
NOVEL COMBINED TEMPLATE FOR AMPEROMETRIC
BIOSENSORS WITH CHANGEABLE SELECTIVITY
Julija Razumiene
1
, Vidute Gureviciene
1
, Jurgis Barkauskas
2
Virginijus Bukauskas
3
and Arunas Setkus
3
1
Institute of Biochemistry, Department of Bioanalysis, Mokslininku 12, 08662 Vilnius, Lithuania
2
Vilnius University, Department of General and Inorganic Chemistry, Naugarduko 24, 03225 Vilnius, Lithuania
3
Semiconductor Physics Institute, Sensors Laboratory, A. Gostauto 11, LT01108 Vilnius, Lithuania
Keywords: Biosensors, Enzymes, Carbon Nnotubes, Medical Application.
Abstract: Present paper describes innovative approach in design of amperometric biosensors useful in various appli-
cations. Original template of the electrodes has been prepared on a base of carbon nanotube support layer
deposited on the polycarbonate membrane. Novel template and changeable enzyme layer give rise to crea-
tion of new family of biosensors acceptable for detection of wide range of carbohydrates. The morphology
and electric properties of the constituent parts of the template electrode are characterized by scanning probe
microscopy. The sensitivity, selectivity and stability are described for typical types of the biosensors.
1 INTRODUCTION
Electrochemical biosensors are the most common
class of biosensors in various practical applications
(A. Chaubey and B. D. Malhotra, 2002). In these
sensors, bio-interaction at specific sites of enzymes
results in extra electric charge. The extra charge can
be transferred from enzyme to electrode and de-
tected by an external electric circuit. Technology of
the electrodes and enzyme immobilization is crucial
for conversion of biochemical interaction into the
response signal and, still, is under intensive studies.
This type of sensors has well known advantages
such as acceptability for functioning in turbid media,
comparable instrumental sensitivity and amenability
to miniaturization, and etc. In this report, we present
an original approach in biosensor technology based
on immobilization of enzymes within special matrix
attachable to carbon nanotube electrode.
2 METHODS
AND EXPERIMENTS
We developed and tested an original technology
acceptable for production of a family of biosensors.
Selectivity of two component biosensors can be
changed simply by replacing special matrix contain-
ing enzymes attached to the single wall carbon na-
notube (SWCNT) based electrode. Therefore the
biosensors can be adjusted for detection of monosa-
charydes and disacharydes such as glucose, lactose,
galactose, maltose and et cetera.
Nanotube support layers on the polycarbonate
membranes were prepared from the industrial
SWCNT (Cheap Tubes Inc., USA). The main para-
meters of these nanotubes ware as follows: diameter
is 2 nm, 5.0 – 30.0 μm length, specific surface area
400 m2/g, electrical conductivity 10-2 S/cm. The
SWCNT were functionalized with carboxyl groups
(2.73 wt%).
In this study we introduced an original protocol
for coating of flexible support by SWCNT layer
acceptable for biosensor electrode. The protocol
includes special filtration of aqueous suspension
448
Razumiene J., Gureviciene V., Barkauskas J., Bukauskas V. and Setkus A. (2009).
NOVEL COMBINED TEMPLATE FOR AMPEROMETRIC BIOSENSORS WITH CHANGEABLE SELECTIVITY.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 448-452
DOI: 10.5220/0001827604480452
Copyright
c
SciTePress

through the isopore polycarbonate membrane and is
crucial for electrochemical properties of biosensors.
The prototype biosensors were based on three
types of enzymes, namely glucose oxidase from
Aspergillus niger and pyrroloquinoline quinone
(PQQ) dependent glucose dehydrogenases and
aldose sugar dehydrogenase. The soluble glucose
dehydrogenase (s-PQQ-GDH) from Acinetobacter
calcoacetics L.M.D. 79.41 was purified by the me-
thod reported in (A. J. A. Olsthoorn and J. J. Duine,
1996). The membrane-bound enzyme (m-PQQ-
GDH) was purified from Erwinia sp. 34-1
(Marcinkevičienė et al., 1999). The water-soluble
aldose sugar dehydrogenase (s-PQQ-ADH) was
purified from Escherichia coli (Southall et al.,
2006). Each of the enzyme types was immobilized
on individual flexible support of polivinylalcohol
coated terylene. Adsorption and cross linking to the
support were the methods for immobilisation of
enzymes.
