
BIOPHYSICAL MODEL OF A MUSCLE FATIGUE PROCESS
INVOLVING Ca
2+
RELEASE DYNAMICS UPON THE HIGH
FREQUENCY ELECTRICAL STIMULATION
Piotr Kaczmarek
Pozna´n Univeristy of Technology, Insitute of Control and Information Engeenering, Piotrowo 3a, 60-395 Pozna´n, Poland
Keywords:
Electrical stimulation, Muscle model, Calcium release, Muscle fatigue.
Abstract:
The aim of this study is to create a model which enables to explain the muscle fibre contraction due to various
stimulation programs. The model accounts for Ca
2+
release dynamics both as a result of an action potential
and of a stimulus shape, duration and frequency. It has been assumed that the stimulus can directly activate
the voltage-dependent receptors (dihydropiridine receptors) responsible for a Ca
2+
release. The stimulation
programs consisted of standard stimulation trains made of low and middle frequency square pulses. High
frequency modulating harmonic signals have been tested to investigate the fibre fatigue effect. It has been
observed that fatigue effect factors depend on the selected stimulation program. The results reveal that the
fatigue effect could be minimized by changing the shape and frequency of the stimulation waveform. Such the
model could be useful for a preliminary selection and optimization of the stimulus shape and the stimulation
trains, thus reducing the number of in vivo experiments.
1 INTRODUCTION
Electrical stimulation is a rehabilitation technique ap-
plied to increase muscles force, reduce spasticity,
muscular atrophy and to decrease pain effects. It is
also used to restitute a motion in handycaped subjects
via Functional Electrostimulation (FES). In order to
get an efficient FES system, the optimal stimulation
programs have to be worked out. The former investi-
gations revealed that muscle fatigue effect is greater
as a result of electrical stimulation than as a result
of a voluntary contraction (Kostyukov et al., 2000;
Gissel, 2000). It has been reported that stimuli train
frequency and a single pulse shape have the signifi-
cant impact on the fatigue effect (Bennie et al., 2002).
Therefore, the optimization of the stimulation pro-
grams is one of the most important aspects of the FES
method. As far, the optimization has been limited to
the identification of the optimal frequency of a stimu-
lation pattern (Ding et al., 2003; Chou et al., 2005)
or to a search for variable frequency pulse trains.
(Mourselas and Granat, 1998).
The studies on the high frequency stimulation pro-
grams (>200Hz) as well as on the single pulse shapes
as related to the muscle fatigue effect are missing.
The dynamics of Ca
2+
ions transportation plays
an important role in the muscle contraction process
(Bottinelli and Reggiani, 2000; Benders et al., 1997;
Delbono and Meissner, 1996). The change of the
Ca
2+
release rate is an important factor of the fatigue
effect (Westerblad et al., 2000; Gissel, 2000). There-
fore a majority of models reflecting potentiation and
fatigue effects have been based on theCa
2+
dynamics.
(Otazu et al., 2001; Ding et al., 2003; Riener and
Quintern, 1997). In these models the impact of the
stimulus shape as well as of the pulse width on the
fatigue effect were not addressed. It is only assumed
there that a single stimulus evokes an action potential
(AP) in the muscle fibre, which activates a voltage-
dependent dihydropiridine receptor (DHPR) resulting
inCa
2+
release from the sarcoplasmic reticulum (SR).
The amount and the release profile of the liberated
Ca
2+
ions are assumed to be constant, even though
the physiological variability of the AP amplitude and
shape (in the t-tubular system) is observed.
The in vivo experiments demonstrated that the
stimulus amplitude and duration affect the calcium
concentration ([Ca
2+
])(Delbono and Meissner, 1996;
52
Kaczmarek P. (2008).
BIOPHYSICAL MODEL OF A MUSCLE FATIGUE PROCESS INVOLVING Ca2+ RELEASE DYNAMICS UPON THE HIGH FREQUENCY ELECTRICAL
STIMULATION.
