
BIOINTERFACES BASED ON IMMOBILIZED BORONIC ACID
WITH SPECIFITY TO GLYCATED PROTEINS
Jan Přibyl and Petr Skládal
National Center for Biomolecular Research, Masaryk University, Kotlářská 2, Brno, Czech Republic
Keywords: Glycated hemoglobin, aminophenylboronic acid, biosensors, quartz crystal microbalance, heterogeneous
affinity assay, microtitre plate.
Abstract: Development of bioanalytical assays for determination of glycated hemoglobin content in blood samples is
reported. First, a combined biosensor setup for determination of total and glycated hemoglobin content was
successfully developed and tested. The effect of various operating parameters, such as ionic strength, flow
rate and instrumental set-up was optimized. The total hemoglobin content was analyzed by measuring of
absorbance of the hemoglobin-cyanide derivative at 540 nm. Only one standard (calibrator), diluted in
various proportions, was necessary for the method calibration. The full range of HbA
1c
content (4 to 15 %)
presented in blood can be analyzed. Only 1 μl of blood was required for analysis. The developed method
was successfully evaluated for analysis of blood samples collected from diabetic patients. Next, the
heterogeneous affinity assay performed in a microtitre plate with an immobilized boronic acid is described.
This assay is based on ELISA (Enzyme-Linked Immunosorbent Assay) principle; however stable
chemiselective ligand is used in this case. The content of glycated hemoglobin is determined according to its
peroxidase activity after attachment to immobilized boronic acid derivative; the total hemoglobin
concentration is measured as an absorbance at 405 nm.
1 INTRODUCTION
Diabetes mellitus is a group of diseases
characterised by high levels of blood glucose
resulting from defects in insulin production, insulin
action, or both. Diabetes can be associated with
serious complications and premature death. Diabetes
was the sixth leading cause of death in USA in 2000.
(National Diabetes Information Clearinghouse,
http://diabetes.niddk.nih.gov). The steps to control
the disease and lower the risk of complications
should be taken. In this way, blood and urine
glucose analysis, cholesterol reduction and blood
pressure control should be mentioned. Analysis of
glycated hemoglobin (HbA
1c
) helps to monitor the
long-term progression of diabetes without influence
of the short-term fluctuations of blood glucose. The
fraction of HbA
1c
is usually indicated as percentage
of its presence in the total hemoglobin content. The
content of glycated hemoglobin in blood should
substitute the term “glycemia”; values lying under
7% indicate good health state of patient and
effective practicing of the proposed therapy
(Marshall and Barth, 2000).
Glycated hemoglobin (GHb) refers to a series of
minor hemoglobin components which are stable
adducts formed by reaction of hemoglobin primary
aminogroups with various sugars. Hemoglobin
HbA
1c
is a stable glucose adduct to the N-terminal
group of the β-chain of HbA
0
. In current opinion,
concentration of the hemoglobin variant HbA
1c
is
considered to be the only specific and stable
indicator of long-term diabetes progress. Neither the
whole glycated fraction of hemoglobin (HbA
1
) nor
fructosamine can be any longer used in the disease
diagnosis.
A wide range of methods for analysis of the
glycated hemoglobin (either HbA
1
or HbA
1c
) has
been reported recently. However, only few of them
were based on biosensor approach and mostly the
chromatographic approaches were employed. From
the area of biosensor development, especially two
concepts should be mentioned. The first one was
based on selection of ligands from the hexapeptide
combinatorial library for binding the glycated
terminus of hemoglobin β-chain; thus found
hexaptides exhibited high specifity and stability
(Chen et al., 1998). However, only the preliminary
study was performed, additional testing of this
107
P
ˇ
ribyl J. and Skládal P. (2008).
