
A MINIMALLY INVASIVE MICROWAVE HYPERTHERMIC
APPLICATOR WITH AN INTEGRATED TEMPERATURE
SENSOR
Guido Biffi Gentili and Mariano Linari
Department of Electronic and Telecommunications, University of Florence, Via S. Marta 3, 50139 Firenze, Italy
Keywords: Interstitial microwave applicator, endocavitary, hyperthermia, temperature sensor.
Abstract: In the field of microwave hyperthermia and thermo-ablation, the use of minimally invasive applicators is
recognized as a very promising means for the treatment of small, early stage, cancer lesions because a very
thin applicator can be easily introduced inside the body and precisely directed towards a deep seated tumour
using the most advanced 3D imaging techniques and surgical stereo-navigation. Minimally invasive
applicators have been successfully employed for the treatment of bladder carcinoma and brain tumours but
the accurate temperature monitoring of the heated tissue volume still remains an open problem. In this
paper we propose a new minimally invasive applicator, integrating a low-cost metallic wired temperature
sensor. The miniaturised endocavitary applicator consists of a asymmetric isolated dipole operating at 2.45
GHz. The very slim shape of the applicator allows to easily insert it into the lesion through a soft plastic
tube (catheter) while a temperature sensor, properly embedded in the applicator body, measures the tissue
temperature at the interface with the catheter surface. An electromagnetic analysis based on the Finite
Integration Technique (FIT) and experimental verifications over a tissue sample proved that a coaxial
choke, enclosing the temperature sensor wires, allows localize the heating pattern in a restrict volume while
drastically reducing measuring artefacts due to the perturbing effects induced by the probe leads.
1 INTRODUCTION
Microwave endocavitary and interstitial
hyperthermia has been widely investigated in the
past decades as localised thermal therapy for cancer
treatment. Many thin applicators have been
developed and used in therapeutic applications with
valuable results. Most of them are essentially
constituted of an insulated monopole, dipole or helix
feed through a thin coaxial cables. However several
technical solutions (Turner, 1986; Tumeh and
Iskander, 1989; Camart et al., 1996; Lin and Wang,
1987; Cerri et al.; 1993; Saito et al., 2000) have
been proposed in order to localize the heating in a
restrict area of tissue around the tumour and to avoid
accidental and unwanted hot spots in the healthy
tissue.
The effectiveness of a microwave thermal
therapy depends not only on the radiative properties
of the applicator used but also on the reliable and
accurate temperature control during the therapeutic
treatment. Impedance tomography, microwave
radiometry, magnetic resonance imaging (MRI) and
also methods based on ultrasounds are very
attractive non-invasive temperature-monitoring
techniques. Despite these promising prospects, up
till now a very accurate measure of depth tumours
temperature can be obtained only by invasive
techniques, because only thermocouples, thermistors
or optical fibres sensors seem to give the required
measuring accuracy, spatial resolution and real-time
response. Temperature monitoring by invasive
sensors is common practice in the hyperthermic
treatments. Usually, very thin probes are separately
inserted in the tissue near the antenna and moved
ahead and back in order to better estimate the
effective heating pattern. In that case only optical
fibre sensors could be employed because metallic
wired probes located near the antenna strongly
interact with the radiating element, producing
uncontrollable electromagnetic fields distortion and
false temperature readings.
When the tumour is at its early stage a single
applicator can be employed to heat the small volume
of the lesion by inserting it as sketched in Figure 1.
113
Biffi Gentili G. and Linari M. (2008).
A MINIMALLY INVASIVE MICROWAVE HYPERTHERMIC APPLICATOR WITH AN INTEGRATED TEMPERATURE SENSOR.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 113-118
DOI: 10.5220/0001052801130118
Copyright
c
SciTePress

In this case it is advantageous to integrate the
temperature sensor directly in the applicator body in
order to avoid additional traumas in the patient and
to simplify the hyperthermic treatments itself.
Figure 1: Single applicator heating a small tumour.
When lesions are more extended in volume an array
of applicators could be required to uniformly heat
the tumor, as sketched in Figure 2.
Figure 2: Array of applicators heating a medium-sized
tumour.
Also in this case applicators with integrated
temperature sensors can be usefully employed
because the temperature distribution inside the
tumor volume can be numerically estimated starting
from the point measurements taken in
correspondence of each radiating element, by
combining tomography algorithms and bio-heat
equation.
From the mechanical and structural point of view
it is not difficult to integrate a very thin fiber-optic
probe inside the core of the applicator without
altering its original diameter. It is worth to note,
however, that fiber-optic sensors are very expensive
and delicate devices and thus not properly suited for
the production of a rugged mono-use applicators, as
required for the routined clinical practice.
