
POLYMERIC FILM SENSORS BASED ON PAH-PAZO IONIC
SELF-ASSEMBLED MULTI-NANOLAYERS
Celso Ribeiro, Paulo J. Gomes, Paulo A. Ribeiro, Maria Raposo
Centro de Investigação em Física Tecnológica (CEFITEC), Physics Department, Faculdade de Ciências e Tecnologia
(FCT), New University of Lisbon (UNL), 2829-516, Caparica, Portugal
Hugo Águas
Department of Material Science and CINEMAT, Faculdade de Ciências e Tecnologia (FCT), New University of Lisbon
(UNL), 2829-516, Caparica, Portugal
Pedro Santos, Beatriz Borges
Department of Electrical and Computer Engineering, Instituto Superior Técnico (IST), 1049-001, Lisbon, Portugal
Pedro Brogueira
Department of Physics and ICEMS, Instituto Superior Técnico (IST), 1049-001, Lisbon, Portugal
Keywords: Sensors, Biosensors, Electronic Tongue, Lab-on-chip, Polymers, polyelectrolytes, PAH, PAZO, Layer-by-
Layer, Nanotechnology, Nanofilms, Thin films, Impedance Spectroscopy, AFM, Roughness, Ellipsometry.
Abstract: A sensor platform composed by interdigitated electrodes covered by a tailored nanofilm of 5 bilayers of
PAH and PAZO polyelectrolytes was successfully built and tested in NaCl solution at relative low
concentrations (10
-5
-10
-6
M). The effect of temperature on the electrical measurements was addressed and
indicated its importance for to sake accuracy reduction in about one order of magnitude. The film
morphology was studied by using ellipsometry and atomic force microscopy techniques. The first inferred
precise and consistent values for the film thickness about 50 nm. With respect to the second, relative low
values of the surface roughness of about few nanometers were measured and also micrometer diameter
agglomerates and channels with a bilayer size depth were identified.
1 INTRODUCTION
Sensing, monitoring, and control are natural tasks
performed by any living organism and also an
increasing part of the humankind activity in which
technology is directly or indirectly involved in day-
to-day life. Numerous sensors are constantly being
developed and the advent of the nanomaterials
increases even further their variety and applicability.
A new branch of potential platform for sensors is
being widely researched by using polyectrolytes
nano-thin films ionically self-assembled in bilayers
via what is so-called layer-by-layer (LbL) technique
(Decher and Hong, 1991, Oliveira Jr., 2001). The
production of a bilayer involves sequential
adsorption of two opposite charged polyelectrolytes
from an aqueous solution, one by one, onto an inert
substrate such as glass or metal. The process is
repeated for attaining multi-layers. This simple and
inexpensive technique is also expected to deliver
films with high thermal stability and robust to
critical solvent and alkaline environments which are
the ideal platform for sensors.
Among several polyeletrolytes bilayer pairs for
building this platform, the ones made by the
polyanic poly[1-[4-(3-carboxy-4-hydroxyphenylazo)
benzene sulfonamide]-1,2-ethanediyl, sodium salt]
(PAZO) and the polycationic poly (allylamine
hydrochloride) (PAH) have a great potential and
results have already been reported (Mermut and
Barrett, 2001 and Ferreira, 2007).
In addition, selective molecules (organic,
inorganic, enzymes, DNA, etc) can be equally
mobilized onto the last layers in order to react
chemically to a particular analyte of interest.
458
Ribeiro C., Gomes P., Ribeiro P., Raposo M., Águas H., Santos P., Borges B. and Brogueira P. (2009).
POLYMERIC FILM SENSORS BASED ON PAH-PAZO IONIC SELF-ASSEMBLED MULTI-NANOLAYERS.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 458-461
DOI: 10.5220/0001830904580461
Copyright
c
SciTePress

The interplay of a broad set of parameters related
to the measurement such as the temperature, pH,
hysteresis, and their time-domain dynamic (drift)
should be carefully quantified aiming to control or
more likely to correct theirs effects via a posteriori
software analysis. This optimization towards
reliability can enhance the enormous commercial
potential of the LbL sensors mainly as a monitor in
an environment with no lab-control.