BIOINTERFACES BASED ON IMMOBILIZED BORONIC ACID WITH SPECIFITY TO GLYCATED PROTEINS.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 107-112
DOI: 10.5220/0001051501070112
Copyright
c
SciTePress

affinity molecules and integration to some analytical
method (e.g. affinity chromatography) is essential
for correct evaluation. A monolayer of boronic acid
conjugate with 11-mercaptoundecaonic acid
immobilised on the surface of gold nanoclusters was
used as recognition element in another study
(Valina-Saba et al., 1999). An easy approach was
reported, when the precipitation reaction between
boronic groups on the particle surface and glycated
protein (horse-radish peroxidase) was visible.
Unfortunately, the application for determination of
HbA
1c
content in whole blood, which is rather
complex mixture, was not tested.
Boronic acid shows ability to bind covalently to
either 1,2- or 1,3-diols and thus forms five- or six-
membered cyclic esters. 3-aminophenylboronic acid
(APBA) binds in this way to the cis-diols of
saccharides, glycated proteins or nucleic acids
(Pickup et al., 2005). The formation of a boronate
ester is usually described as a two step reaction; the
planar boron group initially reacts with hydroxyl
(pH>7.0 is essential) to form tetrahedral boronate
anion, which subsequently binds reversibly to the
positively charged carbon atoms in the diol-
containing structure (Ito et al., 2003). This kind of
ester formation designates boronic acid and its
derivatives to be used as the affinity recognition
elements in variety of applications, such as
construction of sensors for saccharides with
piezoelectric (Lau et al., 2000) and surface plasmon
resonance (Kugimiya and Takeuchi, 2001)
transducers or fluorescent (Kataoka et al., 1995)
detection. Boronic acid derivatives immobilized in
the matrix of columns have formed the basis of a
new field of chromatographic techniques designated
for analysis and separation of sugars and glycated
proteins. This area is commonly known as boronate
affinity chromatography (Bongartz and Hesse,
1995).
The aim of presented study was to continue our
previous attempts with boronate-modified sensors
for sugars (Přibyl and Skládal, 2005) in order to
develop an innovative, easy to handle and cost
effective but reliable biosensor set-up with high
stability and reproducibility for determination of
glycated hemoglobin in blood samples. The
designed system contains two parts, one performs
the analysis of HbA
1c
using a piezoelectric sensor
modified with phenylboronic acid, and the second
one is designed for a photometric determination of
total Hb. The absolute concentration of these blood
components differs in each sample. The percentage
of HbA
1c
presence will be determined as a ratio of
these two concentrations;
(conc
HbA1c
/conc
Total Hb
) x 100%.
Another interesting method for detection of
glycated hemoglobin is reported, too. This
bioanalytical method called AHA (Affinity
Heterogeneous Assay) employs microtitre plates
consisting of the wells covered with
aminophenylboronic acid. The AHA assay allows
determination of total hemoglobin as well as
glycated fraction of hemoglobin in blood samples,
similarly as the biosensor based method.
2 EXPERIMENTAL
2.1 Chemicals and Reagents
Chemicals were obtained from Sigma (St. Luis,
USA) and used as received without any further
purification. Microtitre plates with chemically
reactive surface (NUNC Immobilizer Amino) were
from Nunc (Copenhagen, Denmark).
The special solutions were prepared, stored and
used as officially recommended (International
Committee for Standardization in Haematology,
1978) for analysis of total hemoglobin content in
blood samples.
2.2 Instrumentation
Measurements with the piezoelectric biosensor were
performed using 10 MHz, AT-cut quartz crystals
(ICM, Oklahoma City, OK, USA) with gold-coated
smooth quartz discs (electrode area, 0.8 cm
2
).
In the center of the system, there was placed a
PMMA-made flow-trough cell (internal volume
10 μl) from NanoQ (Brno, Czech Rep.) with the
piezoelectric biosensor mounted between two silicon
rubber O-rings. The cell was supplied with flowing
liquid via two stainless steel tubes (i.d., 0.5 mm).
Sensor was connected to MultiLabPlus instrument
(MultiLab) combining oscillator with high resolution
frequency counter.
Handling of liquids and samples was performed
by the FIAlab 3500b instrument (Alitea, Seattle,
WA, USA).