Another sensor that is practically unaffected by
the strong EM fields existing near the antenna is the
Bowman thermistors (Bowman, 1976) because its
high resistance leads can not carry significant RF
currents. Unfortunately also this sensor type is
delicate and expensive to be fabricated.
Ordinary low-cost and sturdy thermometric
sensors, as thermocouples and thermistors, use high
conductance metallic leads for the connection to the
measuring unit. Unfortunately, when they are placed
too close to the applicator body, metallic wires cause
unwonted electromagnetic (EM) interferences. In
these cases dangerous and uncontrolled hot spots in
the tissue can occur, as well erroneous (or very
noisy) temperature readings.
This work suggests a novel technique for
integrating a low-cost wired temperature sensor
inside the body of a minimally invasive Microwave
Hyperthermic Applicator (MHA) without perturbing
the radiated fields.
2 METHODS
2.1 Applicator Design
The proposed interstitial/endocavitary MHA,
depicted in Figure 3, essentially consists of a coaxial
asymmetric dipole type antenna, radiating in the
biological tissue through an insulating tube
(catheter).
Figure 3: Microwave Hyperthermic Applicator (MHA)
(length: 65 mm; thickness: 2 mm) working at 2.45 CHz.
In order to avoid unwanted heating of the healthy
tissue, a coaxial balun has been introduced to block
the back currents flowing on the surface of the
coaxial feeding line (Longo et al., 2003). The
diameter of the radiating upper arm of the dipole has
been properly increased to improve the matching of
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
114

the MHA input impedance to the tissue (Jones et al.,
1988; Biffi Gentili et al., 1995). Thank to its very
thin shape (65 mm in length and 2 mm in thickness)
the coaxial applicator can be easily introduced inside
a small catheter (3-4 mm in diameter) and
subsequently inserted into the lesion. The position of
the applicator can be fixed in loco using an anchor
balloon.
Starting from this basic applicator configuration,
a wired temperature probe (thermocouple or
thermistor) has been successively introduced into the
catheter inside the balun as depicted in Figure 4.
Figure 4: MHA with a temperature probe embedded inside
the insulator of the coaxial choke.
The temperature sensitive tip of the probe is
embedded in the catheter body in order to measure
the temperature of the tissue at the interface with the
catheter itself, where higher temperatures are
expected. Miniature (SMT) chip inductors can be
also inserted close to the sensing tip of the probe to
isolate it from the radiating conductors of the coaxial
applicator.
2.2 Numerical Analysis
Electromagnetic full-wave analysis has been
employed to investigate the Specific Absorption
Rate (SAR) distribution produced by the applicator
and the perturbations due to the closeness of the
metallic wires of the temperature probe. EM fields
have been calculated with a time domain Finite
Integration Technique (FIT) (CST Studio Suite,
2006) in a three-dimensional spatial domain
constituted by a muscular tissue volume including
the MHA model.
Perfect Matched Layers (PML) boundaries condition
(Berenger, P. 1994) have been used in order to limit the
computational domain to 140 × 60 × 60 mm
3
volume.
However the thin profile of the applicator and the high
permittivity of the human muscle tissue (ε
r
= 52 − j13 @
2.45 GHz) required a very little mesh size (< λ/50 at 3
GHz) which made heavy the computation load. EM
simulations, performed in the 2 to 3 GHz frequency range,
run for 2 hour with an Intel Pentium III @1GHz with 1.5
GB RAM memory
2.3 Experimental Set-up
Different types of soft material phantoms with the
same dielectric properties of human tissue have been
experimented upon in the recent years to investigate
SAR and temperature distribution produced by
microwave applicators. An accurate phantom should
closely represent the electromagnetic properties of
the human body in the frequency range of interest
and it should be easy to prepare and to handle.
When small volume of tissue are involved in the
heating process, true biological tissue sample as a
suine liver or a chicken breast appear more suitable
to investigate power deposition near the applicator.
In our case the realized MHA prototype has been
tested by using chicken breast. The prototype,
ending with a SMA male connector, is connected to
a microwave source working at 2.45 GHz, capable
of a maximum power of 300 W continuous (CW) or
pulsed. The input and reflected power of the MHA
is monitored by a power meter connected to a
bidirectional coupler while the temperature,
measured by a thermocouple or a thermistor, is
acquired by an A/D converter, recorded in a data file
and simultaneously displayed on a PC monitor for
the real-time direct control of the heating process.