In Proceedings of the First International Conference on Bio-inspired Systems and Signal Processing, pages 52-57
DOI: 10.5220/0001062300520057
Copyright
c
SciTePress

Bakker et al., 1996; Benders et al., 1997). However,
the direct influence of a neuro-muscular electrical
stimulation (NMES) on the DHPR receptor behaviour
was ignored in the models. Therefore the applicabil-
ity of these models for testing stimulation trains com-
posed of wider pulses is dubious and the trains fre-
quency should be restricted to the maximal physiolog-
ical frequency of the AP generation (f
stim
< 100Hz).
The aim of this work is to analyse the influence of
the stimulation parameters on the muscle contraction
and fatigue effect. We present a novelmodel of a mus-
cle fibre. The model is an extension of already known
models, by introducing the direct interaction between
the stimulus and the DHPR receptor activity as well as
by incorporating the calcium release dynamics. These
adds-on enable to study the muscle fatigue effect dur-
ing various stimulation programs. In particular we
analyse the influence of a train frequency and a single
pulse-duration on the dynamics of calcium concentra-
tion and on the fatigue effect.
2 PHYSIOLOGICAL
BACKGROUND
2.1 Excitation-Contraction Coupling
Depolarization of sarcolemma due to the physiologi-
cal action potential (AP) or to stimulation, activates a
sarcoplasmic reticulum (SR) Ca
2+
release. The volt-
age signal is transformed into the Ca
2+
release via
a voltage-sensitive dihydropiridine receptor (DHPR),
which activates some of the Ca
2+
channels (ryanoi-
dine receptor - RyR) in SR. This process is called
Dihydripiridine-Induced Calcium Release (DICR).
The amount of the activated RyRs is dependent on
the stimulus intensity and the muscle fibre type. The
number of RyR coupled with DHPR depends strongly
on a fibre type, and is the largest for the slow fibres
(Delbono and Meissner, 1996; Benders et al., 1997).
The uncoupled RyRs are activated as a result of
the sarcoplasmic [Ca
2+
] increase. This effect, called
Calcium-Induced Calcium Release (CICR), generates
a positive feedback in the Ca
2+
liberation process.
Ca
2+
ions are transported by a Ca
2+
-ATPase pump
from cytosol into SR. The pump efficiency is depen-
dent on the [Ca
2+
] in the sarcoplasm. At the rest-
ing state the Ca-ATPasepump maintains theCa
2+
ions
concentration about 10
4
higher in SR than in cytosol
(Bottinelli and Reggiani, 2000).
Ca
2+
diffuses in cytosol from the proximity of SR
surface to the interior of the myofibrils, where a tro-
ponin (TN) is localized. TN is a part of a thin filament
proteins. Whenever TN binds to Ca
2+
, actin (the part
of thin filaments) and myosin (the part of thick fila-
ments) are able to interfere resulting in the myofibril
contraction. In the sarcoplasmic space the Ca
2+
can
be buffered also by parvalbumin (PARV). The CaTN
and CaPARV buffers decrease the concentration of
free Ca
2+
ions in cytosol.
2.2 Fatigue Effect
There is an experimental evidence that the muscles
are subject to the faster fatigue under the electri-
cal stimulation than during the voluntary contraction.
Moreover, the stimulation of muscles having majority
of the fast-type fibres induces stronger fatigue effect
than with the slow-type muscles (Delbono and Meiss-
ner, 1996; Gissel, 2000).
The following reasons of the muscle fatigue are
reported:
1. RyR receptor has an inactivating binding site for
Ca
2+
(Glukhovski et al., 1998) resulting in the in-
hibition of CICR during long-lasting stimulation
as well as in response to APs.
2. The AP amplitude and shape changes in the t-
tubular system under long-lasting AP (Wallinga
et al., 1999; Bakker et al., 1996).
3. TheCa
2+
liberation is inhibited due to the increase
of Mg
2+
concentration and decrease of [ATP]
(Westerblad et al., 2000).
4. Calcium-phosphate precipitation in the SR (We-
sterblad et al., 2000)
5. Structural degeneration of the muscle fibres as a
result of the eccentric, low frequency contraction
(Westerblad et al., 2000).
In this paper only the two first factors will be dis-
cussed.