Optical part for determination of total
hemoglobin content was located in front of the
biosensor cell. The detector consisted of a Z-type
flow-trough absorption cell (optical path, 10 mm)
supplied with flowing liquids trough the Teflon
tubing and standard visible light source and optical
fibre spectrophotometer from Ocean Optics
(Dunedin, FL, USA).
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
108
2.3 Immobilization Procedure
2.3.1 Affinity Biosensor Fabrication
Matrix based layer was prepared when 2% solution
of polyethylene imine (PEI) in methanol (3 μl) was
used to activate the gold surface. The APBA layer
was attached through the glutaraldehyde linker (8%,
8 hours, 4
o
C) In the last step, the recognition layer
was stabilised by the reduction of Schiff bonds with
10 mg/ml solution of sodium borohydride (2 hours).
The thiocompound-APBA conjugates were
prepared in order to modify the gold biosensor
surface with a monolayer of boronate groups. In the
first step, the carboxygroup of mercapto-terminated
(on the opposite side of chain) acids was activated
with carbodiimide (3 hours, 99
o
C). Conjugation of
aminophenylboronic acid to bellow mwntioned
thiocompounds was performed during the next step:
DTSP, 11-MUA, 16-mercaptohexadecanoic acid and
lipoic acid (3 hours, 99
o
C). Final products exhibited
light-yellow color and were stored at -20
o
C prior
use.
A monolayer of boronic groups was prepared,
when the freshly cleaned gold electrodes were
incubated with 15 μl of the conjugate for 24 hours at
laboratory temperature in a closed chamber.
Gold surface modified with
11-mercaptoundecanoic acid and the freshly cleaned
gold electrode were used as reference surfaces. For
comparison of binding specifity to the matrix-
modified surfaces, the polyethylene imine layer was
attached to the piezosensor.
2.3.2 Specific Modification of Microtitre
Plate
The ‘Amino Immobilizer’ microtitre plate from
Nunc shows ability to bind covalently any molecule
containing primary aminogroup. 10 mg/mL solution
of aminophenylboronic acid (APBA) in 50 mM
carbonate buffer pH=9.5 was used to cover the
microtitre plate with boronic groups. The solution of
APBA was kept overnight under ambient
temperature in order to cover the wells of plate with
boronic groups. After 4-times repeated washing
