
DATA COMPLIANCE IN PHARMACEUTICAL INDUSTRY
Interoperability to Align Business and Information Systems
Néjib Moalla
(1,2)
, Abdelaziz Bouras
(1)
, Gilles Neubert
(1)
, Yacine Ouzrout
(1)
(1)
CERRAL/PRISMa, IUT Lumière Lyon 2, 160 Boulevard de l’Université, 69500, Bron, France
Nicolas Tricca
(2)
(2)
Sanofi Pasteur / Campus Mérieux. 1541, Avenue Marcel Merieux 69280, Marcy l’Etoile, France
Keywords: Pharmaceutical Sector, Information Systems, ERP, Marketing Authorizations, Compliance, Interoperability
.
Abstract: The ultimate goal in the pharmaceutical sector is product quality. However this quality can be altered by the
use of a number of heterogeneous information systems with different business structures and concepts along
the lifecycle of the product. Interoperability is then needed to guarantee a certain correspondence and
compliance between different product data. In this paper we focus on a particular compliance problem,
between production technical data, represented in an ERP, and the corresponding regulatory directives and
specifications, represented by the Marketing Authorizations (MA). The MA details the process for
manufacturing the medicine according to the requirements imposed by health organisations such as Food
and Drug Administration (FDA) and Committee for Medicinal Products for Human use (CHMP).
The proposed approach uses an interoperability framework which is based on a multi-layer separation
between the organisational aspects, business trades, and information technologies for each involved entity
into the communication between the used systems.
1 INTRODUCTION
The pharmaceutical industry is distinguished among
process industries by the need to comply with
regulatory constraints imposed by organizations like
Food and Drug Administration (FDA) (FDA, 2004),
Committee for Medicinal Products for Human uses
(CHMP), the guidelines of International the
Conference of Harmonisation (ICH) (ICH6, 2003).
Further constraints are imposed by the conventions
signed with national and international authorities,
called Marketing Authorisation (MA) –
Authorization to Make to Market (AMM) in Europe
– for the manufacture of drugs.
In this operating context, the issue of product
quality is one of high priority for a company in order
to maintain its credibility compared to its customers.
One of the key factors of quality is the good
management of product data. Product data comes in
several types and formats specific to various
business trades and are supported by several
heterogeneous information systems. The challenge is
to enable communication among these systems and
the process of guaranteeing the validity and the
conformity of exchanged information. This
challenge is seldom addressed systematically.
Indeed, considering the complexity of information
systems architectures for the production, there is a
general tendency to check conformance only
between the MA files and the Standard Working
Instructions (SWI).
Our Scope in this paper covers the problem of
communicating product data between information
systems supporting the MA and the ERP for
structuring production data. Delivering a product
according to its description in the MA requires the
right information in the ERP. Otherwise, we risk
manufacturing a non compliant product, to not
deliver our product in time to respect customer
commitments, and in final destroy these products
and lose money.
The pivotal problem of medical data is the
absence of machine readable structures (Schweigera,
2005). Most healthcare data is narrative text and
79
Moalla N., Bouras A., Neubert G., Ouzrout Y. and Tricca N. (2006).
DATA COMPLIANCE IN PHARMACEUTICAL INDUSTRY - Interoperability to Align Business and Information Systems.
In Proceedings of the Eighth International Conference on Enterprise Information Systems - DISI, pages 79-86
DOI: 10.5220/0002460300790086
Copyright
c
SciTePress
often not accessible. Generally, related works
(Schweigera, 2005) have a certain tendency to treat
this problem in structuring drug and other
information using XML standards. This is generally
made using topic Maps (Schweigera, 2003), but
presenting a product XML data models and
connecting them is not sufficient (EBXML, 2001).
Same Standard for the Exchange of Product Model
data (like STEP-ISO 10303) addresses this through
formats and programming interfaces derived directly
from domain-related information models written in
the EXPRESS information modelling language.
However, these formats and programming interfaces
are predetermined (Sang Bong, 2002), and not
always well suited to current information processing
technologies. We can find also Product Data Markup
Language (PDML) (William, 2001) as an Extensible
Markup Language (XML) vocabulary designed to
support the interchange of product information
among commercial systems (such as PDM systems)
or government systems (such as JEDMICS), where
the vocabularies are related via mapping
specifications.