3 PROCESS MODEL
The proposed muscle fibre model is based on the
model of Otazu et al. (Otazu et al., 2001), origi-
nally applied to study a potentiation and a catch-like
effects in muscle fibres. It consisted of two blocks:
the activation dynamics block (AD) and the contrac-
tion dynamics block (CD). The input to the AD sub-
system is a potential of the sarcolemma activating the
voltage-dependent DHPR receptors. In the original
model it has been assumed that the muscle contrac-
tion is evoked only by APs. Each AP generates the
BIOPHYSICAL MODEL OF A MUSCLE FATIGUE PROCESS INVOLVING Ca2+ RELEASE DYNAMICS UPON
THE HIGH FREQUENCY ELECTRICAL STIMULATION
53

same membrane potential profile and thus the ampli-
tude and dynamics of DCICR is kept constant during
simulation.
The model proposed here accounts for the depo-
larization of the sarcolemma under direct influence
of the stimulation pulses. Thereby it takes into ac-
count the fact that DICR profile and amplitude de-
pend on the stimulus shape, amplitude and train fre-
quency. Such a model let to study the muscle fibre be-
haviour under a high-frequency or a wide-pulse stim-
ulation, when APs are not generated. Such the model
could enable the preliminary optimization and selec-
tion of the stimuli and the stimulation trains reducing
the number of in vivo experiments.
The model of a voltage activated channel reflects
some properties of the DHPR receptor recorded in
vivo during the stimulation with a high amplitude and
the long lasting depolarization pulses (Delbono and
Meissner, 1996; Bakker et al., 1996). The AD block
produces the concentration of the TN bounded to the
Ca
2+
ions ([CaTN]).
3.1 Activation Dynamics
In this section the description of the myofibril model
has been limited only to the aspects necessary for the
analysis of stimulation effects. The full model with
parameters values have been presented by Otazu et
al. (Otazu et al., 2001).
The intracellular Ca
2+
concentration is described
by the stoichiometric equation:
d[Ca
2+
]
PROX
dt
= γ
DICR
+ γ
CICR
+ γ
LEAK
− γ
PUMP
−
−
[Ca
2+
]
PROX
− [Ca
2+
]
DIST
τ
PROX
, (1)
where: γ
DICR
is the rate of Ca
2+
liberation pro-
cess elicited by the voltage-dependent DHPR re-
ceptor (see section 3.2), γ
CICR
is a rate of the
Ca
2+
release from SR through uncoupled-RyR, γ
LEAK
denotes a constant Ca
2+
efflux leakage, while γ
PUMP
is a Ca-ATPase pump rate. [Ca
2+
]
PROX
de-
notes a Ca
2+
concentration nearby SR surface, while
[Ca
2+
]
DIST
is a Ca
2+
concentration in the interior of
the myofibrillar space, τ
PROX
denotes a time constant
of a diffusion process.
Previous results (Glukhovski et al., 1998) revealed
that the RyR channel has two calcium binding sites:
the first one for coupleCa
2+
ions (activating site) and
the second one for a single Ca
2+
ion (inactivating
site). The Ca
2+
release rate is described by the prob-
ability of binding of two Ca
2+
ions to the activation
site (a) and the probability that the inactivation site is
bound to a single Ca
2+
molecule (i).
γ
Ca
= f
Ca
(1− i)a (2)
da
dt
= α
a
(1− a)[Ca
2+
]
2
− β
a
a (3)
di
dt
= α
i
(1− i)[Ca
2+
] − β
i
i (4)
where f
Ca
denotes the maximum rate of Ca
2+
release
through the uncoupled-RyR. The probability of bind-
ing of Ca
2+
ion to the activation or inactivation site is
represented by a coefficient α and depends on [Ca
2+
].
A durability of the bond is characterized by β.
3.2 Voltage Activated Channel
It is difficult to evaluate unambiguously a relationship
between the sarcolemma potential and the Ca
2+
li-
beration rate (via the coupled RyRs) based on the re-
cent experimental evidence, because the CICR effect
is strictly dependent on the DICR effect. The inter-
action between the DICR and the CICR results in a
complex dynamical system, therefore the decompo-
sition of these two effects is difficult (Bakker et al.,
1996; Delbono and Meissner, 1996). For the sake of
simplicity, it is assumed that DICR release rate is pro-
portional to the depolarization potential. Model of the
RyR coupled with DHPR receptor reflects a voltage-
dependent factor generating a slow decline in the
Ca
2+
release rate as an effect of the long-lasting depo-
larization (Delbono and Meissner, 1996). Moreover,
the threshold depolarization potential (V
th
), which re-
flects DHPR excitability, is taken into consideration
(Delbono and Meissner, 1996; Bakker et al., 1996).
