
METHOD FOR MEASURING PARYLENE THICKNESS USING
QUARTZ CRYSTAL MICROBALANCE
Henna Heinilä, Maunu Mäntylä and Pekka Heino
Institute of Electronics, Tampere University of Technology, P.O. Box 692, FI-33101 Tampere, Finland
Keywords: Parylene, Quartz Crystal Microbalance, Biomedical Coating.
Abstract: At present, the exact final thickness of parylene coating is difficult to specify in the beginning of the coating
process since the parylene thickness is a function of many components. The elements that control the
thickness are substrate surface area in a vacuum chamber, program parameters, and amount of dimer charge.
This paper describes a method for measuring parylene coating thickness using quartz crystal microbalance.
The thickness is measured by an oscillation frequency change of quartz crystal as parylene deposites on the
quartz crystal plate. These results can be used for specifying the parylene thickness real-time during the
coating process.
1 INTRODUCTION
Biomedical implants have many strict requirements
as they are being implanted for a long time under the
skin. Implantable medical devices need to be coated
hermetically before implantation. The coating
material has many strict requirements. One very
important, biological aspect is that the implant must
be biocompatible. To reduce inflammation, all of the
components of an implant should be nontoxic to
cells (Wolgemuth 2002). Since most of the materials
used in the device's electronics are not
biocompatible, the encapsulation of the device with
nontoxic materials is needed to prevent elusion into
the body. The human body is a very hostile
environment for any foreign materials. Therefore,
the implant must be biostable. This electrical
characteristic means that the device operation must
be protected from the living tissue (Wolgemuth
2002). Otherwise the body tries to destroy or isolate
the device. These two fundamental aspects, the
protection of the device against the biological
environment and the protection of living tissue
against device’s materials must be ensured before
the device is implanted in a human being
(Wolgemuth 2002). In addition, the long-term
stability of the device and the coating needs to be
high and meet the specifications.
Materials, which are used as coating materials
for medical applications and qualify above-
mentioned requirements, include many metals, metal
alloys, ceramics, polymers, and polymer composites
(Ratner et al. 1996). Small and rigid implantable
devices, like pacemakers and drug pumps, could be
coated with a metal case unlike the devices that must
be extremely small scale, like in MEMS
applications, or devices that must conform to the
tissue movements. These latter mentioned devices
like neural prosthesis should be made from flexible
substrate with flexible coating.
Many types of polymers are widely used for
medical purposes. Polymers like epoxies, silicones,
polyurethanes, and parylenes, have many desirable
properties, such as ease of tailoring and processing,
low cost, and an excellent corrosion resistance
(Ratner et al. 1996). Parylene conformal coating is
ideal for the medical applications because of its
many unique properties. Vacuum deposited parylene
is applied in a chamber by means of gas phase
polymerization (Noordegraaf & Hull 1997).
Compared to liquid coating processes, vacuum
deposited parylene coatings exhibit uniform
coverage of medical implants and electronics
components without the presence of pinholes or
pooling. During the coating process, all of the
exposed substrates in evacuated vacuum chamber
are coated and the coating grows as a conformal film
simultaneously on all surfaces and parylene
penetrates into the pinholes (Noordegraaf & Hull
1997). During the parylene coating process, no
impurities are generated and hence parylene coatings
222
Heinilä H., Mäntylä M. and Heino P. (2008).
METHOD FOR MEASURING PARYLENE THICKNESS USING QUARTZ CRYSTAL MICROBALANCE.
In Proceedings of the First International Conference on Biomedical Electronics and Devices, pages 222-226
DOI: 10.5220/0001056102220226
Copyright
c
SciTePress

are, like silicone, one of the highest purity coatings
on the market (Licari 2003). Parylene is flexible
coating material just like silicone, but compared to
silicone, parylene has better moisture and chemical
resistance and also extremely thin coatings are tight
enough for insulation and implants (Stieglitz et al.
2002). In addition, parylene has excellent adhesion
to most surfaces (Licari 2003, p. 157).