Performing data mapping between regulatory
and industrial product definition present a hard task
that requires regrouping efforts from different
sectors like regulatory affairs, industrial operations,
information systems, etc.
Some pharmaceutical industries are specialized
in biologic development of medicines. The
implication of a deviation in manufacturing or
subcontracting can run the gamut from very minor to
catastrophic. Our challenge consists in delivering the
right product data value through manufacturing
states in the production information systems.
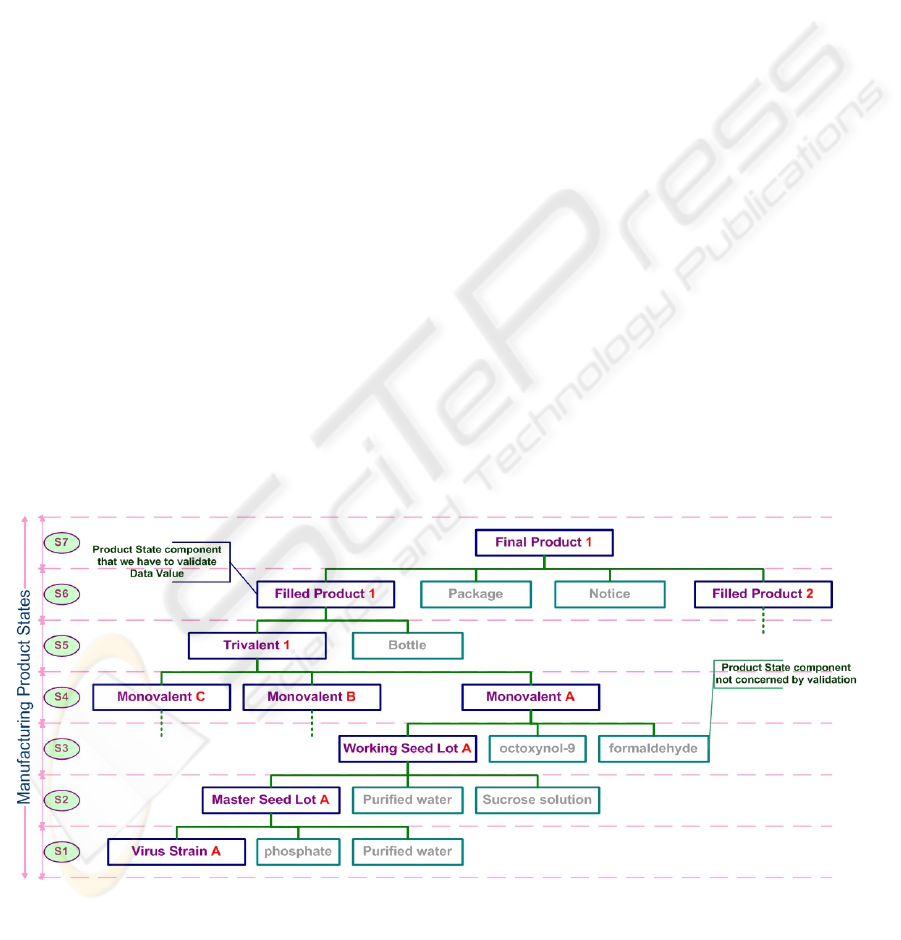
During manufacturing process, the product
passes from one state to another. Each state may
concern one or several components and we have to
validate their corresponding specifications based on
data coming from MA information system. The
following Figure (Figure 1) presents a hierarchical
structure for a product in the ERP (Enterprise
Resource Planning) system of the company.
When we have to ensure compliance for one data
from MA to ERP, it is necessary to find and validate
product data value for each component through
different product states.
Our main contribution in this paper is to use a
modelling approach to handle the communication
between information systems within a
pharmaceutical context. We also propose a
methodology for structuring and exchanging product
data while ensuring their conformance. In the
following section we present some modelling
approaches and adapt them to our problem. In
section 3, we propose a data exchange structure that
ensures compliance between the information
systems. Finally, by using our approach, we present
a case study at Sanofi-Pasteur, a developer and
producer of vaccines for human use.
Figure 1: Manufacturing product states and state components.
