL-based model performance.
The TL-based model on the other was first trained
on skin cancer dataset and the knowledge representa-
tions transferred (as a base model) to a new model.
The latter was fine-tuned by adding two Fully Con-
nected Layers (FC’s), RELU, Dropout and Batch
Norm. Table 2 summarizes the results of the TL-
based model.
6 DISCUSSION
Multitask Learning (MTL) and Transfer Learning
(TL) are key learning methods which have been
widely adopted to enhance model’s performance. In
this study, we designed MTL-based model and TL-
based model and compared their results in detection
of IDC. The results of the two models were tabulated
in Table 1 and Table 2 respectively.
From Table 1, the MTL-based model on IDC de-
tection recorded cross entropy training loss of 0.26,
validation loss: 0.6, validation accuracy:75.99, and
training accuracy of 88.50. Comparatively, the TL-
based model recorded a cross entropy training loss of
0.43, validation loss of 0.51, validation accuracy of
67.38 and training accuracy of 79.12. Moreover, the
results of auxiliary tasks (skin and brain tumor) can
be read from Table 1. In addition, the performance of
base model used to transfer knowledge to TL-based
model can also be read from Table 2.
Based on the results, the accuracy difference be-
tween MTL-based model and TL-based model on
IDC detection was 8.6 on validation set and 9.37 on
the training set. On comparing the loss metric of the
same models, a cross entropy of 0.18 was recorded on
validation set and 0.08 on the training set. Consider-
ing the accuracy of the two models’ on IDC detection,
MTL-based model performed was better as compared
to the TL-based model.
Even though the results follow logically from
the theoretical and empirical foundation presented
by Caruana (1997) and Ruder (2017), more experi-
ments need to be conducted to absolutely conclude
that MTL-based models are generally better than TL-
based models in the context of cancer detection. This
is based on the fact that first: there are other cancer
classes which were not considered in this study. Two,
TL-based model has the potent to yield the quite com-
petitive results (as shown in Table 2). Lastly, the skin
dataset and brain MRI dataset used in this study was
relatively small as compared to datasets which might
be available for other cancer classes.
7 CONCLUSION
In this paper, we investigated how MTL and TL based
models performance compare when applied to the
IDC detection. Following the obtained results, MTL-
based model recorded a better performance. TL-
based model yielded relatively competitive results.
Even though the outcome of this research coin-
cided with the theoretical and empirical underpin-
nings of other studies, there is still the need to con-
NCTA 2019 - 11th International Conference on Neural Computation Theory and Applications
554
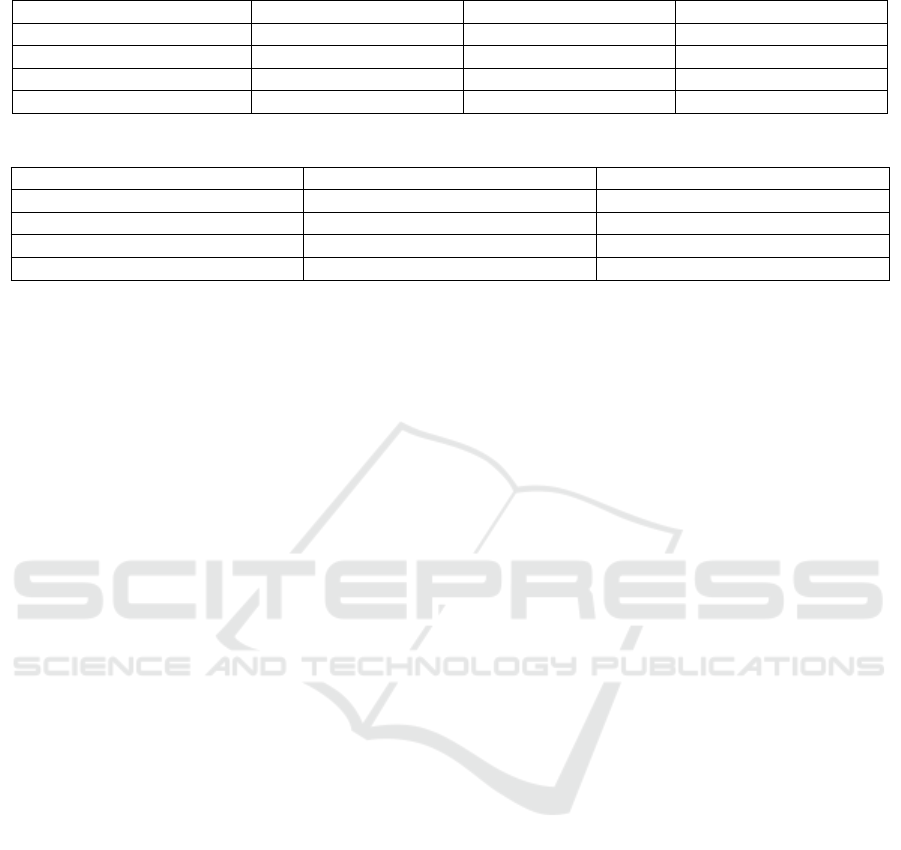
Table 1: MTL-based Model Results.