2 BACKGROUND
2.1 Parylene
Parylene (poly-para-xylylenes) is a universal term
for members of a unique polymer class (Licari
2003). By using dimer of di-para-xylylene, parylene
has the ability to be deposited by vacuum deposition
onto exposed surfaces at room temperature (Licari
2003). Varying the process parameters of deposition
can control the thickness of parylene and the
thickness may vary from 0,025 µm to several tens of
micrometers. According to Licari (2003), the final
thickness of coating can be controlled to ±10 % of
desired thickness. Nevertheless, our laboratory
results have proven that the thickness variance can
be even greater. The parylene coating is inert and
conformal and hence provides dielectric and
environmental isolation. Parylene coating is used in
many applications like aerospace, automotive and
military industry, and also in medical applications.
Nowadays, the four most frequently used
commercially available parylene variations are
parylene N, C, D, and HT. The two first have the
longest history of use and are most commonly used
in medical coating applications. This paper
concentrates on coating with the polymer parylene
C. (Specialty Coating System 2007)
Parylene C can provide extremely thin, uniform,
and pinhole-free coating. It has low electrical
dissipation factor, high dielectric and mechanical
strength, and good chemical, electrical, and
biological stability. It also has significantly lower
moisture, chemical, and caustic gas permeability
than parylene N. Above-mentioned reasons make
parylene C very compatible for medical implants. In
addition, parylene C is not cytotoxic and it is proven
to be compatible with body tissue and blood. (Yang
1998)
2.2 Parylene Coating Process
The parylene coating process can be divided into
three stages. The first stage is vaporization, the
second is pyrolysis, and the third stage is deposit. In
the beginning of the coating process, the raw
material, dimer that is white powder, is vaporized
under vacuum (1.0 mbar) and heated to a dimeric
gas at approximately 150 ˚C. During the second
stage, pyrolysis, the gas is pyrolized to cleave the
dimer to its monomeric form under vacuum (0.5
mbar) to approximately 680 ˚C. In deposition stage
the monomer reaches the room temperature
deposition chamber. The monomer gas
simultaneously absorbs and polymerizes on the
substrate as a transparent parylene film. The
substrate temperature never rises more than couple
of degrees above the room temperature. (Specialty
Coating System 2007; Pang et al. 2005, p. 4)
The surface area of substrates in deposition chamber
can vary a lot. The substrates to be coated are
positioned in a stand that spins in a vacuum
chamber. When a small amount of dimer is used, it
does not matter where the substrates are located in a
stand. Also the stand area might vary a lot. The
stand might for example have several levels and the
grid on each level might be tight. The coating
thickness is mainly a function of substrate surface
area in chamber and amount of dimer charge.
Program parameters have also minor importance.
Even 1 μm thick coating is discovered to be tight
enough for implants (Stieglitz et al. 2002). Thus it is
very important to be able to measure the thickness of
the coating accurately. The process is controlled by
the deposition process parameters. The process
parameters for our measurements are found from
known coating process recipes that are used also in
Para Tech Coating, Inc. in Sweden (Para Tech
Coating, Inc. 2006). Recipes determine the amount
of dimer, process temperatures and times, and
approximate final parylene thickness. It has been
proven that when different recipes are used for same
amount of dimer, the final thickness might be
different. Therefore, in addition to the amount of the
dimer, also the process parameters affect the final
thickness of parylene.
After the coating process, the achieved thickness
could be measured by releasing a sample parylene
film from the top of a preparat glass that has been in
vacuum chamber, and then measuring the thickness
of film. This does not give very accurate results,
since the thin film is charged electrically and it
might be creased. Also, after releasign the film from
the top of a preparat glass, the film surface might
already contain some impurities from the air that
affect the result. Moreover, as the parylene thickness
can depend on the location in the camber, the
thickness of the film on the preparate glass can be
METHOD FOR MEASURING PARYLENE THICKNESS USING QUARTZ CRYSTAL MICROBALANCE
223

different from the thickness of the sample coating.
Since the final thickness is hard to predict before the
coating process or to measure accurately after the
process, a real-time thickness monitoring system
would be useful in many cases.
2.3 Quartz Crystal
A crystal oscillator is an electronic circuit that
creates an electric signal with a certain frequency by
using the mechanical resonance of a vibrating quartz
crystal. The crystal is made of piezoelectric material
and it is placed between a pair of electrodes. When
these two electrodes are connected to an alternating
electric field, the quartz crystal starts to oscillate at
its resonance frequency due to the piezoelectric
effect.