ICEIS 2006 - DATABASES AND INFORMATION SYSTEMS INTEGRATION
80

2 INTEROPERABILITY IN
PHARMACEUTICAL
INDUSTRY
2.1 General Requirements for
Interoperability
The IEEE standard computer dictionary defines
interoperability as “the ability of two or more
systems or components to exchange information and
to use information that has been exchanged”. The
EU Software Copyright Directive (ATHENA, 2005)
gives a similar definition and considers the
interoperability between computing components as
“the ability to exchange information and mutually to
use the information which has been exchanged”.
This does not mean that each component must
perform in the same way, or contain the same
functionality as the other components–
interoperability is not a synonym for cloning.
Rather, interoperability means that components with
different functionalities can share information and
use it according to their needs.
The European Interoperability Framework
definition identifies three separate aspects:
• Organisational – is concerned with defining
business goals, modelling business processes and
bringing about the collaboration of administrations
that wish to exchange information, but that may
have a different internal organisation and structure
for their operations.
• Semantic – is concerned with ensuring that
the precise meaning of exchanged information is
understandable by any other application not initially
developed for this purpose. Semantic
interoperability enables systems to combine received
information with other information resources and to
process it in a meaningful manner.
• Technical – covers the technical issues of
linking up computer systems and services. This
includes key aspects such as open interfaces,
interconnection services, data integration and
middleware, data presentation and exchange,
accessibility and security services
Identification and structuring of these
interoperability types help to perform a better
exchange between systems Therefore, it is necessary
to identify the area of our investigation and its
specifications: structures, business constraints, etc.
To achieve interoperability among divisions
systems in collaborative enterprise, we consider
three challenges (ATHENA, 2005):
• Heterogeneity, incoherent information,
different systems and software infrastructures,
different working practices, etc.
• Flexibility, information reuse, following of
variations in documents versions, etc.
• Complexity, definition granularities,
dependency between different components, etc.
Heterogeneity, flexibility and complexity must be
managed at different levels:
• Knowledge, approaches, methods and skills
needed for innovation, shared languages.
• Process, planning’s, coordination and
management of cooperative and interdependent
activities.
• Infrastructure, information formats,
software tools, interoperability technologies.
In an industrial framework, structuring business
knowledge in an information processing system does
not imply facilitation of communication with
another business system. Data interpretation changes
according to the business and the challenge is in the
ability to preserve information semantics when
communicating.
Building interoperability architecture for
communication can align Business, Knowledge and
ICT through semantic framework to ensure
compliance when exchanging data. In the following
section, we will explain a deployment of the
interoperability framework to present a
communication architecture adapted to our context.
2.2 Characteristics of
Pharmaceutical Industry
Product data is compiled from various functional
divisions which interact between each others for the
creation and manufacture of the product. Each of the
following divisions contributes by introducing
different types of data and information:
• Research division: looks for new drugs or
substances that can contribute to the creation of new
drugs. At this stage conducted studies are reported
and indexed in the form of technical reports.
• Research & Development division:
conducts specific research, and is interested in the
development of mixture processes of excipients,
tests and stability conditions of the final solution that
can be defined as a drug. The information system is
used to structure data about clinical trials and tests
for validity. At this stage starts the definition of an
explicit product structure.
• Industrialization division: defines the
industrial infrastructure which will support the
DATA COMPLIANCE IN PHARMACEUTICAL INDUSTRY - Interoperability to Align Business and Information
Systems
81

production of a defined product quantity on the basis
of a definition of product solution. At this stage, we
define technical data describing the product
manufacturing operations and the used process and
tools.
• Production division: deals with planning,
scheduling and follow-up of production based on the
data describing industrial infrastructure and product
composition. At this stage, we identify static data
compared to external dynamic data like work orders
or those generated by the ERP such as buying orders
of raw material.
• Distribution division: defines the conditions
for handling the product for customer delivery in
accordance with the description of the conditions of
manufacture, which is given by R&D division. At
this stage, product handling information is
documented.
From one stage to another, product data are
recorded using a specific structure and format. Each
division information system is defined in accordance
with the needs which are relevant to the business
trades.
The definition of a product for pharmaceutical
industry is not tied to physical shape except in the
packaging stage.
The company submits to the Health Authorities
entire product specifications along with documented
information. These deposed documents constitute
the request of Marketing Authorization. When health
authorities approve this request, they give the
Marketing Authorization. In the delivered
documents to authorities, it is necessary to present
all the information which justifies the product
creation process, including pre-clinical tests, clinical
trials, tests of validity and the appendices such as
bibliography. Only after reaching the
industrialisation stage that MA documents can get
defined.
