
PERSPECTIVES OF ELECTROCOAGULATION IN
WATER DISINFECTION
C. Ricordel
1,2
, D. Hadjiev
2
and A. Darchen
3
1
Ecole des Métiers de l’Environnement, Campus de Ker Lann, 35170 Bruz, France
2
Laboratoire de Biotechnologie et Chimie Marine, Université de Bretagne Sud
Centre de Recherche, rue Saint Maudé, 56132 Lorient, France
3
UMR CNRS Sciences Chimiques de Rennes, ENSCR, avenue du Général Leclerc, 35700 Rennes, France
Keywords: Water disinfection, E. Coli, Electrocoagulation, Aluminum, Kinetic.
Abstract: Today effective electrochemical disinfection system seems to be an alternative to conventional chlorination,
ozone or UV processes. This paper reports on a series of experiments evaluating the disinfection efficiency
of electrocoagulation cell using Al electrodes. Solutions contaminated by E. coli and surface waters were
used as test media. The process significantly outperformed chemical coagulation reducing the amount of
aluminum used. In all cases, the treated solutions were effectively disinfected and almost total removal of
coliforms and algae were rapidly performed. A relationship for the disinfection rate of E. coli cells has been
proposed. This equation takes into account the electrophoretic migration of the cells and the subsequent
release of discharged cells into the bulk.
1 INTRODUCTION
Nowadays water quality and available quantity are
being challenged by increased pollution from
various sources such as industry and agriculture.
Innovative, cheap and efficient methods of purifying
water as well as to clean wastewaters from industrial
effluents are needed. Electrochemical techniques
such as electroflotation, electrodecantation or
electrocoagulation offer the possibility to be easily
installed and they require few chemicals. According
to Rajeshwar et al. (Rajeshwar 1994) benefits from
using electrochemical techniques include:
environmental compatibility, versatility, energy
efficiency, safety, selectivity and cost effectiveness.
In addition to these, the systems employ only
electrons to facilitate water treatment. Of the known
techniques, there is a considerable interest in using
electrocoagulation. The process is well known for
the treatment of suspended particles (
Domini 1994,
Abuzaid 2002), phosphates (Idermez 2006a, Idermez
2006b) and oil-in-water emulsions (Canizares 2008 ,
Asselin 2008
, Un 2006). Another approach of the
electrocoagulation is water disinfection where this
technique can reduce notably the amount of chlorine
used. It has been reported that electrochemical and
magnetic fields can destroy a wide variety of
microorganisms from viruses (
Zhu 2005) to bacteria
(
Dao 2004, Li 2004).
In this study, Escherichia coli cells were used to
show the disinfection effect of electrocoagulation
using aluminum electrodes. The technique was then
used to treat total coliforms and algae.
2 THEORETICAL
Electrocoagulation is a process involving many
chemical and physical phenomena that use soluble
(“sacrificial”) anode to supply ions into the treated
water. In the electrocoagulation process the
coagulation ions are produced in situ. The process
involves three successive stages: coagulant
formation by electrolytic oxidation of the soluble
anode; contaminants destabilization and emulsion
breaking; aggregation of the destabilized phases
leading to floc formation.
In the case of an electrocoagulation, the reactor
contains one cathode and one anode (Figure. 1).
When a potential is applied with a current generator,
the anode material undergoes oxidation, while water
254
Ricordel C., Hadjiev D. and Darchen A. (2009).
PERSPECTIVES OF ELECTROCOAGULATION IN WATER DISINFECTION .