A quartz crystal microbalance (QCM)
measurement technique is based on the oscillation at
a precise frequency. When any type of mass is added
on the surface of the crystal, the resonance
frequency of the quartz crystal decreases. According
to the Sauerbrey equation,
2
2
2
2
op
o
qq qq
o
f
f
f
mx
Av v
ff
ρ
ρρ
=−Δ = Δ = Δ−
(1)
the change in mass, Δm, is proportional to the
change in frequency, Δf. Here f
o
is the initial
resonant frequency of the crystal, f the resonant
frequency of the coated crystal, A is the effective
area of the crystal (between electrodes), ρ
q
is the
density of quartz, and v
q
is the shear velocity in
quartz. The change of mass is written in terms of
parylene thickness, Δx and density, ρ
p
. When very
accurate measurements of very small mass changes
need to be performed, the QCM technology is very
appealing and it has already been studied as a system
for measuring film thickness during deposition of
different materials. (Eggins 2002; Gulati, Auras, &
Rubino 2006) In addition to rigid deposits, the QCM
has been widely used for its respond to changes in a
liquid's viscoelastic properties. Some targets of using
QCM have been for example humidity sensor (Ito et
al. 2003), bacterial spores detector (Lee et al. 2005),
and proteins detector.
3 MEASUREMENTS
3.1 Preliminary Study
The starting point for the real-time thickness
measurements method was the conclusion that if
parylene penetrates inside the crystal oscillator and
covers the quartz crystal (Fig. 1), the mass of the
quartz crystal must increase. Based on the QCM
technology, the mass change of the crystal should be
directly proportional to its frequency change.
Moreover, since the mass change is proportional to
crystal area and parylene thickness, the frequency
change is proportional to parylene thickness, as
given in eq. (1). By increasing the parylene
thickness, the frequency of crystal oscillators placed
in vacuum chamber should linearly decrease.
Figure 1: A quartz crystal covered with parylene.
In order to enable parylene penetration to the surface
of the quartz crystal, small holes must be drilled to
the metal case of the crystal oscillator. As a
preliminary study for the measurements, the number
of holes on the sides of the oscillator required to
achieve the largest frequency change, was
determined. It seemed that three 1 mm holes
generated larger resonance frequency change than
one or two holes, even if all of the crystal oscillators
were in the same run of parylene equipment in
vacuum chamber. At the same time making four
holes on both sides gave the same result as three
holes so there was not need to increase the hole
number to more than three. Thus it seems that full
covering of the quartz resonator was not obtained
with only one or two holes in the resonator case.
These results bear evidence that parylene can not
penetrate through all pinholes easily.
3.2 Measurement Set-up
For the measurements, 21 crystal oscillators were
used. The resonance frequency of these crystal
oscillators was 3.579 MHz. Three 1 mm holes were
drilled on both sides of the crystals' metal case. Each
crystal was numbered with consecutive numbers.
After drilling the holes, the resonance frequency of
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
224
each oscillator was measured with a network
analyzer.
The measurement arrangement was carried out in
the following order. After measuring the initial
frequency, all of the crystals in metal case were
coated with parylene. Model 3000 Labtop, Parylene
Deposition System, Para Tech Coating (Aliso Viejo,
USA) equipment was used for coating. The
resonators were coated using 1.7 g of dimer and
process parameters for 2 μm coating. Therefore the
estimated thickness of the parylene film was 1.7–2.0
μm. The same procedure was repeated 11 times for
all crystals. Each time there was an estimated 1.7–
2.0 μm growth on the preceeding coating. The
frequency of each crystal was measured with
network analyzer after each coating run, and results
were documented. The frequency decreased due to
the mass increment on the quartz crystals.
To be able to analyse the results, an accurate film
thickness after each parylene coating must be
known. These measurements were done visually, by
using microscope Olympus BX60M. For reference
data, silicon samples were coated in the same
processes with the crystal resonators. Eleven silicon
chips (approximately 1.0 cm x 1.0 cm), in addition
to crystals, were placed into the vacuum chamber in
the beginning of the first coating. One silicon chip
was taken away from the chamber after every
coating and marked with consecutive numbering.
Meaning chip having number i should have
approximately i × 1.7–2.0 μm parylene film on the
silicon. To be able to define the accurate thicknesses
of parylene coats, each chip was placed in a mould
and covered with epoxy. The cross-sectional
samples were then mechanically prepared for
microscopic thickness examination by grinding and
polishing.