Once approved in one country, this MA is used
as a reference document to manufacture the product.
It is considered as a contract between the authority
of a given country and the company, implying the
respect of the regulatory constraints. For the
American market for example, the Food and Drug
Administration (FDA) is responsible for the
checking of the adequacy of the delivered product
and manufacturing processes to the acquired
authorization.
The major quest for each pharmaceutical
company is product quality. This objective is
achieved only by ensuring a better degree of
compliance between existing information in these
MA documents and those used for the production.
We propose hereunder the means to use the MA
data, which can be read only by pharmacists, to
adapt them to logisticians needs. The used approach
makes it possible to ensure interoperability between
the supporting information systems, while satisfying
some business constraints.
3 INTEROPERABILITY AND
COMPLIANCE
In our context, the objective behind the
establishment of the communication between the
information systems is to ensure the conformity of
the product data in one system in relation to each
other. Based on the description of information in an
Marketing Authorization, it is necessary to return the
product data values, useful for the production, to the
ERP.
3.1 From MA to ERP
As we mentioned before, the following systems are
involved in our context:
• Marketing Authorization (MA) information
system: generally managed by the regulatory affairs
division of the company and constitutes a collection
of different information. A MA is composed of
electronic documents coming from several sources
and contains, for example, scanned documents,
reports and attached papers. The semantic
structuring of these authorisations documents
provides a format and content which are harmonized
according to a pharmaceutical vision. It specifies the
Common Technical Document (CTD), defined by
the International Conference of Harmonisation
(CTD, 2005)
(ICH, 2000). In the CTD it is not
always easy to find all the information needed for
production, and some pharmaceutical background is
necessary to find the needed information from
regulatory data. Even with a very large number of
MA documents – that’s run into thousands of pages
– it is very difficult to find all information needed
for production. MA presents regulatory aspect of
product data.
• ERP (Enterprise Resource Planning)
system: related to different divisions of company
and regroups complex functionalities of
“provisioning and scheduling” and generates new
dynamic data, such as working orders, based on the
product definition. When the ERP data are non-
conform to the right product data definition, it
necessarily produces a non conforming product.
ICEIS 2006 - DATABASES AND INFORMATION SYSTEMS INTEGRATION
82

Each division presents a specific vision of the
product with local knowledge tied to its business
needs. To ensure the conformity at the product data
definition level during its translation (from the
regulatory systems to the ERP), it is necessary to
define a communication platform to include the
different viewpoints: organisational, business,
informational, and technical (Gao, 2003).
3.2 Type of Data to be Translated
The product structure is defined in both MA and
ERP systems as a specific series of “product states”.
The pharmaceutical description of the product and
its various states related to the manufacturing phase
are presented in the CTD “product quality”
documents of the MA. These states are not
necessarily coherent with the actual production
states. To guaranty the product data coherence, it is
suited to organize these data according to the
product states. However, the problem still concerns
the conformance of data values for each product
states during the translation process. We should take
care about the definition of these states and data
semantic in each one. For example the shelf life of
an intermediate product substance (state) is 3 years,
at a storage temperature of -70°C if it was preserved
with no alteration (as is) and 1 year if it was stored
at 5 °C.
In the manufacturing phase, we assume that the
product has a fixed number of states (reflected into
the information system). It is necessary to identify
from the ERP and the regulatory information system
the entire specification of each state. This is
achieved by what we call “product states reference
frame”. The reference frame represents the
structuring of one product datum that assigns for
each product state, the data value, rules applied to
extract data from the information system, and
business constraints helping to understand the choice
of data value. For each product state, we need to
define also some components of the bill of materials
of this state. For example, when our final product is
presented (at its final state) in the form of two
substances (i.e. powder and liquid), we need to
specify shelf life for these two substances.
The application of this reference frame to
product data consists in seeking data values of all
states in accordance to rules and business constraints
already identified. Figure 2 illustrates this
structuring.
This reference frame represents the data profile
in both information systems. It must be updated
during a potential modification of the structure of
the product. It can also be published in the
organization to ensure better comprehension and
exploitation of the product data.
Each line of this reference frame contains the
product state components and for each one of them,
the value to be validated, the rules which allow to
extract and transform data and business constraints.
