
SCENARIO-BASED REQUIREMENTS ELICITATION IN
A PAIN-TELETREATMENT APPLICATION
I. Widya, R. G. A. Bults
University of Twente, Centre for Telematics and Information Technology
Remote Monitoring & Treatment Group, The Netherlands
M. H. A. Huis in ’t Veld, M. M. R. Vollenbroek-Hutten
Roessingh Research and Development, The Netherlands
Keywords: Requirements engineering, Requirements elicitation, Telemedicine, Trial design, Goal-oriented, Task
analysis.
Abstract: This paper proposes a way to elicit requirements in the domain of eHealth, in particular telemedicine
treatment, that is in alignment with the evidence based working practice in medicine. In collaboration with
ICT developers, medical professionals co-shape the intended system, which has to support the telemedicine
application. These professionals develop a scenario and provide feedback to the subsequent requirements
elicitation process which is based on the developed scenario. We propose a mix of methods and techniques
to elicit requirements holistically to achieve the previously mentioned alignment. The requirements
elicitation applies basically a top-down scenario based approach, however complemented with additional
methods to overcome the inherently incompleteness of scenarios. In the proposed approach, we for example
analyze treatment tasks and their goals that are not only identified or inferred from the scenario but also
from the treatment protocol defined in an associated treatment trial design. This paper only addresses the
early phase of requirements engineering, later phases, which require refinements of associated use cases, are
beyond the scope of this paper.
1 INTRODUCTION
The early stages of the development of a software
based system are considered crucial to a successful
design (Yu, 1997). Requirements wished by users or
involved stakeholders of the system to be designed
have to be elicited, analyzed, negotiated, specified
and evaluated in these stages. The engineering
activities on requirements for e-Health application
supporting systems in general, and telemedicine
treatment supporting systems in particular, are
challenging research topics which deserve more
attention.
Design of a telemedicine application supporting
system is a complex multidisciplinary effort
involving medical professionals and Information,
Communication and Technology (ICT) developers.
Evidence based medicine (Sackett and Rosenberg,
1995) requires the collection of medical treatment
evidences, each one of which typically is acquired in
stages. One of these stages includes the design of
trials and the trialling of the proposed treatment
protocol on humans. Trial results will then become
evidence for practitioners to enable evidence based
treatment of their patients in the post trial phase.
Trial design (Meinert, 1986) defines the proposed
treatment protocol and the setting of the trial, for
example the trial inclusion and exclusion criteria,
which in turn define certain characteristics of
participating patients like age, gender and
disabilities. These trial design components are
therefore important for requirements elicitation.
Accordingly, ICT developers have to hook into the
trial design to improve requirements elicitation. On
the other hand, medical professionals who design
trials (i.e. trial designers) need ICT based systems to
enable teletreatment in accordance with the
proposed treatment protocol. This intertwinement
requires close multidisciplinary collaboration
between the experts of the two domains.
406
Widya I., M. R. Vollenbroek-Hutten M., H. A. Huis in ’t Veld M. and G. A. Bults R. (2009).
SCENARIO-BASED REQUIREMENTS ELICITATION IN A PAIN-TELETREATMENT APPLICATION.
In Proceedings of the 4th International Conference on Software and Data Technologies, pages 406-413
DOI: 10.5220/0002287404060413
Copyright
c
SciTePress

This paper discusses how to elicit requirements
for a telemedicine application supporting system in a
multidisciplinary collaboration setting, in which
medical professionals co-shape the system to be
designed. It only addresses the early phase of
requirements engineering, later phases which for
example address requirements on detailed look and
feel of screen windows human computer interfaces
are beyond the scope of this paper. In particular, this
paper describes the requirements elicitation in a
work-related neck-shoulder pain teletreatment trial.
The requirements elicitation basically applies a
top down development approach in which a scenario
plays an essential role as a common universe of
discourse to bridge the collaboration gap between
the trial designers (i.e. medical professionals) and
the ICT developers (see also Cysneiros (2002), Go
and Carroll (2004), and Van Helvert and Fowler
(2003)). Since scenarios are inherently incomplete
and a holistic solution is necessary in evidence based
medicine, other methods have been used to
complement our scenario based requirements
elicitation process. We investigate constraints
imposed by identified stakeholders of the trial, who
are not necessarily users of the intended system. ICT
developers study parts of the trial design and also
cross disciplinary literature on pain treatment.
Furthermore, we analyze the objectives of the
treatment tasks and activities, which are not only
identified or inferred from the scenario but also from
the trial design. This is in line with the way of
working in medicine and if viewed from the
mentioned analysis perspective we apply a goal
oriented requirements elicitation approach (cf. Letier
and Lamsweerde (2004), Mylopoulos et al. (1991)
and Yu (1997).