This work reports preliminary results on the
temperature effects on a LbL film sensor plataform
built with PAH/PAZO bilayers and tested in rather
simple aqueous solutions containing sodium choride
(NaCl) via impedance spectroscopy technique. The
polyelectrolytye PAZO which is at the last surface
of the multilayers should attract electrostatically the
cation Na
+
, so the global electrically properties of
the multilayer are expected to change according to
the concentration of this cation and therefore NaCl
concentration is sensed. In addition to this,
prelimilary analysis on the sensor film thickness and
morphology are presented by using ellipsometry and
atomic force microscopy (AFM) techniques.
2 EXPERIMENTAL
The sensor was produced from polyelectrolytes LbL
films deposited onto substrates of BK7 optical glass
where gold interdigitated electrodes were deposited
by vacuum evaporation. The sensor effective area
was about 2x5mm
2
, the interspace between the lines
was 20μm and their width and thickness were 2μm
and 0.2μm, respectively. These dimensions were
measured by a Dektak perfilomoter and an optical
microscopy Olympicus SZ-PT.
The polyelectrolytes poly [ 1-[4-(3-carboxy-4-
hydroxyphenylazo) benzenesulfonamido]-1,2-
ethanediyl, sodium salt] (PAZO) and the
poly(allylamine hydrochloride)(PAH) (average M
w
=
50,000-60,000g/mol) were acquired from Sigma-
Aldrich. The PAH polyelectrolyte aqueous solution
with concentration of 10
-2
M was prepared by
dissolving this polyectrolyte in deionised water with
a resistivity of 18.2MΩcm supplied by a Millipore
system (Milli-Q, Millipore GmbH). The PAZO
aqueous solution also with a concentration of 10
-2
M
was obtained by dissolving this polyelectrolyte on an
aqueous buffer solution of pH=10. The
polyelectrolyte concentrations were based on the
molecular weight repeat unit and the buffer solution
was prepared mixing a 0.05M sodium hydrogen
carbonate (NaHCO
3
) aqueous solution with a 0.1M
sodium hydroxide (NaOH) solution in a proportion
of 500:107(v/v) (Ferreira (a), 2007). The PAZO
solution was also filtered with a 5 mm thick and 50
μm porous diameter ceramic filter.
The LbL films were prepared by immersing the
substrate with the interdigitated electrodes into the
PAH solution for 5 minutes, washed 3 times into
water for a total of 10s, and then immersed into the
PAZO solution for the same 5 minutes and equally
washed but into the buffer solution instead of water.
This procedure leads to a production of a bilayer and
repeated until the 5 bilayers were obtained. Finally,
the thin film was dried with a nitrogen flux.
The sensor impedance measurements were
carried out by a Precision Impedance Analyser
Agilent 4294A (40Hz-110MHz, 1mHz resolution,
GPIB connection). The root mean square oscillator
voltage signal level was 50 mV.
The film thickness was measured using a
spectroscopic ellipsometer model HORIBA Jobin
Yvon UVISEL. A three layer model was used
assuming the sensor is composed by a film layer on
the top of a 1 mm thick BK7 glass substrate and
another on its back. The spectral range used was 1.5-
6.5 eV (531-2302 nm) with a 0.025 eV increment.
The AFM measurements were performed by a
Dimension 3100 SPM with a Nanoscope IIIa
controller from Digital Instruments (DI) under
ambient conditions in tapping mode
TM
. A
commercial tapping mode etched silicon cantilever
probe from DI (constant force of 42N/m, resonance
frequency of 320kHz) and a 90x90 μm
2
scanner
were used. The scan rate was 1.51 Hz. The image
resolution was fixed to 256×256 pixels.