3 RESULTS AND DISCUSSION
In order to accurately define the heating pattern
volume and avoid unpredictable field distortion or
undesirable tissue overheating, back currents
flowing on the surface of the coaxial feeding line
and currents induced on the metallic wires of the
thermocouple was blocked by a common coaxial
balun. As a result, at the 2.45 GHz operative
frequency, the power deposition in the tissue is
confined within a small well defined ellipsoidal
volume wrapping the radiating section of the
applicator body as shown in Figure 5.
As expected, numerical simulations evidence a
focusing point of the EM fields near the sensing tip
of the sensor that could be responsible of a localised
hot spot and temperature overestimation. In order to
reduce this unwonted spot, miniaturised chip
inductors have been properly inserted in series to the
leads of the temperature sensor near the tip to block
any RF current flowing in the sensing element. In
A MINIMALLY INVASIVE MICROWAVE HYPERTHERMIC APPLICATOR WITH AN INTEGRATED
TEMPERATURE SENSOR
115
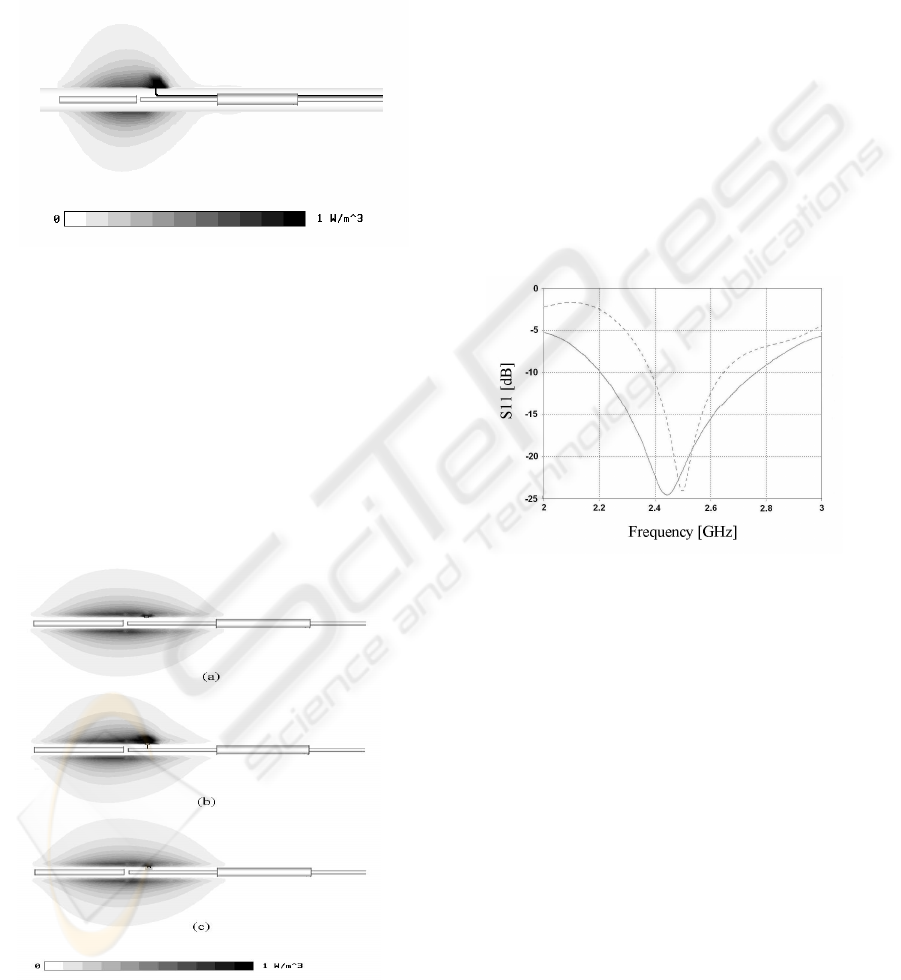
our EM model we used lumped inductive elements
with an inductance of 1 nH and we compared
numerically this solution (Figure 6c) with the
reference cases where the tip was kept floating
(Figure 6a) or short circuited to the external coaxial
conductor of the applicator (Figure 6b) that
schematizes the applicator shown in Figure 5.
Figure 5: Normalized SAR distribution of a MHA with a
temperature probe embedded inside the insulator of the
coaxial choke.
If a thermistor is used as temperature sensing
element instead of a thermocouple, the sensor tip is
constituted by a semiconductor with conductivity
ranging between 10
-2
and 10
-4
S/m. In both cases the
simulation result of Figure 6c shows that the
insertion of the micro-chokes practically eliminates
the arising of hot spots near the sensing termination
and therefore drastically reduces temperature
measuring errors.