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing, pages 254-258
DOI: 10.5220/0001511602540258
Copyright
c
SciTePress
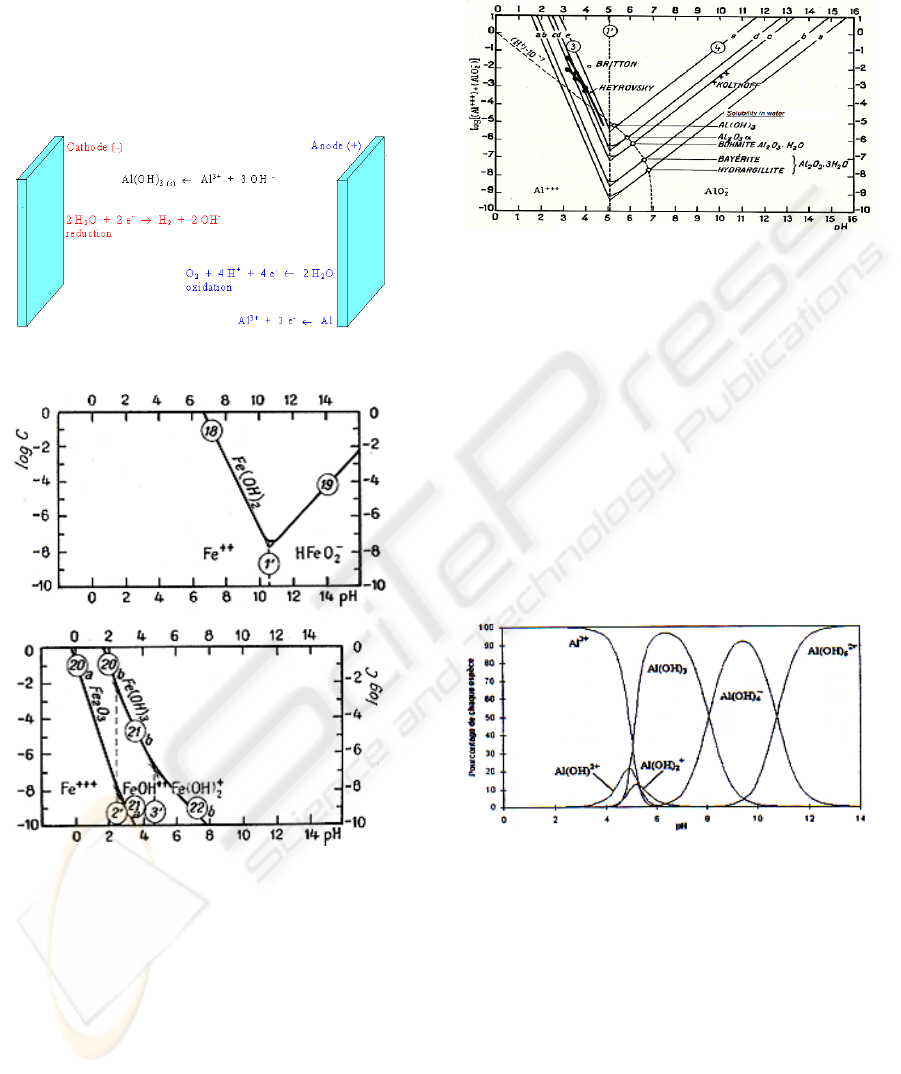
reduction occurs at the cathode. The reactions can be
presented as follows:
At the anode: M
s
= M
aq
n+
+ ne
-
2H
2
O = 4H
+
+ O
2
+ 4e
-
At the cathode: 2H
2
O +2e
-
= H
2
+ 2OH
-
Figure 1: Schematic diagram of an electrocoagulation cell.
Figure 2: Influence of pH on the solubility of iron
hydroxides.
Generally iron or aluminum electrodes are
proposed both allowing good process performance.
These metal ions generated in the solution react in
solution and afford corresponding hydroxide and/or
polyhydroxide compounds. These compounds have
strong affinity for suspended particles, droplets and
eventually bacteria to cause coagulation. In fact, the
choice of the sacrificial electrode is a very important
parameter which depends on the operating
conditions, the goal being to use all the metal
hydroxides dispersed in the solution. The solubility
of these hydroxides depends on the pH of the treated
solution as it can be seen in Figure 2 and Figure 3.
Figure 3: Influence of pH on the solubility of aluminum
hydroxides.
It is clear that aluminum hydroxides have a lower
solubility than ferrous hydroxide, and therefore a
better efficiency in wastewater treatment. This is
why aluminum electrodes have been used in this
study.
When aluminum electrodes are used, the
generated Al
aq
3+
ions undergo spontaneous reactions
to generate:
Mononuclear complexes :
Al(OH)
2
+
,Al(OH)
4
-
, Al (OH)
2+
Polynuclear complexes : Al
2
(OH)
2
4+
, Al
2
(OH)
5
+
,
Al
6
(OH)
15
3+
, Al
13
(OH)
34
5+
Figure 4: Al hydroxides and polyhydroxides species as
function of pH.
For pH between 5 and 8, the predominant specie
is Al(OH)
3
(almost 95% when pH = 6.5) with small
amounts of Al(OH)
2
+
and Al(OH)
2+
. These
compounds show strong affinity for counter ions to
cause coagulation. The gases evolved at the
electrodes may impinge on and cause flotation of the
coagulated material. It can be assumed that the
process may be summarized as follows: charge
neutralization resulting from the production of
counter ions during the electrochemical dissolution
of the sacrificial anode, van der Waals interactions
followed by coagulation and floc formation.