(PBS pH=7.4), the unreacted surface group were
saturated with glycine (25 mg/mL in PBS buffer
pH=7.4) during 2 hours reaction performed under
ambient temperature. After thorough washing with
PBS, the plates were dried in the air stream of
ambient temperature (4 hours). Such modified plates
can be long-term stored in a well sealed box (4
o
C)
without any significant loosing of their binding
activity.
2.3.3 Biosensor Setup - Measuring
Procedure
A similar protocol was used for all experiments:
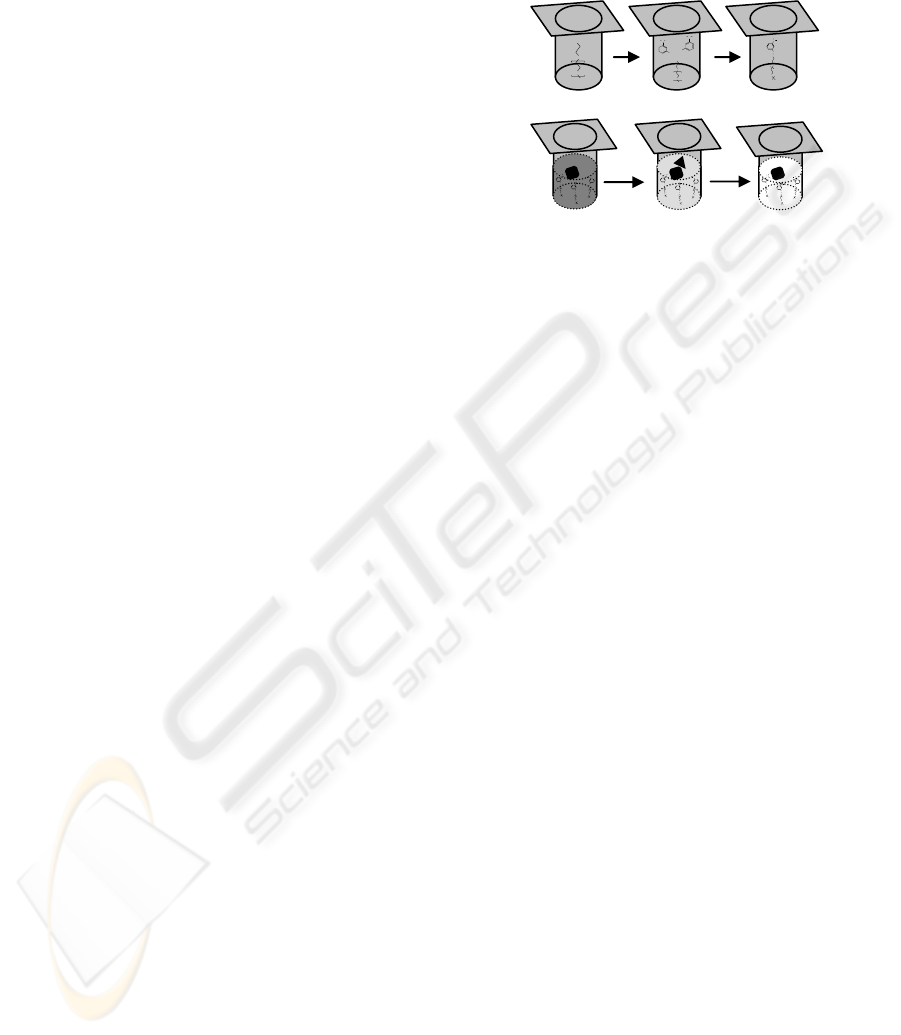
Figure 1: I – Immobilization of the APBA molecule to the
activated surface of microtitre plate (A, activated surface;
B, APBA solution added; C, APBA-modified surface. II –
Procedure of total and glycated hemoglobin determination
in boronic acid modified plate (A, well filled with diluted
blood sample – total hemoglobin determination during
binding of GHb to the surface; B, peroxidase activity of
bound GHb is used to oxidase a substrate; C, reaction
stopped and the overall activity measured).
after 5 min of the base-line signal stabilisation with
the carrier buffer, the flow of sample - glycated
hemoglobin dissolved in the carrier buffer
(alternatively supplied by sorbitol solution) followed
for 7 min. For the next 7 min, the flow cell was
washed with the carrier buffer in order to equilibrate
the signal (non-specifically adsorbed molecules
dissociated from the biosensor surface). Injection of
200 mM aquatic solution of HCl for 120 seconds
disintegrated the complex formed between glycated
hemoglobin and monolayer of boronic acid groups;
complex [GHb-matrix immobilized APBA]
dissociated spontaneously. Washing with working
buffer for a few minutes followed (new base-line
stabilization) prior performing the next measuring
cycle.
2.3.4 AHA Analysis
Another way of determination of total hemoglobin in
blood samples is, comparing to the previously
employed conversion to a cyanomethemoglobin, its
conversion to alcalic hematin. The carbonate buffer
pH=9.0 was used for this purpose, when the blood
samples were diluted 400-times in this medium, the
most of hemoglobin molecules was converted to the
hematin. This can be quantified by measuring of
absorbance at 405 nm. Moreover, the alcalic pH is
an optimal value to support the affinity interaction
between the boronic group and GHb in a solution.