Figure 6: Normalized SAR distribution of a MHA with the
integrated temperature probe: (a) floating tip (ideal case),
(b) tip short circuited to the coaxial conductor (worst
case), (c) tip RF isolated with inductors (actual case).
At a radial distance of 5 mm from the applicator, in
the direction of maximum radiation, SAR is less
than 50 % of the maximum value calculated at the
surface of the catheter and reduces to 90 % at a
distance of 10 mm. This confirms that the applicator
can be used both for hyperthermic treatments of
small tumours ad also for thermo-ablation surgery,
depending on the maximum applied power and time.
It is worth to note that the device can tolerate 30 W
CW or average power and up 150 W pulsed power
without any damage or excessive self-heating.
The MHA input matching is also numerically
calculated in the frequency range from 2 to 3 GHz,
in absence of the thermocouple and in presence of a
temperature sensor with the probe close to the
applicator and the metallic leads embedded into the
coaxial balun. In both cases a reflection coefficient
less than -20 dB is assured at the 2.45 GHz working
frequency as shown in Figure 7.
Figure 7: Input reflection parameter vs. frequency of the
MHA with (continuous line) and without the integrated
temperature probe (dotted line).
Power distribution in the tissue was experimentally
evaluated by applying 20 W continuous microwave
power to the MHA over a period of about 15
minutes (Figure 8) and a pulse power of 60 W for 10
seconds. The results are in good agreement with the
numerical predictions and proved that the induced
heating pattern in the biological medium assumes
the typical ellipsoidal shape around the applicator
radiating end. The overall dimension of the
ellipsoidal heating pattern clearly depends on the so
called thermal-dose, i.e. on the quantity of the EM
energy delivered to the medium. It is also evidenced
that the metallic sensor tip (thermocouple or
thermistor) do not produces a local hot spot,
authorizing us to state that an accurate temperature
monitoring can be obtained. This was afterward
confirmed through a measure made with an auxiliary
fiber-optic temperature sensor. Using the integrated
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
116

sensor, the temperature at the interface between the
catheter and the tissue can be carefully monitored
during the microwave heating process because not
significant self-heating of the sensor has been
observed. Figure 9a shows the evolution of the
temperature vs. time when 20 W CW power is
applied for 15 minutes at the input of the applicator,
while Figure 9b depicts the thermal response of the
medium to a 10 second pulse of 60 W peak power.
It is worth note that the increase of the
temperature is very fast in both cases because the
chicken breast used as phantom for our heating
experiments is not perfused by the blood and
therefore the thermal response of the tissue is
determined only by its thermal conductivity.
(a)
(b)
Figure 8: Thermal pattern in a splitted chicken tissue
obtained by applying 20 W CW power to the MHA for 15
minutes (a) and 20 minutes (b) respectively.
(a)
(b)
Figure 9: Temperature evolution at the tissue/applicator
interface obtained by applying 20W CW input power to
the MHA for 15 minutes (a) and a 10 second pulse of 60W
peak power (b).
4 CONCLUSIONS
The proposed minimally invasive MHA integrates a
very cheap temperature sensor and therefore it is
very suited for the mass-production of mono-use
devices. The integration of the radiating element and
the temperature sensor inside the same applicator
case allows to heat a small tissue volume (target)
and to measure the temperature accurately at the
same time. Due to the use of a simple coaxial balun
the microwave energy is confined around the
applicator body reducing the risk of accidental
overheating of healthy tissue close to the tumour.
The thermistor (or the thermocouple junction) peeps
out from the catheter surface in the point where the
EM field, and hence the temperature, reaches the
maximum. Temperature in deeper zone of the tissue
surrounding the applicator can be extrapolated by
mathematical models based on the bio-heat equation
(Pennes, 1948) if the EM and thermal parameters of
the tissue are known.
A MINIMALLY INVASIVE MICROWAVE HYPERTHERMIC APPLICATOR WITH AN INTEGRATED
TEMPERATURE SENSOR
117

Multiple applicators (arrays) with integrated
temperature sensors could be used in order to treat
larger tissue volumes and more accurately estimate
the temperature distribution through the combined
application of bio-heat equation and tomography
algorithms.
The pliability of the miniaturized coaxial cable
and of the associated silicon catheter make easier the
insertion of the applicator using the natural way of
the body or minimally invasive surgical procedures.