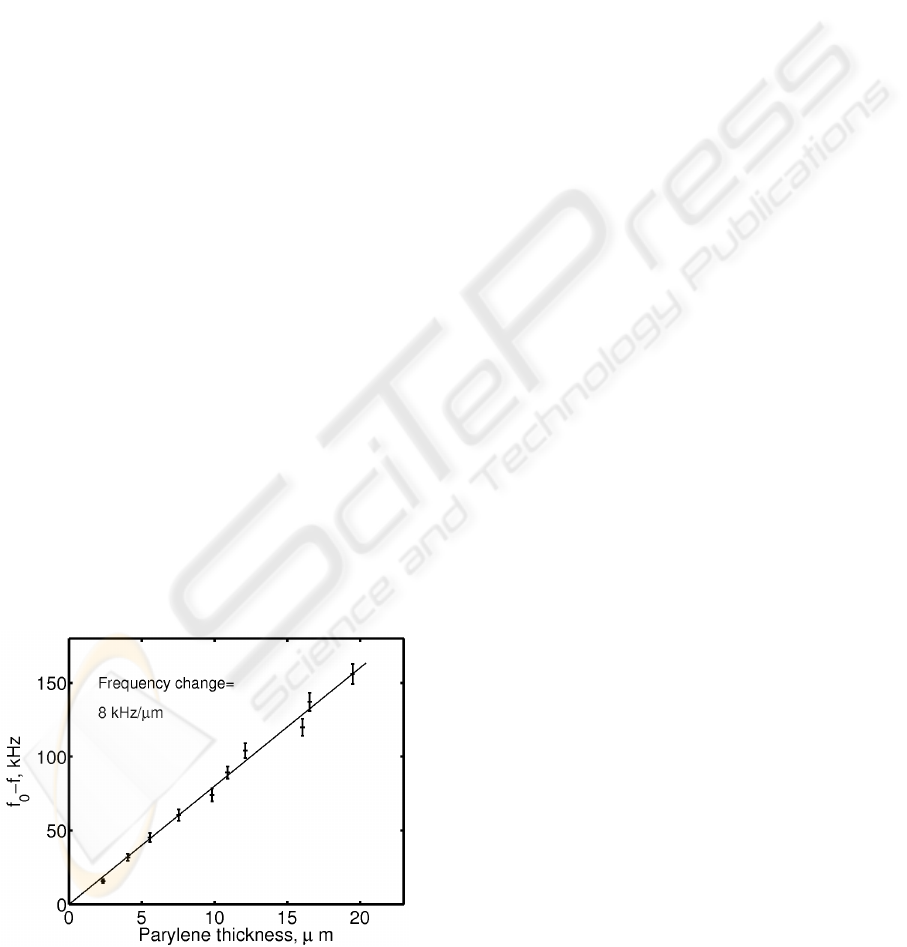
Figure 2: The frequency change of crystals as a function of
parylene thickness. The vertical lines indicate the average
value ± standard deviation of 21 crystal samples.
4 RESULTS
After 11 coating runs, measurement results proved
that there is a certain interrelation between the
resonance frequency and the thickness of parylene.
The measurement results are illustrated in Fig. 2.
The frequency change of crystals increases linearly
as parylene thickness increases. The slope of this
line is 8.0 kHz/μm.
The coefficient appearing in the Sauerbrey
equation, (1), is 3.64 kHz/μm. As compared to this
value, the frequency change in Fig. 2 is quite a lot
larger. There are several possibilities that can
explain this discrepancy.
On each coating run, parylene was added on the
last coat of parylene. It is possible that these layers
or the interfaces had collected some impurity that
affected the results; though impurities were not seen
in visual microscope examination. Another source of
discrepancy might be in the coating process
parameters. When different recipes for same amount
of dimer are used, the final thicknesses are unequal.
Therefore, it seems that the process parameters
affect the final density of parylene, and/or the
parylene coats different locations in the chamber
with different thicknesses. Furthermore, the parylene
density after each coating is not measured, and
hence it is unreliable to use the literature value for
parylene density in Sauerbrey equation. Finally,
when the crystal is coated, parylene covers the entire
quartz crystal area including the sides of the crystal
and the electrodes connected to the quartz crystal.
Hence it might prevent the vibration of the quartz
crystal and measurement results in frequency change
are not equal to frequencies measured with
Sauerbrey equation.