Our prototype amperiometric biosensors con-
sisted of SWCNT based electric charge drain and
changeable biosensitive detector. The sensor con-
struction is illustrated by a sketch in Fig. 1.
Surfaces of the sensor components, namely, elec-
trode support, SWNT coatings and matrix without
and with immobilized enzymes, were analyzed by
scanning probe microscope (SPM) D3100 / Nanos-
cope IVa (Veeco Instruments Inc.). Standard AFM
methods such as contact and tapping mode surface
scanning were used for visualization of the surface
morphology. The surface electrical characteristics
were evaluated from measurements of tunneling
current obtained in contact mode. Conductive probe
of the SPM was firmly pressed to the surface so that
it was not damaged. Special module SPM D3100
TUNA (Veeco Instruments Inc.) was used for these
experiments. The maps of the current and local point
volt-amperic characteristics (VACh) were obtained
for the components of the biosensor electrodes in
these experiments. The data and SPM mages were
processed by the NanoScope Software 6.14 (Veeco
Instruments Inc.).
Electrochemical experiments were performed us-
ing a conventional three-electrode system containing
a screen-printed carbon electrode as a working elec-
trode, a platinum wire as a counter electrode and an
Ag/AgCl in saturated KCl as a reference electrode
(all potential values presented in the text are vs. this
reference electrode). 0.05 M acetate buffer (pH 6.0)
containing 1 mM of Ca
2+
and 0.2 mM N-
methylphenazonium methyl sulphate was used as a
default buffer. Steady state currents of the biosen-
sors were recorded at 0.4 V using a polarographic
analyzer “PARSTAT 2273” (Princeton Applied
Research, USA).
Figure 1: General side and top views (A) and the compo-
nents (B) of the biosensor: 1 – insulating film, 2 – enzyme
immobilized on terylene film, 3 – contact zone, 4 –
SWCNT-polycarbonate membrane, 5 – insulating film.
3 RESULTS AND DISCUSSIONS
Characteristics of the biosensor family were ob-
tained only for four types of the biosensors based on
the original prototype structure in present study. The
SWCNT layer on polycarbonate membrane and
changeable enzyme based detector are the most
important results of the sensor technology in present
study. It was proved by experiments with the proto-
type biosensors that SWCNT based structure is
acceptable for the sensor electrode and immobiliza-
tion of enzymes. The attachable enzyme detectors
were reproducible and stable for comparatively long
time.
3.1 Surface Properties of the
Electrodes
The morphology and electric properties were de-
scribed for separate components of the template
electrodes by the SPM experiments. The results
were obtained for the components at intermediate
stages of the technology.
Typical structure of the SWCNT coating is illu-
strated by a SPM image in Fig. 2. It is seen in Fig. 2
the SWCNT were found in vertical and horizontal
positions on the membrane. Since the membrane
contained the pores deep valleys were found in the
nanotube layer. It was revealed by high aspect ratio
SPM tests that SWCNT are in vertical position in
the areas corresponding to the pores in the mem-
brane. On the flat surfaces of the membrane there
were no preferable orientations of the SWCNT with
respect to the membrane surface. The SWCNT layer
NOVEL COMBINED TEMPLATE FOR AMPEROMETRIC BIOSENSORS WITH CHANGEABLE SELECTIVITY
449
was comparatively thick and at least several layers
of horizontal nanotubes were detectable.
It was proved by measurements of tunneling cur-
rent that electric conductivity highly depends on the
structure of the SWNT layer. The areas with vertical
nanotubes were more conductive that that with hori-
zontal SWCNT. Electrical properties of individual
areas of the SWCNT layer are compared in Fig. 3 by
typical voltamperic characteristics (VACh) that were
measured by special SPM module TUNA in contact
mode. The tunneling current was measured by the
SPM conductive cantilever tip diameter of which is
about 20 nm.
Figure 2: The SPM image of the surface of the SWCNT
coating on polycarbonate membrane. The surface area of
the image is 5x5 μm
2
and the maximum height is 400 nm.