γ
DICR
= g
DHPR
(1− i
V
)(V
m
− E
rest
) (5)
di
V
dt
= α
V
(1− i
V
)(V
m
− E
rest
) − β
V
i
V
(6)
where V
m
is the sarcolemma potential, E
rest
denotes
a resting potential of the sarcolemma, g
DHPR
denotes
a proportional coefficient, i is related to the voltage-
dependent DICR decline.
The parameters in eq. (5) and (6) were estimated
based on in vivo results available for a soleus muscle
(Delbono and Meissner, 1996), under the assumption
that the refractory period of the DHPR is similar to a
refractory period of sarcolemma (8ms). The value of
g
DHPR
was calculated assuming that AP (which am-
plitude reaches 20mV (Wallinga et al., 1999; Bakker
et al., 1996)) generates the Ca
2+
release according to
Otazu et al. (Otazu et al., 2001). The V
th
is calcu-
lated from the Voltage dependent of SR Ca
2+
release
results and the coefficients α
V
and β
V
were estimated
by using least square method and digitalized results
of the time dependence Ca
2+
release. The obtained
estimates are presented in tab. 1
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
54

Figure 1: The contraction profiles (A) and [Ca
2+
] concentration (B) recorded at the beginning and at the end of 100s stimu-
lation period for 10,50,100Hz trains of square-wave and modulated harmonically pulses (500Hz).
Table 1: Parameters of the voltage activated channel.
g
DHPR
α
V
β
V
E
rest
V
th
M(mV · s)
−1
(mV · s)
−1
s
−1
mV mV
1.0e− 3 1.29 125 −80 −50
3.3 Contraction Dynamics
The input to the block modelling the contrac-
tion dynamics is a concentration of TN bound to
Ca
2+
([CaTN]) (Otazu et al., 2001). The contrac-
tion dynamics is described by a linear second-order
element connected with two nonlinear elements: a
threshold-type (connected to the input) and a Hill-
type saturation (connected to the output). Such the
behavioural model, accounts for the following phys-
iological observations: the threshold level of the
[CaTN] above which the contraction occurs, and the
saturation of the [CaTN]-Force curve (Bottinelli and
Reggiani, 2000).
4 SIMULATIONS
4.1 Comparison of Two Modes of
Stimulation
In our experiment the fatigue effect was studied dur-
ing stimulation of the myofibril model lasting 100s.
The standard stimulation with short stimuli (0.1ms)
and frequency in the range of (2÷100)Hz was used.
Each pulse was assumed to trigger an AP. More-
over, the persistent stimulation by (250÷1500)Hz si-
nusoidal trains was investigated. It was assumed
that during transcutaneous NMES, the muscle fibre
was depolarized by both positive and negative half-
periods. The pulse polarity has a little influence on
muscle activation as compared to the pulse amplitude.
The intensity magnitude must be above the DHPR
threshold (V
th
) (Green and Laycock, 1990). The stim-
ulation amplitude was selected in order to obtain my-
ofibril contraction at the level observed with a tradi-
tional stimulation at the range (50Hz÷100)Hz. It has
been assumed that the persistent stimulation inhibits
the generation of APs (as in a TENS effect)(Bakker
et al., 1996).
4.2 Evaluation of Fatigue Effect as
Related to the Pulse-width
The influence of the depolarization on the fatigue
effect was investigated in the following experiment.
First the square stimulation pulses at the frequency
10, 30 and 50Hz with varying width in the range
of 4÷20ms have been applied. Then, the modula-
tion of the corresponding stimulation pulses with the
harmonic 500Hz signal were applied with respect to
30Hz stimulation sequence. In both cases the genera-
tion of AP at the beginning of each stimulation period
(30Hz) was enabled. The aim of this study was to
determine whether the pulse-width or the pulse mod-
ulation can reduce the fatigue effect.
In our paper, the fatigue effect is characterized by
two parameters: the relative force decrease (RFD)
and the relative Ca
2+
concentration decrease (RCD).