The interoperability process is supported by the link
between these values, rules and constraints.
3.3 Rules Definition
The definition of the rules is a tedious phase and
requires three levels:
3.3.1 Production Information Rules
These are rules specify when to extract or to insert
data into the ERP. Some difficulties arise when
attempting to insert data because ERPs are
characterized by the re-use of product states
information. Taking a close look into two drugs
pharmaceutical solution, there is a great probability
to find the same excipients. In this case, there are
invariably one or more specific common production
states with the same coding in the system.
In the ERP, and following a request for
modification of a data value of a product state, it is
necessary to check if the reference for this state is
already used by another product. Considering the
complexity of the ERP architecture and overlapping
between the product states information, it is difficult
to seek products by a simple indication of an
“intermediate” state. For example, such
identification can take up to two days to find all
Figure 2: product states reference frame.
DATA COMPLIANCE IN PHARMACEUTICAL INDUSTRY - Interoperability to Align Business and Information
Systems
83
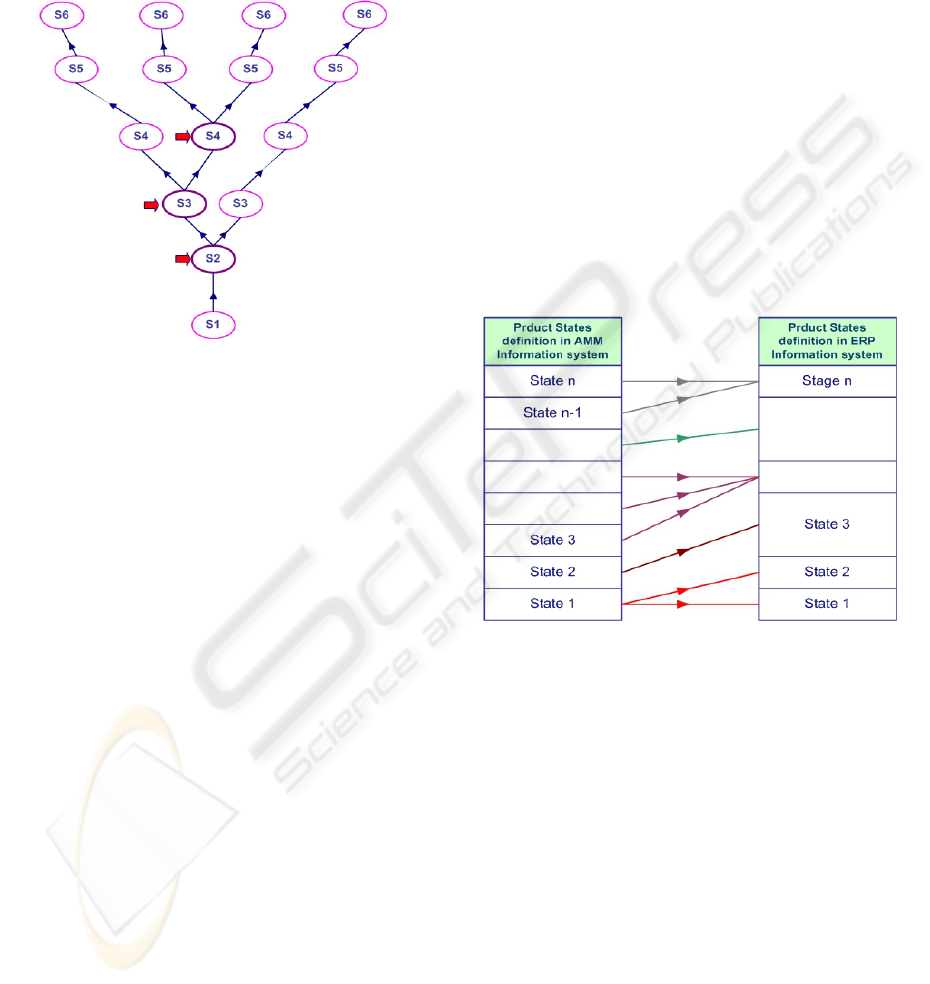
concerned product states and theirs dependencies. If
we schematize product states by a tree structure, the
overlapping between branches can be possible
everywhere except at the top level (tree leaves).
Figure 3 shows an example of these overlapping.
Each product has 6 states: S1 to S6.