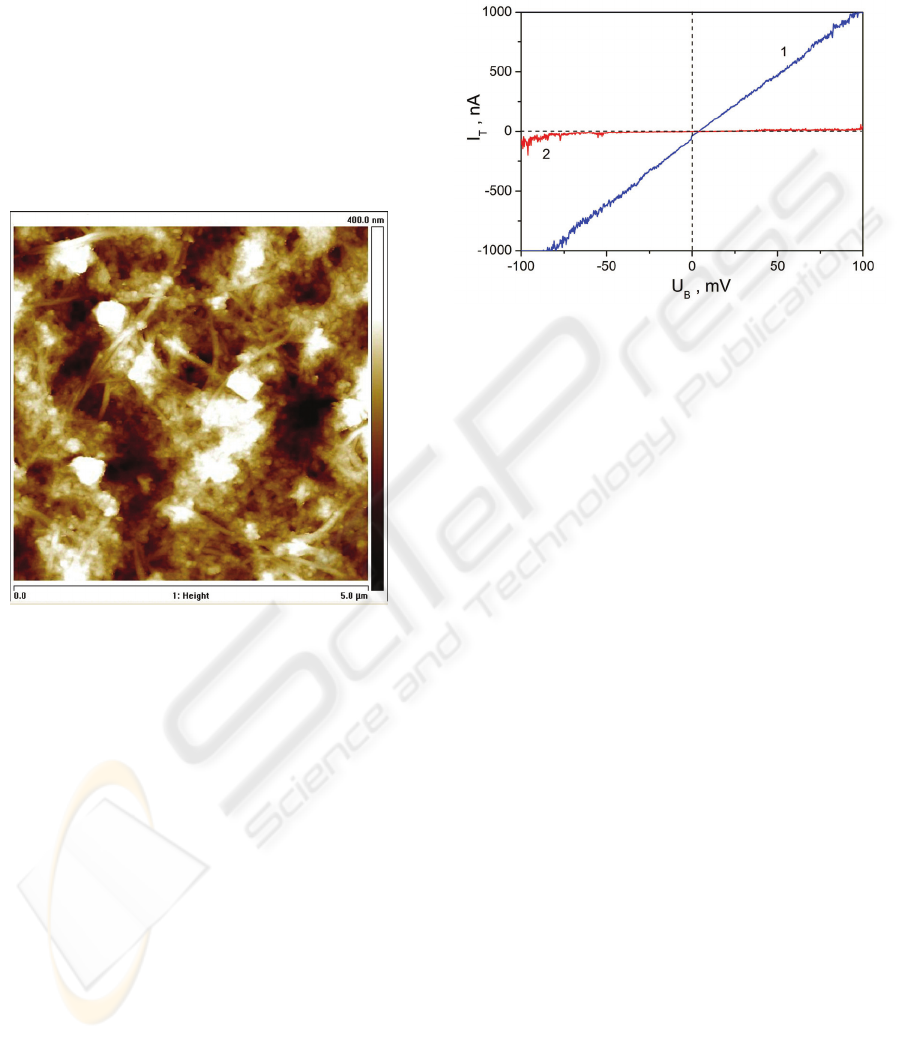
In Fig. 3, the surface areas with the lowest and
the highest conductivity are represented by the
VACh measured at the tip-points of individual sur-
face area. The lowest conductivity was obtained for
the tip attached to the horizontal SWCNT (2 in
Fig. 3). The highest conductivity of the SWCNT
layer was found in the areas corresponding to the
pores in the membrane (1 in Fig. 3).
Detailed distribution of the electrical conductivi-
ty over the surface of the SWCNT layer was visua-
lized by scanning of the surfaces with the SPM
TUNA. It was found that the spots of high conduc-
tivity are measured over the flat surface of the mem-
brane if vertical SWCNT are detected in this area.
Comparatively large areas were characterized by
intermediate electric conductivity. It was supposed
that only part of the SWCNT are connected so that
produce conductive mesh of the electrode. The ma-
jor part of the vertical SWCNT is only partly con-
nected to this mesh and, therefore, limits electric
charge transfer from the enzymes to the measure-
ment circuit. This electric limitation reduces the
effectiveness of the biosensor electrode.
Figure 3: Tunneling current versus dc-potential between
the SPM cantilever and the sample.