Model Skin Brain IDC
Training Loss 0.27 0.6 0.26
Validation Loss 0.48 0.9 0.6
Validation Accuracy 75.92 62.5 75.99
Training Accuracy 86.90 60.7 88.50
Table 2: TL-based Model Results.
Model Base Model (on skin dataset) IDC
Training Loss 0.19 0.43
Validation Loss 0.17 0.51
Validation Accuracy 81.12 67.38
Training Accuracy 93.77 79.12
duct more experiments with different types of cancer
on much larger datasets. This would allow drawing
more general conclusions about the performance of
MTL-based and TL-based models in the context of
cancer research.
8 FUTURE WORK
Following the obtained results, MTL-based model
yielded much better outcome compared to TL-based.
In future work, we seek to research on the mod-
els’ performance based other cancer sub-types and on
much larger datasets. This would allow drawing con-
clusive remarks on TL-based or MTL-based model
performance in the context of cancer research.
REFERENCES
(2019). (1) (PDF) A brief review on multi-task learning.
[Online; accessed 10. Jul. 2019].
Asgarian, A. (2019). Transfer Learning—part 1. Medium.
Bi, W. L., Hosny, A., Schabath, M. B., Giger, M. L., Birk-
bak, N. J., Mehrtash, A., Allison, T., Arnaout, O.,
Abbosh, C., Dunn, I. F., et al. (2019). Artificial in-
telligence in cancer imaging: clinical challenges and
applications. CA: a cancer journal for clinicians,
69(2):127–157.
Caruana, R. (1997). Multitask learning. Machine learning,
28(1):41–75.
He, K., Zhang, X., Ren, S., and Sun, J. (2016). Deep resid-
ual learning for image recognition. In Proceedings of
the IEEE conference on computer vision and pattern
recognition, pages 770–778.
Khosravan, N. and Bagci, U. (2018). Semi-supervised
multi-task learning for lung cancer diagnosis. In 2018
40th Annual International Conference of the IEEE En-
gineering in Medicine and Biology Society (EMBC),
pages 710–713. IEEE.
Klang, E. (2018). Deep learning and medical imaging.
Journal of thoracic disease, 10(3):1325.
Liu, X., Wang, C., Hu, Y., Zeng, Z., Bai, J., and Liao, G.
(2018). Transfer learning with convolutional neural
network for early gastric cancer classification on mag-
nifiying narrow-band imaging images. In 2018 25th
IEEE International Conference on Image Processing
(ICIP), pages 1388–1392. IEEE.
Ruder, S. (2017). An overview of multi-task learn-
ing in deep neural networks. arXiv preprint
arXiv:1706.05098.
Torrey, L. and Shavlik, J. (2010). Transfer learning. In
Handbook of research on machine learning appli-
cations and trends: algorithms, methods, and tech-
niques, pages 242–264. IGI Global.
Xie, S., Girshick, R., Doll
´
ar, P., Tu, Z., and He, K. (2017).
Aggregated residual transformations for deep neural
networks. In Proceedings of the IEEE conference on
computer vision and pattern recognition, pages 1492–
1500.
Zhang, Y. and Yang, Q. (2017). An overview of multi-task
learning. National Science Review, 5(1):30–43.
Multitask Learning or Transfer Learning? Application to Cancer Detection
555