Integration rules are used to control the
existence of any overlapping between the tree
branches (resulting in common states) as well as the
impact of any data modification on the product
structure and its states. The impact of some
modifications or transformations at the data level is
sometimes governed by informal business
constraints. For example, the manufacturing date of
a product is notified as the starting date of the first
valid test of stability. If we want to change the shelf
life of a state, the expiry date must be revalidated.
This aspect is important for data understanding. This
is why we added informal business constraints to
each product state. Moreover, these constraints help
understanding the context in which the product
states rules are used, and in mapping the ERP
“product states reference frame” to its corresponding
reference frame in the MA information system.
3.3.2 Mapping Rules
These are rules for mapping between “product states
reference frames” by establishing links between
“active product states”. From all predefined product
states in one reference frame, active product states
present significant states with data value. Performing
these links present a regulatory and pharmaceutical
responsibility that is necessary to share with
production, to ensure the coherence of rules. The
product states are not the same across information
systems and across reference frames. From one
product to another, a state may or may not exist. We
use different business knowledge as references to
create these links of communication between active
states. We notify these information on both MA and
production reference frames (ERP).
The mapping rules allow the formalization of the
fields of the data to be inter-connected (links n .. n).
Active states data values in regulatory reference
frame generate corresponding values in the product
states reference frame of the ERP. Figure 4
illustrates examples of connection modes. One state
in each reference frame can correspond to one or
more states in the second and vice versa. To
generate mapping rules, we should analyze data and
rules from the two reference frames. For example,
mapping rules could be the adding of states data
values, the calculation of their average or their
minimum, etc.
3.3.3 Regulatory Information Systems Rules
According to pharmaceutical data structuring, the
information system which manages the MA is not
able to be directly interfaced to the regulatory
product states reference frame. It is possible to have
several MAs for only one product, and conversely,
one MA for several products. These characteristics
are relocated on product states, which increase the
complexity of the information retrieval. It is very
frequent to find for example two product
authorizations with various destinations (country) or
presentations (packaging containers) and having a
common product state but with different data values.
This difference is due to the history of the
negotiations between the company and health
authority about the MA content.
Figure 4: Mapping links.
Figure 3: overlapping between product states fo
r
different products in the ERP system.
ICEIS 2006 - DATABASES AND INFORMATION SYSTEMS INTEGRATION
84

In the following, we will explain the need for
defining different rules types and later (in a future
work), we will present, through a multi level
modelling approach, different kinds of rules we need
to create.
4 CASE STUDY
This case study presents an illustration of an
application developed within Sanofi Pasteur
Company, a firm specialised on biologic
development and the production of vaccines for
human use. The purpose of this application is to
ensure compliance, from the MA to the ERP, for
three data: Site of Manufacturing, Shelf Life, and
Storage Condition.
All MA data were structured in e-TRAC (Electronic
Tracking of Registrations and Commitments) MA
information systems. Access to these data is ensured
through web interface allowing us to export the
defined report from RA-Cockpit reporting module.
As presented in figure 5, we can:
a) export data for one product line to create the
report ,
b) distribute this report by product licence number as
criteria to identify different product data,
c) for one product data, instantiate three reference
frames for regulatory product states,
d) apply mapping rules to generate corresponding
ERP (here SAP) product states reference frame,
e) use the same specific criteria for data structuring
in SAP to validate data (comparing to those coming
from SAP reference frame generated after mapping).
4.1 Validate Data in SAP
As mentioned before, there is a great probability to
have the same product state in different product
states decompositions. So, we can find the same
value for the same product state in different SAP
reference frames. In SAP system, we identify each
entity, called item, by one code. That is why, in
addition to the first SAP reference frame generated
after mapping, we instantiated a second SAP
reference frame with only SAP code and
corresponding data value field. In this second
reference frame it is necessary to find, from SAP,
the code and value of each product state. Due to
specific information structuring in SAP at Sanofi-
Pasteur Company, we can find the item code for the
last state (final product) and use “item code
filiations” (Where-Used technology) to find the code
for previous product states and their data values
starting from the last.
Actually we have two SAP reference frames: one
with data values generated after mapping from the
regulatory reference frame, and the second with data
values and item code coming from SAP. We define
here some new rules of coherence:
R1: For the same product state, there is necessarily
the same data value, otherwise notify a compliance
exception,
R2: The same item codes in the second SAP
reference frames (corresponding to different
products) should have the same associated data
values, otherwise notify a compliance exception,
Figure 5: Communication scenario.