Moreover, our approach applies a mix of
methods and techniques known in the area of
requirements engineering or the medical domain.
The considered added value of this work is therefore
more on the application and the combination of the
methods and techniques in a way in accordance with
the working practices in medicine. Such alignment
with medical working practices is considered one of
the success factor conditions for usable telemedicine
systems (Wootton et al., 2006).
This paper is organized as follows. The next
section describes the teletreatment application.
Thereafter, we discuss the scenario based
requirements elicitation approach and the elicitation
results. Section 4 discusses some of the lessons
learned and in the last section we present the
conclusion of the work.
2 TREATMENT APPLICATION
This paper addresses a work-related neck-shoulder
pain teletreatment application of the European eTEN
project MyoTel (Myofeedback-based Teletreatment
service) (MyoTel, 2009). This project investigates
the feasibility of deployment of a myofeedback
based teletreatment service that enables patients with
neck-shoulder complaints to receive personalized
remotely supervised treatment during their daily
activities at their place of work. It validates the
teletreatment service in four European countries.
The MyoTel service uses clinical data, in
particular surface-ElectroMyoGraphy (s-EMG) that
represents the trapezius muscle activity. Measures of
muscle activation and relaxation patterns are fed
back to the patient and also presented to a remote
treating myofeedback therapist. This service
provides three feedback loops to enable control of
patient’s muscle activation and relaxation patterns
(Figure 1):
• a micro-loop provides local tactile feedback in
the form of vibration from a body-worn device
to indicate insufficient muscle relaxation results
averaged over a moving time window,
• a meso-loop provides visual feedback on a PDA
screen to indicate near real time muscle
activation performance of the left and the right
trapezius muscles, and
• a macro-loop provides supervised feedback
from a remote therapist during planned
consultation sessions.
The MyoTel telemedicine system consists of the
following: 1) a Body Area Network (BAN)
consisting of body-worn s-EMG sensors and
vibrating actuator and a gateway PDA with wireless
public network access, 2) a MyoTel m-Health portal,
3) a Terminal of a therapist, and 4) a hybrid data
Communication Infrastructure consisting of public
and private wireless networks, the internet and
optionally an intranet.
Figure 1: MyoTel neck-shoulder pain teletreatment system.
SCENARIO-BASED REQUIREMENTS ELICITATION IN A PAIN-TELETREATMENT APPLICATION
407

3 ELICITATION APPROACH
As mentioned earlier our elicitation approach uses a
mix of methods and techniques known in the
literature in the area of requirements engineering or
medical trial design. In this section, we describe and
justify how we combine them to elicit requirements
in the domain of telemedicine.
In a multidisciplinary collaboration, trial
designers and ICT developers firstly develop a
scenario which reflects the treatment protocol
proposed in the trial design. Based on this scenario,
the requirements for the intended system will be
elicited. As discussed earlier, this elicitation process
is complemented with a stakeholder analysis and
also a task and task objective analysis. To improve
the process, ICT developers study pain treatment
literature and parts of the trial design.
During the elicitation process, the ICT
developers conduct several e-mail exchanges and
(semi) structured interviews with the trial designers.
Group discussions in a participatory design setting,
for example conducted during plenary project
meetings, are in our case used for feedback on the
scenario in development and feedback on identified
(and prioritized) requirements. The elicitation, which
depends on feedback of the trial designers who are
myofeedback treatment professionals, uses tables,
textual descriptions, and window screen mock-ups to
specify the requirements. Analyzed and prioritized
requirements in each iteration cycle of the early
phase requirements elicitation are checked by the
trial designers to validate the derived telemedicine
system functionality with respect to their
expectation. During these interviews and group
discussions the trial designers, who are also treating
therapists, therefore validate the requirements.
3.1 Scenario
Different definitions, scopes and objectives of
scenarios are given in the literature. In this paper, we
adopt the definition of Van Helvert & Fowler
(2003): “a scenario is defined as a concrete
description of an activity that the users engage in
when performing specific tasks, a description that is
sufficiently detailed so that design implications can
be inferred or reasoned about”.
In accordance with Go & Carroll (2004) but
slightly adapted here, scenarios can be categorized
as follows:
• Year in the life scenario: typically used in the
area of strategic planning;
• Day in the life scenario: which illustrates user’s
daily activity, for example, in the setting of the
envisioned application of the system to be
designed;
• Activity in the life scenario: which describes the
use of the system to be designed. These
scenarios are also called use-cases, which
specify user’s interactions with the intended
system.