These parameters are defined as:
RFD =
F
max
− F
min
F
max
· 100% (7)
RCD =
[Ca
2+
]
max
− [Ca
2+
]
min
[Ca
2+
]
max
· 100% (8)
where F
max
denote maximal force and [Ca
2+
]
max
is
a maxiumum calcium concentration, while F
min
and
BIOPHYSICAL MODEL OF A MUSCLE FATIGUE PROCESS INVOLVING Ca2+ RELEASE DYNAMICS UPON
THE HIGH FREQUENCY ELECTRICAL STIMULATION
55
[Ca
2+
]
min
are maximal a force and a calcium concen-
tration, respectively at the end of stimulation experi-
ment lasting 100s.
5 RESULTS AND CONCLUSIONS
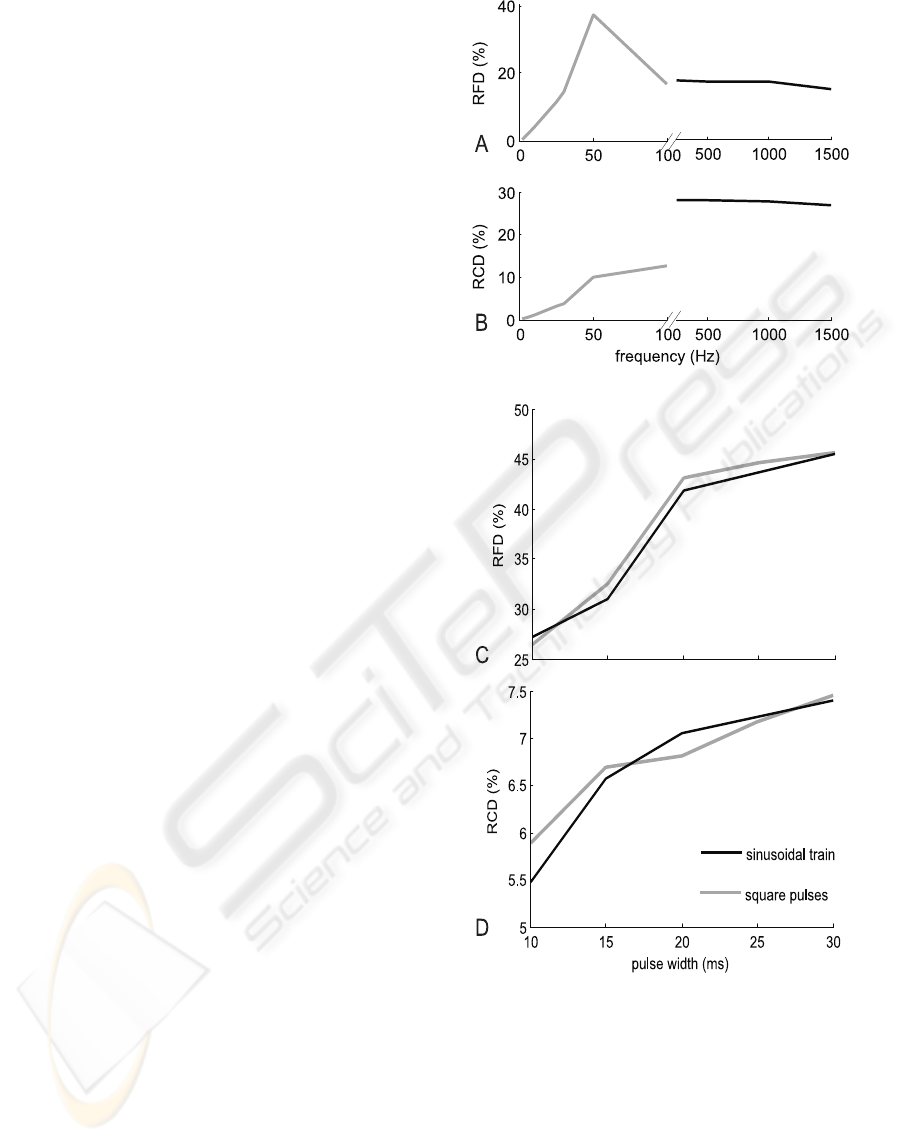
5.1 Frequencial Effects
The fatigue effect under the traditional square-wave
stimulation (1÷100)Hz is similar to the results of
in vivo experiments (Westerblad et al., 2000; Chou
et al., 2005). The relative force decrease (RFD) is
greater for sub-tetanic (50Hz) contractions than for
the fused tetani (100Hz) stimulation (fig. 1A and,
2A). However, this result does not reflect the change
in Ca
2+
concentration. The relative [Ca
2+
] decrease
(RCD) is greater for the 100Hz than for the 50Hz
stimulation (fig. 2B). The muscle stimulated with
100Hz pulses is more fatigue-resistant due to the non-
linear relationship between the [CaTN] and the con-
traction force. The saturation of this function ensures
that during fused contractions, the force changes are
small even if the calcium concentration changes are
significant (Westerblad et al., 2000). In the case of
unfused contractions (1-30Hz) the rise of the stim-
ulation frequency increases the fatigue effect (RFD)
and RCD as well (fig. 2). However the RFD and
the RCD values are lower in that case than during
sub-tetani contractions (50Hz). In each case, the cal-
cium concentration decrease is due to the inhibition
of uncoupled-RyR (see eq. 4). The inhibition level
depends on mean as well as on maximal calcium con-
centration. This can be observed in the frequency-
RCD relation (fig. 2). Moreover such a significant
force decrease in the case of sub-tetani contraction
(50Hz) is due to the decay of the potentiation effect
(Otazu et al., 2001). The results obtained with the har-
monic high-frequency stimulation (HFS) reveal that
the observed RFD is similar as for the 100Hz tra-
ditional stimulation (fig. 1A) and slightly depends
on the pulse base-frequency (fig. 2A). However the
RCD value is two times larger here than in the case
of the traditional stimulation (fig. 2B). The calcium
concentration decrease cannot be explained here as a
result of uncoupled-RyR inhibition, because the max-
imal [Ca
2+
] level is significantly lower than during