O
n
B
NH
OH
O
O=C
O
O
n
B
NH
OH
OH
O=C
O
n
B
NH
OH
OH
O=C
T
M
B
+
H
2
O
c
o
l
o
r
O
n
B
NH
OH
O
O=C
O
O
n
B
NH
OH
OH
O=C
O
n
B
NH
OH
OH
O=C
5 minutes
37
o
C
O
n
B
NH
OH
O
O=C
O
O
n
B
NH
OH
OH
O=C
O
n
B
NH
OH
OH
O=C
H
2
SO
4
O
O
NHS
n
B
NH
OH
OH
O
O
O=C
n
O
O
NHS
n
B
NH
2
OH OH
B
NH
2
OHOH
A B
C
A B
C
II
I
BIOINTERFACES BASED ON IMMOBILIZED BORONIC ACID WITH SPECIFITY TO GLYCATED PROTEINS
109

The blood solution (in a carbonate buffer) was left to
interact with the surface immobilized boronic groups
for 60 minutes, at ambient temperature, in a closed
box. The total hemoglobin content was quantified as
change of A
405
during 20 minutes following the
reagent addition. Afterwards, the wells were washed
4-times with PBS buffer pH=7.4 and the peroxidase
substrate solution, containing 0.075% hydrogen
peroxide and 105 ug/mL of tetramethylbenzidine in
50 mM acetate buffer pH=4.5 (solution freshly
prepared before each experiment). The enzymatic
reaction releasing intensive blue color was left to
proceed for 5 minutes in a dark box heated to 37
o
C.
The reaction was stopped by addition of 50 uL of 1
M H
2
SO
4
solution to each well. The color of solution
in wells turns yellow immediately. The amount of
the enzymatic reaction product was measured as
absorbance at 405 nm in a microtitre plate reader.
The absorbance of the whole blood solution
corresponds to a total hemoglobin in a sample, the
enzymatic activity of bound hemoglobin (measured
as A
405
in a next step) is proportional to a glycated
hemoglobin content. The percentage of GHb
presence in the total hemoglobin can be easily
calculated by simple dividing of those two values.
3 RESULTS AND DISSCUSSION
3.1 Biosensor based Experiments
The amount of boronic groups deposited on the
surface of piezoelectric sensors was first monitored
during the immobilisation procedure. The deposited
mass was calculated according to Saurbray equation
from the difference of resonant frequency during
deposition. These results indicate that the highest
amount of boronic groups was coupled to the
biosensor surface via 3,3′-Dithiodipropionic acid
di(N-hydroxysuccinimide ester (DTSP),
11-mercaptoundecanoic acid (11-MUA) and mainly
inside the polyethylene imine structure.
However, the evidence of optimal affinity for
samples containing glycated hemoglobin provided
the comparative experiments. Within those, the eight
types of prepared biosensors with either specific or
reference surfaces were consequently mounted into
the flow-trough cell and the response to GHb sample
(410 μg/ml, constant concentration) was monitored.
The experiments were done in duplicate with each
sensor; the equal scheme of experiment was used in
all cases. The lowest response provided the Gold-
PEI-GA-APBA sensor (together with the appropriate
reference one). Therefore these were tested for their
ability to bind sorbitol (low molecular compound
containing vicinal diol group) in concentration of
10 mg/ml (in phosphate buffer pH=9.0). Response of
the specific sensor (229.6 Hz) together with the
reference one (19.8 Hz) showed correctness of
theoretical predictions. Low density of boronate
groups presented on the top of PEI-matrix (low
affinity to glycated hemoglobin) allowed only low
binding of glycated hemoglobin. Moreover, the
difference between specific and non-specific
response (66.5 vs. 44.6 Hz, respectively) was the
next, and probably main, reason to exclude the PEI-
GA-APBA recognition layer from further use in
GHb analysis.
As it was commonly considered the boronic
acid-diol interaction is not substantially affected by
ionic strength of environment. However, most recent
publications (Zhong et al., 2004) indicated a
substantial increase of boronate affinity to diol group
in low ionic strength solutions (co-solute
concentrations up to 0.25 M). Determination of the
ionic strength effect on glycated hemoglobin
interaction with immobilised boronic groups was not
the principal aim of our study. However, the
examination of influence of the used various
reagents on interaction were carried out prior to the
final analysis. The ionic strength of tested reagents
proceeded from 0.9 to 84.3 mM (Modified Drabkin
reagent and 50 mM phosphate buffer, respectively),
pH was in range 7.4 - 9.6. Low ionic strength, i.e.
use of Modified Drabkin Reagent, supports the
affinity interaction. This result well correlates with
findings of other authors.