3 RESULTS AND DISCUSSION
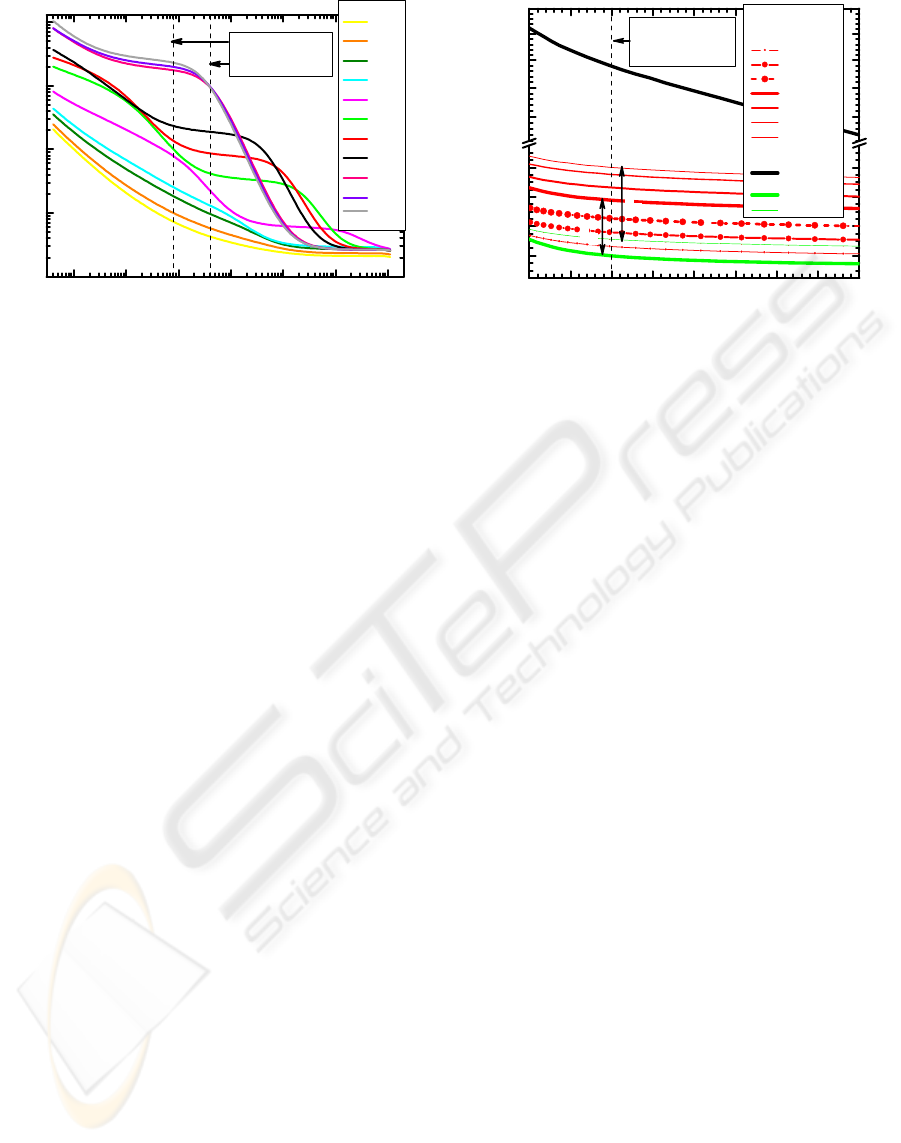
The influence of the NaCl concentration on the
sensor impedance (Z=Z′+iZ″) were analysed. Real
(Z′) and imaginary (Z″) parts, module and phase of
Z, and Nyquist representation were all studied. The
clearest way for visualise that influence was by
using the Z′ part against the impedance analyser
frequencies which is presented in the graph of fig.1.
The sensor was immersed into solutions of different
NaCl concentrations from the lowest concentration,
virtually null corresponding to Milli-Q pure water,
to the highest of 1M. Impedance spectra
measurements within several weeks show systematic
drifts for all concentrations but not to a point to
overlap any two consecutive values, excepted at low
frequencies below 5 kHz.