PERSPECTIVES OF ELECTROCOAGULATION IN WATER DISINFECTION
255

In the present study we are presenting results
obtained with artificial and real aqueous solutions
for E. coli and indigenous flora to illustrate the
possibilities offered by an electrochemical
disinfection process using aluminium anode.
3 EXPERIMENTAL
3.1 Artificial Solutions containing E.
Coli
These solutions were prepared by diluting an E. coli
culture in a PCA medium. The E. coli was grown on
a shaker at 37°C for 24 hours and then 100 µl were
used to inoculate the medium.
Surface waters were obtained from local river
and pool.
E. coli, total coliforms and algae were counted in
accordance with standard methods.
3.2 Pilot Plant
Figure 5: Schematic diagram of the bench-scale reactor
with monopolar electrodes.
The experiments were performed with two types of
reactor. The first one was composed of two
electrodes which were plunged in a beaker (V = 1
L). Different distances between the electrodes have
been tested (1, 2 and 4 cm). The electrodes ware
connected to a direct current power supply able to
furnish a controlled tension or current up to 30 V or
10 A, respectively. Applied tension, current,
temperature, conductivity and pH were measured.
After each run, the electrodes were dried and
weighted.
The obtained results have been used to build the
pilot plant shown in Figure 5. It consists of an
electrocoagulation reactor (V = 2 L) having two
pairs of monopolar aluminum electrodes and a
decantation chamber with V = 10 L. The flow in the
pilot is controlled with a centrifugal pump (Prolabo,
France). All tests were performed at a constant flow
rate of 100 L.h
-1
(flow velocity of 1.85 x 10
-3
m.s
-1
).
4 RESULTS AND DISCUSSION
4.1 Comparison between Coagulation
and Electrocoagulation
In order to illustrate the interest of the technique, a
comparison between the chemical coagulation and
the electrocoagulation is presented in Table 1. The
chemical coagulation was performed using the same
amount of aluminum as in the electrocoagulation. It
can be seen that the electrocoagulation improves
considerably thee bacteria removal. Using the same
amount of aluminium a 3-log (99.9%) reduction can
be achieved in this case, while only 1-log bacteria
reduction was reached with the chemical coagulation
in the same Al dosage range.
Table 1: Comparison between chemical and electro-
coagulation (Coa = coagulation).
Coa 1 Coa 2 Coa 3 Electo
coagulation
T0
(CFU/mL)
5.75E+04 2.38E+04 8.30E+04 3.00E+05
Tf
(CFU/mL)
9.75E+03 1.12E+04 1.40E+04 5.40E+02
E (%) 83 53 83 99.9
The fact that the electrocoagulation significantly
outperformed chemical coagulation deserves
explanation. According to Oss (Oss 1994) bacterial
adhesion to surfaces results from the Lifshitz-Van
der Waals interaction free energy and the Lewis
acid-base interaction free energy. Bacteria either
donate or accept electrons to the surface of the
substrate (in this case the gas bubbles). Adhering
bacteria may decrease electrostatic repulsion
BIOSIGNALS 2009 - International Conference on Bio-inspired Systems and Signal Processing
256

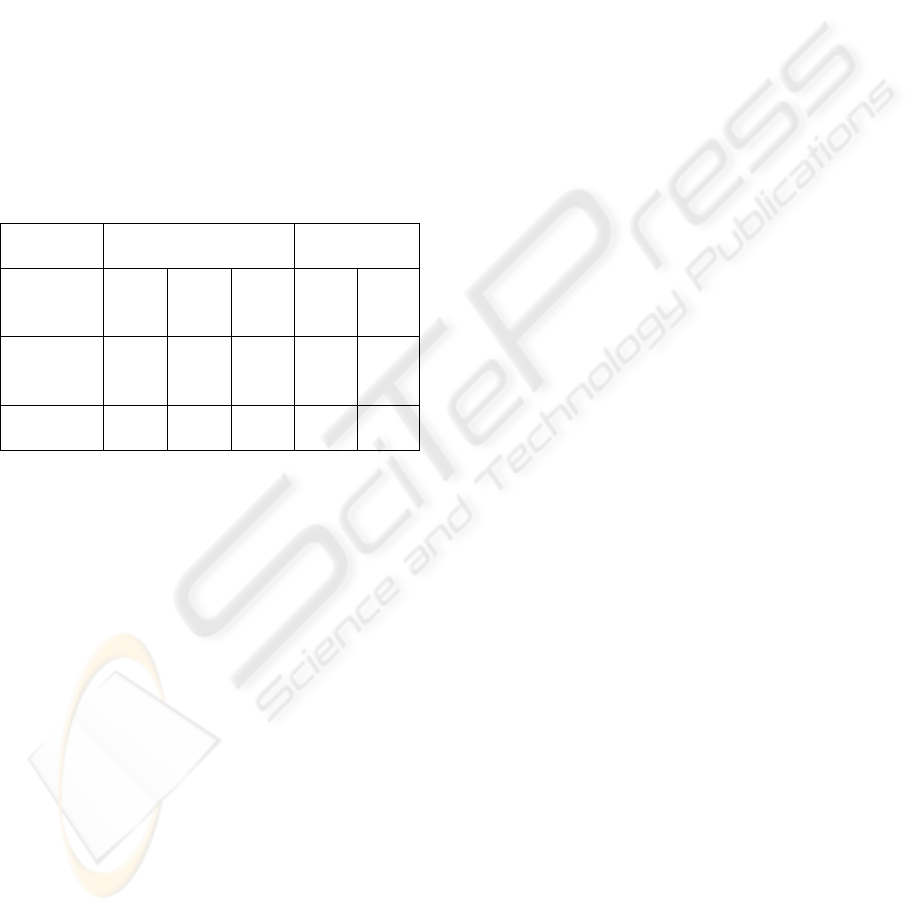
Table 2: Reduction of the cellular concentration of E.coli as function of the gap between the electrodes.
Distance 1 c
m
2 c
m
4 c
m