The biosensor Gold-MUA-APBA and the
previously optimized conditions (peristaltic pump;
flow rate of 100 μl/min; Modified Drabkin reagent
as the working medium and the 2 min regeneration
of sensing surface with 200 mM HCl) were used in
all calibration experiments. The presented method
shows the advantage of calibration using only one
standard solution – blood sample with defined
content of glycated hemoglobin. After dilution in
various proportions, thus obtained standards were
used for calibration. The response of the
piezoelectric biosensor as well as photometric sensor
was increasing with increasing concentration of
glycated and total Hb, respectively. The percentage
of glycated hemoglobin was calculated as the
glycated hemoglobin / total Hb ratio (x 100%). Both
values (glycated and total hemoglobin concentration,
respectively) were taken from the calibration curves,
constructed as the response of biosensor and
photometric sensor to concentration of glycated and
total hemoglobin, respectively. The biosensor can
not be calibrated only using samples containing
various percentage of glycated hemoglobin, the
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
110

10 20 30 40 50
0.1
0.2
0.3
0.4
0.5
0.6
A
405
GHb [μg/ml]
APBA
Glycine
( )
amount of total hemoglobin should be considered,
too.
A blood sample of diabetic patient with high
content of glycated hemoglobin (14.3%; determined
by the ion-exchange HPLC) was used for calibration
of our setup. The set of six samples for calibration of
analyser was prepared by dilution of blood with the
Modified Drabkin reagent in the following
sequence: 300, 375, 500, 600, 1000 and 2875-times.
Thus prepared samples were placed to the
autosampler and after 15 min of preincubation
(including the base-line stabilisation) were
consequently measured. The combined calibration
graph was constructed using the responses of
photometric and piezoelectric sensors in the time
5 min (Fig. 2).
Figure 2: Calibration of a combined setup, upper curves
show absorbance changes due to the different hemoglobin
content in samples, the curves below were recorded as a
result of binding of various concentration of glycated
hemoglobin.
3.2 AHA Experiments
The AHA method was optimized according its
ability to bind glycated hemoglobin. Buffers of
various pH (7 and 9, respectively) were used to
maximize the surface affinity to GHb. Although
there was found higher adsorption of glycated
hemoglobin at pH=7, the next experiment showed
the low specifity of binding at this pH. On the other
hand, use of buffer of pH=9.0 provides quite a lower
capacity of the surface, however, the binding is
highly specific (Fig. 3). The reference surface,
covered only with glycine, was employed to
compare specifity of binding.
In the further experiments the assay was calibrated
by use of hemoglobin standard solution (total Hb
calibration) and blood sample (with known GHb
content, determined by a standard method), both
diluted in various ratio in order to get at least five
points in a calibration graph. The total hemoglobin
assay provided a linear response in range 10 – 1000
µg/mL; the GHb analysis can be performed in the
concentration range varying between 10 and 40 µg
GHb/mL.
Figure 3: Measured enzymatic activity as result of binding
of glycated hemoglobin to reference (modified with
glycine) and specific surface (modified with
aminophenylboronic acid, APBA). Experiments
performed at pH=9.0.
4 CONCLUSIONS
Two methods for analysis of hemoglobin A
1c
are
presented. First, a combined biosensor for
determination of glycated and total hemoglobin in
blood is reported. The amount of total hemoglobin is
measured in flow-through photometric sensor,
concentration of the glycated fraction is
subsequently monitored by its binding to the
APBA-modified piezoelectric biosensor (higher
content cause higher damping of resonant
frequency).
The other method is based on ELISA principle
(AHA, Affinity Heterogeneous Assay) in either
direct or indirect arrangement. Boronic acid
derivative, capturing the glycated fraction of
hemoglobin, is immobilized on the surface of the
microtitre plate. Amount of glycated hemoglobin is
visualized by measuring of its peroxidase activity.