POLYMERIC FILM SENSORS BASED ON PAH-PAZO IONIC SELF-ASSEMBLED MULTI-NANOLAYERS
459
10
2
10
3
10
4
10
5
10
6
10
7
10
8
10
100
1k
10k
100k
possible values to
discriminate the
concentrations
Real component of the Impedance Z' (Ω )
frequency (Hz)
NaCl in M
1
5x10
-1
2x10
-1
10
-1
10
-2
10
-3
10
-4
10
-5
10
-6
10
-7
milli-Q
water
Figure 1: Real part, Z′, of the sensor impedance versus the
impedance analyser frequencies for the sensor immersed
in NaCl aqueous solutions with different concentrations.
The overall Z′ curves show a complex behaviour
with the frequency and concentration leading to a
short window for an effective analysis which is
empirically delimited (e.g. by the two lines drawn in
this figure). In the lower limit at 8kHz, a
concentration up to 10
-5
M can be easily measured
and possibly 10
-6
M too if sufficient statistics and at
least temperature measurements of the aqueous
medium are available as will be seen further on.
At the upper frequency of 40 kHz the sensor is
robust since the Z′ values are well distinguished for
each concentration, in addition to be less sensitivity
to the frequency. Values of 10
-5
M can not be really
exceeded. However, in this region the sensor thermal
stability is very high as it is shown in the plot of
fig.2, where the impedance spectra are shown for
NaCl solutions of 10
-4
M and 10
-3
M concentrations
over a temperature range of 9 to 62°C and 9.0 to
26°C, respectively. Around 40kHz, Z′ variation is
almost the same for temperatures close to 9°C (Δ
2
)
or to 26°C (Δ
1
) for these concentrations. However, it
is clear that the 10
-4
M concentration can be
mistaken for the 10
-3
M if the right temperature
curve is not used. An overlap of these curves occurs
below 20kHz, even for small temperature variation.
Therefore, the possibility to measure lower
concentrations (e.g. down to 10
-6
M) really demands
the precise knowledge of the temperature and the
statistical values of the concentration in a laboratory
environment. A more feasible alternative to this
might be to increase the sensor sensitivity by
increasing the number of bilayers, for example.
Ellipsometry measurements inferred a precise
and consistent sensor film average thickness of
49±4nm. The ellipsometric measurements were
20k 30k 40k 50k 60k 70k 80k 90k 100k
400.0
600.0
800.0
1.0k
2.1k
2.2k
2.3k
2.4k
2.5
k
Δ
2
possible value
to discriminate
the concentrations
Real component of the Impedance Z' (Ω )
frequency (Hz)
Temperature (
o
C)
at 10
- 4
M
62
44
33
26
15
11
8.8
------------------------
26 (10
- 5
M)
26 (10
- 3
M)
9.0 (ibidem)
Δ
1
Figure 2: The real part, Z′, of the sensor impedance versus
the impedance analyser frequencies for the sensor
immersed in NaCl aqueous solutions with few different
concentrations and temperatures.
carried out about 8 mm away form the interdigitated
electrodes where only the PAH/PAZO film
deposited onto the glass substrate was presented.
Topography measurements of the PAH/PAZO
films were obtained by AFM scanning over the film
sensor at 2 mm and 8 mm away from the
interdigitated electrodes, again in regions where only
the PAH/PAZO film deposited onto the glass
substrate was presented. In the fig.3 two typical 3-D
reconstructions of the topography, both with a scan
window of 5x5μm
2
, are presented in a sensor used in
a NaCl aqueous solution. In the fig.3a, a relative
smooth surface is observed except for the presence
of agglomerates with a micrometer size diameter and
at least 60 nm height. These structures are possibly
made or a result of NaCl attachment to the surface.