In the future, these measurement results are
usable basis for developing the parylene thickness
measurement set-up, though additional experimental
results will be needed for accurate reference data.
Idea is to control the deposition thickness of
parylene during the deposition process by
monitoring the resonance frequency change of
crystal oscillator in vacuum chamber. The real-time
measurement set-up would be composed of the
crystal oscillator placed in vacuum chamber, the
network analyser, and the measuring cables for
generating the connection between the crystal
oscillator's electrodes and the network analyser. The
possibility to produce sealed hole, for example to the
observation window of vacuum chamber to allow
measuring cables to pass through, should be studied.
In real-time measurement, the network analyser
measures the resonance frequency change and as the
METHOD FOR MEASURING PARYLENE THICKNESS USING QUARTZ CRYSTAL MICROBALANCE
225

earlier defined frequency change for target parylene
thickness is reached, the coating run could be cut
off.
5 SUMMARY
We have presented a method to measure the
thickness of parylene coating, especially for medical
electronic devices. The method is based on
frequency change of coated quartz crystals. We
proved that the frequency change is proportional to
the parylene thickness, and determined the factor
relating the thickness and frequency change. The
applicability of this factor to different parylene
coating processes was discussed. The method is
applicable also for real-time measurements enabling
the measurement of parylene thickness during the
growth process. In real-time measurements, the
growth process could be stopped after the target
thickness has been reached.
REFERENCES
Eggins, B. R. 2002, 'Chemical Sensors and Biosensors',
John Wiley & Sons. 291 p.
Gulati, N., Auras, R., Rubino, M. 2006, 'Determination of
barrier properties of poly(lactide) polymers using a
quartz crystal microbalance', ANTEC 2006 Platics,
North Carolina, 2006, p. 1530–1534.
Ito, H., Kakuma, S., Ohba, R., and Noda, K., 2003,
'Development of a Humidity Sensor using Quartz
Crystal Microbalance', SICE Annual Conference,
Fukui, Japan, August 4.-6., 2003, pp. 1175–1178.
Licari J., 2003, Coating Materials for Electronic
Applications: polymers, process, reability, testing,
Noyes/William Andrew, New York, pp. 154–168
Noordegraaf J. and Hull, H. 1997, 'C-shield parylene
allows major weight saving for EM shielding of
microelectronics', 1st IEEE International Symposium
on Polymeric Electronics Packaging, Norrkoping,
Sweden, October 26.-30., 1997, pp. 189–196.
Pang, C., Cham, J. G., Nenadic, Z., Musallam, S., Tai, Y-
C., Burdick, J. W., and Andersen, R. A. 2005, 'A New
Multi-Site Probe Array with Monolithically
Intergrated Parylene Flexible Caple for Neural
Prostheses', 27
th
IEEE Annual Conference on
Engineering in Medicine and Biology, Shanghai, 2005, 4p.
Para Tech Coating, Inc. 2006. Retrieved September 7,
2007, from http://www.parylene.com/index.html.
Ratner, B. D., Hoffman, A. S., Schoen, F. J., and Lemons,
J. E., 1996, 'Biomaterials science: an introduction to
materials in medicine', Academic press, San Diego,
484 p.
Lee, S.-H., Stubbs, D. D., Cairney, J., and Hunt, W., D,
2005, 'Rapid Detectioon of Bacterial Spores Using a
Quartz Crystal Microbalance (QCM) Immunoassay',
IEEE Sensors Journal, vol. 5, no. 4, pp. 737–743.
Specialty Coating System 2007, Retrieved March 27,
2007, from http://www.scscoatings.com/.
Stieglitz, T., Kammer S., Koch K. P., Wien S., Robitzki A.
2002, 'Encapsulation of Flexible Biomedical
Microimplants with Parylene C', International
Functional Electrical Stimulation Society
(IFESS):231-3.
Wolgemuth, L. 2002, 'The Surface Modification
Properties of Parylene for Medical Applications',
Medical Device Manufacturing & Technology, pp. 1–4.
Yang, G. R., Ganguli, S., Karcz, J., Gill, W. N., and Lu T.
M. 1998, 'High deposition rate parylene films'. Journal
of crystal growth, vol. 183, pp. 385–390.
BIODEVICES 2008 - International Conference on Biomedical Electronics and Devices
226