Total hemoglobin concentration is measured
photometrically at 405 nm.
11
31
52
63
84
104
50 H
z
1 min
A
B
76
219
367
444
587
730
2 min
0.1 A.U.
(540 nm)
sample
buffer
11
31
52
63
84
104
50 H
z
1 min
11
31
52
63
84
104
50 H
z
1 min
A
B
76
219
367
444
587
730
2 min
0.1 A.U.
(540 nm)
sample
buffer
BIOINTERFACES BASED ON IMMOBILIZED BORONIC ACID WITH SPECIFITY TO GLYCATED PROTEINS
111

Both methods present promising approach in
diagnosis of glycohemoglobin. The first one presents
fully automatic, low-cost instrument, the other one
offers the possibility to monitor simultaneously
HbA
1c
content in 96 blood samples within a
relatively short time (2 hours).
Possible use of those methods in routine analysis
of blood samples and their comparison with
conventional methods (HPLC) was shown, too.
ACKNOWLEDGEMENTS
The work was supported by the grant no.
KJB401630701 of the Grant Agency of the Czech
Academy of Science.
REFERENCES
Bongartz, D., Hesse, A., 1995. Selective extraction of
quercetrin in vegetable drugs and urine by off-line
coupling of boronic acid affinity chromatography and
high-performance liquid chromatography. J.
Chromatogr., B. 673, 223-230.
Chen, B., Bestetti, G., Day, R.M., Turner, A.P.F., 1998.
The synthesis and screening of a combinatorial peptide
library for affinity ligands for glycosylated
hemoglobin. Biosens. Bioelectron. 13, 779-785.
International Committee for Standardization in
Haematology, 1978. Recommendations for reference
method for hemoglobinometry in human blood (ICSH
Standard EP 6/2: 1977) and specifications for
international hemoglobincyanide reference preparation
(ICSH Standard EP 6/3: 1977). J. Clin. Pathol. 31,
139-143.
Ito, H., Kono, Y., Machida, A., Mitsumoto, Y., Omori, K.,
Nakamura, N., Kondo, Y., Ishihara, K., 2003. Kinetic
study of the complex formation of boric and boronic
acids with mono- and diprotonated ligands. Inorg.
Chim. Acta 344, 28-36.
Kataoka, K., Hisamitsu, I., Sayama, N., Okano, T.,
Sakurai, Y., 1995. Novel sensing system for glucose
based on the complex formation between phenylborate
and fluorescent diol compounds. J. Biochem. 117,
1145-1147.
Kugimiya, A., Takeuchi, T., 2001. Surface plasmon
resonance sensor using molecularly imprinted polymer
for detection of sialic acid. Biosens. Bioelectron. 16,
1059-1062.
Lau, O.W., Shao, B., Lee, M.T.W., 2000. Affinity mass
sensors: determination of fructose. Anal. Chim. Acta
403, 49-56.
Marshall, S.M., Barth, J.H., 2000. Standardization of
HbA
1c
measurements: a consensus. Ann. Clin.
Biochem. 37, 45-46.
Pickup, J.C., Hussain, F., Evans, N.D., Rolinski, O.J.,
Birch, D.J.S., 2005. Fluorescence-based glucose
sensors. Biosens. Bioelectron. 20, 2555–2565.
Přibyl, J., Skládal, P., 2005. Quartz crystal biosensor for
detection of sugars and glycated hemoglobin, Anal.
Chim. Acta 530, 75-84.
Valina-Saba , M., Bauer , G., Stich , N., Pittner , F.,
Schalkhammer T., 1999. A self assembled shell of 11-
mercaptoundecanoic aminophenylboronic acids on
gold nanoclusters. Mat. Sci. Eng. C 9, 205-209.
Zhong, H., Li, N., Zhao, F., Li, K., 2004. Determination of
proteins with Alizarin Red S by Rayleigh light
scattering technique. Talanta 62, 37–42.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
112