3.2 Biosensor Characteristics
Since the main advantage of PQQ-dependent dehy-
drogenases is functional independence of oxygen
these enzymes are highly attractive for development
of biosensors (Razumiene et al., 2005; Razumiene et
al., 2006). All these enzymes were chosen also due
to different ability to oxidise a number of carbohy-
drates. Thus, integration of these biosensors in
whole sensing system allows detecting broad range
of sugars, encompassing clinically important such as
lactose, galactose, maltose and et cetera that is
usually not detectable in body fluids although are
associated with several diseases. In spite of numer-
ous modifications of these enzymes that can be
acceptable for detection of various important com-
pounds we probed only a few types in this study.
Typical calibration curve for glucose obtained
using s-PQQ-GDH based biosensor is shown in
Fig. 4.
In Fig. 4, the current generated at the electrode
during electrocatalytic oxidation of glucose by the
enzymes was measured as a function of glucose
concentration in the solution. Similar dependences
were measured for all types of biosensors manufac-
tures and probed in this work.
Kinetic characteristics, namely the apparent Mi-
chaelis constant (K
M
app
) and maximum current (I
m-
ax
app
), calculated for each type of the biosensors are
summarized in Table 1.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
450

Table 1: Kinetic characteristics of SWCNT-based biosen-
sors with different enzymes.
Biosen-
sor type
K
M
app
,
mM
I
max
app
,
μA
n r
2
s-ADH 305.4 42 7 0.9913
s-GDH 5 27 12 0.9945
m-GDH 0.11 10 8 0.9986
GOx 5.8 260 7 0.9870
Results in Table 1 shows that enzymes possess
different kinetics of action. From the functional
standpoint, there are also different, i.e. (s-) soluble
types operate in cytoplasma and (m-) membrane-
bound is tightly bound to the outer surface of the
cytoplasmic membrane (Matsushita et al., 2003). It
has been shown that they are different enzymes with
different pH-optima, molecular weights and sub-
strate specificity.
Figure 4: The calibration curves of the biosensors based
on direct immobilization of s-PQQ-GDH on SWCNT (1)
and with changeable s-PQQ-GDH film attached to
SWCNT electrode (2). C
g
is glucose concentration in the
solution.
In our previous paper (Laurinavicius et al., 2004)
has been demonstrated that due to the immobiliza-
tion the active center of enzyme can be distorted that
leads to different affinity to substrates as in the case
of the native enzyme. Aiming to evaluate the affinity
of enzymes operating in heterogeneous biosensing
systems, the selectivity to clinically important meta-
bolites such as glucose, lactose, galactose and mal-
tose were investigated for all types of the proposed
biosensors. The responses to individual metabolites
were represented by the signal ratio with respect to
the detection of 100 % glucose. The results are
summarized in Table 2.
In order to understand an influence of the en-
zyme immobilization method of the of s-PQQ-GDH
on the the selectivity and main kinetic parameters of
the biosensor we investigated the electrodes based
on SWCNT and carbon paste electrodes (CE) and
manufactured by the method previously described in
(Razumiene et al., 2006). The K
M
app
and I
max
app
pa-
rameters for three types of the electrodes are sum-
marized in Table 3.
The I
max
app
for all substrates can be explained by
the s-PQQ-GDH catalyzed oxidation of main sub-
strates such as glucose, lactose, and galactose with
almost the same rate ratio for all probed types of
biosensors (Table 3). However, the kinetic parame-
ters are individual for the biosensors with differently
immobilized enzymes (Table 3). The increase in
K
M
app
results in extension of the interval of the linear
calibration curve. We associate it with diffusion
limited access of the substrate.
The stability of the s-PQQ-GDH and GOx based
biosensors was investigated during couple of weeks.
The responses to the standard glucose solution (5
mM) were periodically recorded at room tempera-
ture equal to about 25 °C during these experiments.
The residual response of the probed biosensors was
not less than about 80 % of initial magnitude over
the period of the tests.
4 CONCLUSIONS
Original technology was developed and probed for
manufacturing of prototype biosensors with change-
able selectivity. The template of the electrodes has
been prepared on the basis of SWCNT conductive
layer deposited on the polycarbonate membrane.
The attachable flexible matrix with immobilized
enzymes was proved functionally acceptable for
catalysis of biochemical reactions and detection of
these reactions. Vertical arrangement of the SWCNT
in the electrodes was related to the areas of high
electric conductivity of the electrodes that was as-
sumed essential for functioning of the biosensors.