Run N° 1 2 3 1 2 3 1 2
T
0
(CFU/mL) 6.60E+04 1.20E+04 3.95E+04 1.60E+04 5.35E+04 2.48E+04 2.50E+04 5.00E+04
T
f
(CFU/mL) 3.50E+02 9.30E+02 6.35E+02 7.70E+01 2.85E+03 4.45E+02 8.30E+01 1.70E+01
E (%) 99.5 92 98 99.6 95 98 99 99
N/N
0
0.0053 0.0775 0.0161 0.0048 0.0518 0.0179 0.0033 0.0003
allowing floc formation. The charge transfer,
however, takes place over a range shorter than 0.5
nm, so close contact is needed. This contact is
realized easily in electrocoagulation process where
the negatively charged bacteria could
electrophoretically move resulting in higher bacteria
concentrations near the positively charged anode. At
the same time, the coagulation (chemical or
electrochemical) creates a sludge blanket that
entraps and can bridge colloidal particles as well as
bacteria still remaining in the aqueous solution.
At the same time, aluminum cation is generated
continuously over the extended area of the anode in
electrocoagulation compared with the point addition
in chemical coagulation. So aluminum is more
effectively used. Thus, locally higher coagulant and
bacteria concentrations might have been one reason
of the better performance of electrocoagulation
compared to chemical coagulation.
4.2 Influence of the Gap between the
Electrodes
The tests were performed at an initial pH = 7.2 at
15.6°C. The initial conductivity of the medium was
0.4 mS/cm. The applied tension was 30 V and the
current 0.22 A. The electrocoagulation time was
estimated according to the well-known sterilization
chart assuming a first order reaction for the cell
elimination and an imposed efficiency of
disinfection of N/N
0
= 0.001. The experiment
duration was 30 min. Three distances between the
electrodes have been tested: 1, 2 and 4 cm. The
obtained results are presented in Table 2.
As it can be seen, the best results have been
obtained with the distance of 4 cm where a 3-log
reduction was always obtained so this distance was
used in the experiments with real effluents. These
results show that electrical phenomena occurring in
the solution, and particularly the ion
electromigration, are involved in the disinfection
process, may be thanks to an electroporation
mechanism.
4.3 Coagulation Rate in a Batch
Electrocoagulation
The electrocoagulation of E. coli cells in a batch
continuously flowing system consisting of two cells
in series has been studied using the pilot plant shown
in Figure 5. The results given in Figure 6 show the
reduction in cell concentration in time as well as the
coagulation rate. The coagulation rate can be
described by a first order rate equation (1):
dC/dt = k
1
e
-k2t
(1)
where C is the concentration at time t, k
2
=
4kT/3µ (k is the Boltzman’s constant and µ the
viscosity of the aqueous medium), k
1
depending on
the electrophoretic velocity of the cells and the
geometric parameter of the electrode size. This
equation takes into account the electrophoretic
migration of the cells and the subsequent release of
discharged cells into the bulk. According to
Matteson et al. (Matteson 1995), the constant terms
in this equation derive from the basic electrophoretic
and coagulation phenomena, which are depending
on the zeta potential of the cells, the applied voltage,
the initial concentration and the electrode geometry.