Many coaxial applicators for hyperthermic
treatments show a heating pattern characterized by a
typical tear drop shape. The implementation of a
coaxial choke in the MHA investigated in this paper
reduces appreciably the drop tail and allows to
precisely localize the tissue volume involved during
the microwave treatment. Moreover the high degree
of miniaturization due to the availability of
miniaturized coaxial cable to use in medium-high
power applications (dimensions about 1-2 mm in
diameter are easily available), permits to extend the
clinical applications of this minimally invasive
applicator. Hyperthermic treatments of bile-ducts in
cancer therapy, impracticable in the past for the
restrict dimensions of the ducts, could be possible
nowadays as well other delicate surgical
interventions that require high precision and reduced
invasivity.
The originality of this MHA, from an
engineering point of view, lies in the peculiar
integration of the metallic wires of a low cost
temperature sensor inside of the choke body without
perturbing the SAR distribution.
The invasivity of the clinical hyperthermic or
thermo-ablation treatment is highly reduced using
this type of applicator because in fact no separated
insertions for temperature probes, no additional
external electrodes as for RF treatments or any other
kind of devices are required. Therefore many deep-
seated tumors (e.g. certain brain, liver,
gastrointestinal or gynaecological tumours), could
be effectively and easily treated with the proposed
MHA.
The integrated temperature sensing element
permits to accurately monitor the maximum
temperature reached into the tissue and it can be
used to close the control loop of a specific
microwave hyperthermic or ablative process by
defining the appropriate thermal-dose to be
administered to the lesion.
By monitoring the temperature in time domain,
very useful data on blood perfusion rate, thermal
conductivity and specific heat could be directly
extrapolated by the bio-heat transfer equation and
used to construct more complex and realistic
biological tissue models. As well thermal properties
difference between healthy and pathological tissue
could be relieved in order to extrapolate diagnostic
information.
REFERENCES
Turner, F., 1986. Interstitial equal-phased arrays for EM
hyperthermia. IEEE Trans. Microwave Theory Tech.,
vol. 34, no. 5, pp. 572-578.
Tumeh, A.M., Iskander, M.F., 1989. Performance
comparison of available interstitial antennas for
microwave hyperthermia. IEEE Trans. Microwave
Theory Tech., vol. 37, no. 7, pp. 1126-1133.
Camart, J.C., Despretz, D., Chive, M., Pribetich, J., 1996.
Modeling of various kinds of applicators used for
microwave hyperthermia based on the FDTD method.
IEEE Trans. Microwave Theory Tech., vol. 44, no. 10,
pp. 1811-1818.
Lin, J.C., Wang, Y. 1987. Intertitial microwave antennas
for thermal therapy. Int. J. Hyperthermia. vol. 3, no. 1,
pp. 37-47.
Cerri, G., De Leo, R., Primiani, V.M. 1993. Thermic
endfire’ interstitial applicator for microwave
hyperthermia. IEEE Trans. Microwave Theory Tech.,
vol. 41, no. 6, pp. 1135-1142.
Saito, K., Hayashi, Y., Yoshimura, H., Ito, K., 2000.
Heating characteristics of array applicator composed
of two coaxial-slot antennas for microwave
coagulation therapy. IEEE Trans. on Microwave
Theory and Techniques, vol. 48, no. 11, pp. 1800-
1806.
Bowman, R.R. 1976. A probe for measuring temperature
in radio-frequency-heated material. IEEE Trans. on
Microwave Theory and Techniques, pp. 43-45.
Longo, I., Biffi Gentili, G., Cerretelli, M., Tosoratti, N.,
2003. A Coaxial Antenna with Miniaturized Choke for
minimally Invasive Interstitial Heating. IEEE Trans.
on Biomedical Engineering, vol. 50, no. 1, pp. 82-88.
Jones, K., Mechling, J.A., Trembley, B.S. 1988. SAR
distributions for 915 MHz interstitial microwave
antennas used in hyperthermia for cancer therapy.
IEEE Trans. on Biomedical Engineering, vol. 35, no.
10, pp. 851-857.
Biffi Gentili, G., Leoncini, M., Trembly, B.S., Schweizer
S.E. 1995. FDTD Electromagnetic and Thermal
Analysis of Intertitial Hyperthermic Applicators. IEEE
Trans. on Biomedical Engineering, vol. 42, no. 10, pp.
973-980.
CST Studio Suite 2006, Computer Simulation Technology
GmbH, D-64289 Darmstadt, Germany.
Berenger, P. 1994. A perfectly matched layer for the
absorption of electromagnetic waves. J. Computat.
Phys. vol. 114, no. 2, pp. 185-200.
Pennes, H.H. 1948. Analysis of tissue and arterial blood
temperatures in the resting human forearm. J. Appl.
Physiol. vol. 85, no. 1, pp. 93-122.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
118