DATA COMPLIANCE IN PHARMACEUTICAL INDUSTRY - Interoperability to Align Business and Information
Systems
85

It is frequent to find two or more MAs or
registrations that differ just by product name from
one country to another. For example we can define
influenza (Flu) vaccines for entire Europe, but
during the structuring of the product information in
the e-TRAC, we should separate the products by
country.
R3: Validating the three data (Site of
Manufacturing, Shelf Life, Storage Condition) for
grippe in a particular region, requires the same data
values in e-TRAC reference frame for all countries
of this region, otherwise notify a compliance
exception.
Finally, within this Flu line product vaccines
case study, the applied architecture and its rules
provided an interesting solution by ensuring
compliance of 94,6% of the final products for the
used three data: Site of Manufacturing, Shelf Life,
and Storage Condition. One of the reasons of non-
total compliance is related to the existence of quality
level information in the MA system that has no
correspondence in the ERP system.
5 CONCLUSION
In this paper, we presented a methodology to
communicate between information systems. We
particularly focused on product structuring and
explained dependencies between product data in the
pharmaceutical field. Our main objective is to ensure
data compliance between two information systems,
one related to the Marketing Authorizations and the
other related to production, through the
establishment of communication architecture. We
based our work on the mapping between product
“states” information along the product
manufacturing life cycle. In spite of differences in
their business visions, both systems use the product
manufacturing decomposition as guide-line for
structuring the information.
Our methodology treats only the information
coming from Marketing Authorizations systems to
map and validate it in the ERP systems. However it
does not treat product information that exists in the
ERP systems and is not related to any MA system.
The next step of this work will focus on the
generalization of the used rules and constraints, not
only to extract or integrate data through reference
frames, but also between product states in a same
reference frame.
REFERENCES
ATHENA, 2005, “Second Version of State of the Art in
Enterprise Modelling Techniques and Technologies to
Support Enterprise Interoperability”, Version 1.3.1,
212 pages.
ATHENA, 2005, “Interoperability Roadmap up date”,
Version 1.0, 30 pages.
CTD Format: http://www.aboutctd.com/resource.htm and
http://www.ich.org/cache/html/1208-272-1.html,
accessed on October 2005.
EBXML, 2001, “Technical Architecture Specification
V1.0.4”, ebXML Technical Architecture Project Team,
39 pages
Gao X., Hayder A., Maropoulos P. G. and Cheung W. M.,
2003, “Application of product data management
technologies for enterprise integration”, International
Journal of Computer Integrated Manufacturing,
Taylor & Francis, Vol 16 N° 7-8, pages 491 – 500.
ICH6 - Sixth International Conference on Harmonisation,
2003, “New Horizons and Future Challenges”,
Summary Report, Osaka Japan, pages 13-15
International Conference on Harmonisation, Harmonised
Tripartite Guideline, 2000 “Organisation Of The
Common Technical Document For The Registration
Of Pharmaceuticals For Human Use”, International
Conference On Harmonisation Of Technical
Requirements For Registration Of Pharmaceuticals
For Human Use, 14 pages.
Sang Bong Y. and Yeongho K., 2002, "Web-based
knowledge management for sharing product data in
virtual enterprises", International Journal of
Production Economics, Volume 75, Issues 1-2, Pages
173-183
Schweigera R., Brumhardb M., Hoelzerc S., Dudecka J.,
2003,” Linking clinical data using XML topic maps”,
Artificial Intelligence in Medicine, Volume 28, Issue
1, Pages 105-115
Schweigera R., Brumhard M., Hoelzerc S., Dudecka J.,
2004, “Implementing health care systems using XML
Standards”, International Journal of Medical
Informatics (2005) 74, pages 267—277
U.S Food and Drug Administration, 2004,
“Pharmaceutical CGMPS For The 21st Century — A
Risk-Based Approach Final Report”, Department of
Health and Human Services, 32 pages
William C. Burkett, 2001, "Product data markup language:
a new paradigm for product data exchange and
integration", Computer-Aided Design, Volume 33,
Issue 7, Pages 489-500
ICEIS 2006 - DATABASES AND INFORMATION SYSTEMS INTEGRATION
86