the traditional stimulation (fig. 1B), so the inhibition
level must be lower as well. Therefore the main factor
resulting in RCD increase must be the coupled RyRs
habituation (eq. 6).
Figure 2: A relative force decrease (RFD) (A,C) and
Ca
2+
concentration decrease (RCD) (B,D) as a function of
the stimulation frequency (A,B) and the pulse-width (C,D).
5.2 Pulse width Effect
The analysis of the pulse width influence on the mus-
cle fatigue does not reveal any significant differences
between the square pulses and the modulated sinu-
soidal stimulation (fig. 2B,C). However the sinus-
modulated trains seem to be slightly better. Fatigue
effect increases here as the pulse width grows, how-
BIOSIGNALS 2008 - International Conference on Bio-inspired Systems and Signal Processing
56

ever for short pulses (10-15ms) it is significantly
lower then for the traditional stimulation at 50Hz (fig.
2). In case of the modulated HFS, the RCD is over
five times lower in comparison to the results of the
harmonic persistent stimulation. This observation can
be explained on the basis of the DICR model, because
the modulated sinusoidal stimulation ensures the re-
fractory period for the DHPR receptor.
5.3 Discussion
Presented myofibril model reflects effects of Ca
2+
re-
lease from SR as a result of sarcolemma depolariza-
tion. It does not take into consideration the proper-
ties of the sarcolemma and other tissues which are
stimulated during NMES. Thereby, the effect of di-
rect influence of a transcutaneous stimulus on DHPR
receptor can not be clearly established. It could be ex-
plained only on the basis of in vivo experiment results
and on a muscle model reflecting myofibril proper-
ties, muscle fibres recruitation during stimulation and
electrical properties of the skin and other tissues com-
bined.
Modulated HFS trains seem to do better than the
traditional stimulation programs, however the influ-
ence of such a stimulation on the fibre degeneration
process should be investigated. Although the ampli-
tude of repolarization pulses during HFS stimulation
are 50% lower as compared to the short-pulses stim-
ulation, the mean stimulation current is significantly
higher (Bennie et al., 2002). In comparison with the
wide-pulse stimulation the modulated HFS seems to
be less painful due to the lower tissue impedance at
a higher frequency. It should be mentioned that the
presented model and results can be useful to evalu-
ate stimulation programs under the hypothesis that the
transcutaneus stimulation can trigger the DICR effect.