Figure 6: Cell reduction and coagulation rate for E. coli
cells.
PERSPECTIVES OF ELECTROCOAGULATION IN WATER DISINFECTION
257
4.4 Electrocoagulation of Surface
Waters
In order to confirm the electrocoagulation
performances, two surface waters were studied. A
river water (conductivity 0.4 mS/cm, pH = 7.2)
containing indigenous coliforms and a pool water
(conductivity 0.55 mS/cm, pH = 6.8) containing
algae were used. The former was disinfected at I =
0.35 A, the latter at I = 0.22 A. In both cases the
applied tension was 30 V. In both cases after 10 min
a froth layer appears at the surface increasing in time
with flocs floating on the surface. The obtained
results after 30 min of electrocoagulation are given
in Table 3. They show a total coliform removal and
a good disinfection efficiency for the algae
population.
Table 3: Coliform and algae reduction in surface waters.
River water Pool water
T
0
(CFU/mL)
1.12
E+02
1.12
E+02
1.12
E+02
4.15
E+04
6.05
E+05
T
f
(CFU/mL)
0 0 0
2.50
E+02
2.20
E+02
E (%)
99.99 99.99 99.99
99.4 99.6
It can be assumed that the applied current
originate potential difference from an extremity to
the other of the cellular membrane on account of its
electrical resistance. This potential difference
modifies consequently the trans-membrane potential
producing destruction of the cellular membrane (Li
2004). The cell membrane is constituted essentially
by layers of phospholipids and proteins can be
included allowing ionic change with the cell
environment. Proteins are easily destroyed by direct
effect of an electric field while the phospholipidic
membrane is not easily oxidable. So, some cells can
be reactivated in a favorable medium. This is
probably the case of the algae present in the
solution.
5 CONCLUSIONS
The effects of disinfection of E. coli cultures,
coliforms and algae using aluminum
electrocoagulation were studied. The process
significantly outperformed chemical coagulation
reducing the amount of aluminum used. In many
cases, E. coli cells in the treated model solutions
were effectively disinfected and almost total
removal of coliforms and algae were rapidly
reached. A relationship for the coagulation rate of E.
coli cells has been proposed. This equation takes
into account the electrophoretic migration of the
cells and the subsequent release of discharged cells
into the bulk.
REFERENCES
Abuzaid N.S., Bukhari A.A., Al-Hamouz Z.M., Adv.
Environ. Res., 6 (2002) 325.
Asselin M., Drogui P., Brar S.K., Benmoussa H., J.F.
Blais, J. Haz. Mat., 151 (2008) 446.
Canizares .P, Martinez F, Jimenez C., Saez C., Rodrigo
M., J. Haz. Mat., 151 (2008) 44.
Dao H.F., Li X.Y., Gu J.D., Shi H.C., Xie Z.M., Proc.
Bioc., 39 (2004) 1421.
Domini J.C, Kan J, Hassan T.A., Kar K.L, Can. J., Chem.
Eng., 72 (1994) 667.
Idermez S., Yildiz Y., Tasunoglu V. , Sep. Purif. Technol.,
52 (2006a) 394.
Idermez S., Demircioglu N., Yildiz Y., Bingül Z., Sep.
Purif. Technol., 52 (2006b) 218.
Li M., Qu J.H., Peng Y.Z., J. Environ. Sci, 16 (2004) 348.
Matteson M.J., Dobson R.L., Glenn Jr R.W., Kukunoor
N.S., Colloids Surf. A.: Physicochem. Eng. Aspects,
104 (1995) 101.
Oss C.J.V., Interfacial Forces in Aqueous Media, Marcel
Decker Inc, New York (1994) p 440.
Rajeshwar K., Ibanez J., Swain G.M., J. Appl.
Electrochem., 24 (1994) 1077.
Un U.T., Koparal A.S., Ogutveren U.B., J. Environ. Man.,
(2008) 1.
Zhu B., Clifford D.A., Chellam S., Wat. Res., 39 (2005)
3098.
BIOSIGNALS 2009 - International Conference on Bio-inspired Systems and Signal Processing
258