The root mean square roughness (Rrms) varied over
7-13nm for this type of scan window. These values
further reduce to 3-4nm for scan windows of
1×1μm
2
. In the fig3b, clear channels were shown at
the surface possibly due to the effect of the electrical
currents, since the electrical field created was
relatively high, i.e. of the order of 2.5kV/m. The
creation of such structure should be avoided in order
to keep the multilayer integrity and thus the sensor
reliability. A worthwhile attempt to minimise this
might be the reduction of oscillator voltage applied
by the impedance analyser. In addition, the reduction
of the upper frequency could also help since no
useful information can be extracted for values much
beyond the optimum frequency (by 40kHz in this
case) where Z′ substantially reduces and the current
flow through the sensor increases accordingly.
The channels depth can be roughly estimated as
10nm from the topographic AFM measurements
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
460

shown in fig.3b. This corresponds to the size of one
bilayer assuming a linear scaling between the
number of bilayers with the film thickness.
Figure 3: AFM 3-D topography of the PAH/PAZO film:
(a) in a typical region; (b) where channels were formed.
As a remark, the AFM morphology of samples
that have not been immersed into a NaCl aqueous
solution showed no agglomerates such as the ones
discussed earlier and their Rrms values are lower,
regardless the scan window values used, i.e. they are
3-5nm, 1.5nm, and 1.6nm for 5×5μm
2
, 2×2μm
2
, and
1×1μm
2
, respectively. The particular value of 1.6nm
here is about one magnitude smaller from that of
~13nm, obtained from previous studies (Ferreira,
2008). This indicates that filtering the polyetrolytes
solution such as for the PAZO here may play an
important role for reducing the roughness.
4 CONCLUSIONS
A sensor platform composed by an interdigitated
electrode covered by a tailored film of 5 bilayers of
polyelectrolytes PAH-PAZO was successful built
and tested in NaCl aqueous solutions at relative low
concentrations around few micro moles per litre.
The effect of temperature on the impedance
measurements was addressed in a limited range of
this variable. Nevertheless, the preliminary results
indicate that the knowledge of this parameter is vital
for attaining accuracy close to one order of
magnitude in useful range of concentrations.
The film thickness and morphology were
characterized by using ellipsometry and atomic force
microscopy techniques. The first allowed to obtain
precise and consistent values for the multilayer film
thickness of about few tens of nanometers. From the
second, relative low values of surface root mean
square roughness, about few nanometers, were
measured and channels with a bilayer size depth and
micrometer diameter agglomerates were also
identified.
ACKNOWLEDGEMENTS
The authors thanks the plurianual financial support
from "Fundação para a Ciência e Tecnologia-FCT",
Portugal. CR is supported by the programme
“Compromisso com a Ciência’’ also from FCT.
REFERENCES
Decher, G. and Hong, J.D., 1991. Ber. Bunsen-Ges. Phys.
Chem., 95, 1430.
Ferreira, Q., A., 2008, Estudo da Formação de Filmes
Nanoestruturados para Aplicação em Fotônica, PhD
thesis, FCT, New University of Lisbon, 97p.
Ferreira, Q., Gomes, P.J., Raposo, M., Giacometti, J. A.,
Oliveira Jr., O. N. and Ribeiro, P.A., 2007. J. Nanosci.
Nanotechnol., 7, 2659.
Ferreira, Q., Gomes, P.J., Maneira, M.J.P., Ribeiro, P.A.,
Raposo, M., 2007 Sensors and Actuators B: Chemical,
126, 311.
Ferreira, Q., Gomes, P.J., Nunes, Y., Maneira, M.J.P.,
Ribeiro, P.A., Raposo, M., 2007, Microelectronic
Engineering Journal, 84(3), 506.
Mermut, O. and Barrett C. J., 2001, Analyst, 126, 1861.
Oliveira Jr., O. N., Raposo, M. and Dhanabalan, A., in
Handbook of Surfaces and Interfaces of Materials, H.
S. Nalwa, Ed. Academic Press, New York (2001),
Vol. 4, Chapter 1. p.1-63.
(a)
(b)
POLYMERIC FILM SENSORS BASED ON PAH-PAZO IONIC SELF-ASSEMBLED MULTI-NANOLAYERS
461