Using glucose oxidase, two types of pyrroloqui-
noline quinone dependent glucose dehydrogenases
(namely s-PQQ-GDH, m-PQQ-GDH) and water-
soluble aldose sugar dehydrogenase s-PQQ-ADH
four versions of the prototype biosensors were
manufactured and investigated in this work. In the
tests, the responses to clinically important metabo-
lites such as glucose, lactose, galactose, arabinose,
manose and glucose-6-phosphate were measured. It
was
NOVEL COMBINED TEMPLATE FOR AMPEROMETRIC BIOSENSORS WITH CHANGEABLE SELECTIVITY
451

Table 2: Responses to different substrates of SWCNT-based biosensors.
Biosensor type Glucose Lactose Galactose Manose Arabinose Glucose-6-
phosphate
s-ADH 100 61 120 7 102 60
s-GDH 100 95 99 87 68 15
m-GDH 100 0 76 57 72 91
GOx 100 0 3 1 0 0
Table 3: Kinetic parameters of SWCNT-based and CE-based biosensors with differently immobilised s-PQQ-GDH.
Substrate
CE, enzymes on tery-
lene
SWNT, enzymes on
terylene
enzymes adsorbed on
CE
I
max
app
,
μA
K
M
app
,
mM
I
max
app
,
μA
K
M
app
,
mM
I
max
app
,
μA
K
M
app
,
mM
Glucose 5.2 4.8 23.4 9 0.37 5.7
Lactose 4.7 8.1 21.7 7.2 0.3 8
Galactose 3.6 10.1 10.4 4.2 0.24 4
proved that the prototype biosensors are sufficiently
stable so that can be acceptable for practical use.
ACKNOWLEDGEMENTS
The study was partly supported by the Lithuanian
State Science and Studies Foundation contracts no.
N-08007 and N-09/2008. It was also partly sup-
ported by COST programme contract no. 31V-119.
REFERENCES
Chaubey, A., Malhotra, B.D.: Mediated biosensors. Bio-
sensors & Bioelectronics Vol. 17 (2002) 441–456
Olsthoorn, A. J. A., Duine, J. J.: Production, characteriza-
tion and reconstitution of recombinant quinoprotein
glucose dehydrogenase (soluble type; EC 1.1.99.17)
apoenzyme of Acinetobacter calcoaceticus. Archives
of biochemistry and biophysics Vol. 336 (1996) 42–48
Marcinkevičienė, L., Bachmatova, I., Semėnaitė, R.,
Rudomanskis, R., Bražėnas, G., Meškienė, R.,
Meškys, R.: Purification and characterization of PQQ-
dependent glucose dehydrogenase from Erwinia sp.
34-1. Biotechnol. Lett. Vol. 21 (1999) 187–192
Southall, S.M., Doel, J.J., Richardson, D.J., Oubrie, A.:
Soluble Aldose Sugar Dehydrogenase from Escheri-
chia coli. A high exposed active site conferring broad
substrate specificity. Journal of Biological Chemistry.
Vol. 281, 41 (2006) 30650–30659
Razumiene J., Barkauskas J., Kubilius V., Meskys R.,
Laurinavicius V.: Modified graphitized carbon black
as transducing material for reagentless H2O2 and en-
zyme sensors. Talanta Vol. 67(2005) 783–790
Razumiene J., Vilkanauskyte A., Gureviciene V., Bar-
kauskas J., Meskys R., Laurinavicius V.: Direct elec-
tron transfer between PQQ dependent glucose dehy-
drogenases and carbon electrodes: An approach for
electrochemical biosensors. Electrochimica Acta Vol.
51 (2006) 5150–5156
Matsushita, K., Toyama, H., Yamada, M., Adachi, O.:
Quinoproteins: structure, function, and biotechnologi-
cal applications. Appl. Microbiol. Biotechnol. 58, 13–
22 (2002) 8. (3) Davis, J.J., Coleman, K.S., Azamian,
B.R., Bagshaw, C.B., Green, M.L.H. Chemical and
Biochemical Sensing with Modified Single Walled
Carbon Nanotubes. Chem. Eur. J. Vol. 9 (2003) 3732–
3739
Laurinavicius, V., Razumiene, J., Ramanavicius, A.,
Ryabov, A.D.: Wiring of PQQ-dehydrogenases. Bio-
sensors and Bioelectronics Vol. 20 (2004)1217–1222
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
452