ACKNOWLEDGEMENTS
The work was partially supported by the Polish
Ministry of Education and Science, project no.
1445/T11/2004/27
REFERENCES
Bakker, A. J., Head, S. I., and Stephenson, D. G. (1996).
Measurement of membrane potential and myoplasmic
[ca2+] in developing rat myotubes at rest and in re-
sponse to stimulation. Cell Calcium, 19(5):409 – 418.
Benders, A. A., Oosterhof, A., Wevers, R. A., and
Veerkamp, J. H. (1997). Excitation-contraction cou-
pling of cultured human skeletal muscle cells and the
relation between basal cytosolic ca2+ and excitability.
Cell Calcium, 21(1):81 – 91.
Bennie, S. D., Petrofsky, J. S., Nisperos, J., Tsurudome, M.,
and Laymon, M. (2002). Toward the optimal wave-
form for electrical stimulation of human muscle. Eur
J Appl Physiol, 88(1-2):13 – 19.
Bottinelli, R. and Reggiani, C. (2000). Human skele-
tal muscle fibres: molecular and functional diversity.
Progress in Biophysics & Molecular Biology, 73:195
– 262.
Chou, L.-W., Ding, J., Wexler, A. S., and Binder-Macleod,
S. A. (2005). Predicting optimal electrical stimulation
for repetitive human muscle activation. J Electromyo-
graphy Kinesiology, 15:300–309.
Delbono, O. and Meissner, G. (1996). Sarcoplasmic reticu-
lum ca2+ release in rat slow- and fast-twitch muscles.
J. Membr. Biol., 151(2):123 – 130.
Ding, J., Wexler, A. S., and Binder-Macleod, S. A. (2003).
A mathematical model for fatigue minimization dur-
ing functional electrical stimulation. Journal of Elec-
tromyography and Kinesiology, 13:575–588.
Gissel, H. (2000). Ca2+ accumulation and cell damage in
skeletal muscle during low frequency stimulation. Eur
J Appl Physiol, 83(2-3):175 – 180.
Glukhovski, A., Adam, D., Amitzur, G., and Sideman, S.
(1998). Mechanism of ca2+ release from the sar-
coplasmic reticulum: a computer model. Ann Biomed
Eng, 26:213–229.
Green, R. and Laycock, J. (1990). Objective methods for
evaluation interferential therapy in the treatment of in-
continence. IEEE Trans Biomed Eng, 37(6):615–623.
Kostyukov, A. I., Hellstrom, F., Korchak, O. E.,
Radovanovic, S., Ljubisavljevic, M., Windhorst, U.,
and Johansson, H. (2000). Fatigue effects in the cat
gastrocnemius during frequency-modulated efferent
stimulation. Neuroscience, 97(4):789 – 799.
Mourselas, N. and Granat, M. H. (1998). Evaluation of pat-
terned stimulation for use in surface electrical stim-
ulation systems. Medical Engineering and Physics,
20:319–324.
Otazu, G. H., Futami, R., and Hoshimiya, N. (2001). A
muscle activation model of variable stimulation fre-
quency response and stimulation history, based on
positive feedback in calcium dynamics. Biol Cybern,
84(3):193 – 206.
Riener, R. and Quintern, J. (1997). A physiologically based
model of muscle activation verified by electrical stim-
ulation. Biochemistry and Bioenergetics, 43:257–264.
Wallinga, W., Meijer, S. L., Alberink, M. J., Vliek, M.,
Wienk, E. D., and Ypey, D. L. (1999). Modelling ac-
tion potentials and membrane currents of mammalian
skeletal muscle fibres in coherence with potassium
concentration changes in the t-tubular system. Eur
Biophys J, 28(4):317 – 329.
Westerblad, H., Bruton, J. D., Allen, D. G., and Lannergren,
J. (2000). Functional significance of ca2+ in long-
lasting fatigue of skeletal muscle. Eur J Appl Physiol,
83(2-3):166 – 174.
BIOPHYSICAL MODEL OF A MUSCLE FATIGUE PROCESS INVOLVING Ca2+ RELEASE DYNAMICS UPON
THE HIGH FREQUENCY ELECTRICAL STIMULATION
57