Benyon & Macaulay (2002) categorize scenarios
amongst others into user-centred perspective PACT
and designer-centred perspective FICS scenarios.
PACT stands for People, Activity, Context and
Technology, for example related to the users using a
technology in their daily activity within a certain
context. FICS stands for Function and events,
Interactions and usability issues, Content and
structure, Style and aesthetics, for example related to
system use.
In the MyoTel project, we develop a day in the
life scenario of a patient but combined with
corresponding activities of the treating therapist
(Figure 1). The purpose is to present the end-to-end
interrelations of the treatment activities of the two
actors that to some extent reflect the teletreatment
protocol.
The development of the scenario is moreover
iterative. First, the trial designers specify a day in the
life scenario in terms of PACT. In the setting of a
participatory design, the ICT developers provide
feedback by proposing FICS extensions to the
scenario. As owner of the scenario the trial designers
provide an updated scenario, eventually yielding a
mixed form day in the life scenario containing
PACT elements combined to some extent with FICS
elements. The benefits of this approach are that
• the scenario preserves its role as an embodiment
of (part of) the treatment protocol and it also
preserves the way of working in pain treatment
as defined by the trial designers;
• ICT developers understand the implication of
the scenario in respect of the required system
support functionality. This is also confirmed by
the trial designers who incorporate those FICS
feedback in the scenario and thereby implicitly
agreeing with the interpretation of the ICT
developers.
In this way, the scenario acts as a common universe
of discourse yielded by the discussed handshake
protocol. This scenario therefore bridges the
multidisciplinary collaboration gap between the trial
designers and the ICT developers.
The developed scenario is shown below.
ACT4SOC-EHST 2009 - 4th International Conference on Software and Data Technologies
408

MyoTel Scenario. Lisa is 35 years old. She is
working at a large administrative company and
predominantly performs computer work. She suffers
from neck-shoulder pain which is, to Lisa’s opinion
related to the computerwork she performs because
during holidays the complaints reduce. Because of
this, she was allowed to have a new treatment
approach; the MyoTel myofeedback treatment
service. By means of the MyoTel service subjects
are taught to relax their neck-shoulder muscles (so-
called trapezius muscle). During her daily work,
Lisa carries her Myotel BAN with her. The BAN
consists of a garment, in which dry surface
electrodes are incorporated, which continuously
measure the muscle tension of her trapezius muscle.
The garment can be worn under the clothes. The
garment is connected to a processing and feedback
unit which vibrates when an insufficient amount of
muscle relaxation is measured. The vibration of this
unit provides feedback on results (sufficient or not
sufficient relaxation). Because of this vibration, Lisa
knows her muscle relaxation has been insufficient
for a while. In order to stop the vibrating signal, Lisa
has to relax her trapezius muscle for instance by
means of the relaxation exercises she has learnt from
her myofeedback therapist. She is able to check her
muscle relaxation patterns (parameters: Root Mean
Squares (RMS) and Relative Rest Time (RRT)) for
both her right and left side of her trapezius muscle
live on the visual display of the PDA within the
BAN. This visual information is considered to be
very important as it provides Lisa more detailed
information about her performance, compared to the
vibration of the processing unit. The PDA also
automatically sends the measured EMG or the
derived muscle relaxation pattern data via a safe
wireless communication infrastructure to a secured
MyoTel portal which is accessible for remote
consultation purposes. Lisa receives four weeks of
treatment during which she wears the MyoTel BAN
and notes her activities and pain intensity in a diary
on the MyoTel portal. Weekly counseling sessions
of approximately 30 minutes with the myofeedback
therapist take place. At the start of the MyoTel
treatment, Lisa and the Myotel therapist meet in vivo
(face-to-face). During this so-called introduction
session (in vivo), the myofeedback therapist gives
instructions about the MyoTel system and explains
the principles and course of remotely supervised
myofeedback treatment. Lisa can read all the
instructions in the manual which is provided along
with the MyoTel equipment. In addition, the
therapist ensures that Lisa’s work station comply
with the ergonomic guidelines and uses a checklist
for the main work times, work tasks, working hours,
workload, and work style. On the visual display of
the PDA the therapist views the muscle activation
patterns to check whether the garment which is worn
by Lisa for the first time is properly adapted to her
anatomy. Thereafter, at least three weekly remote
counseling sessions take place (by telephone) in
which Lisa is taught about personal work style in
relation to muscle tension and beginning techniques
to manage actual stressors at work and at home that
may affect her musculoskeletal health. Prior to the
remote counseling session (conducted by telephone),
the therapist prepares the consultation. This means
the therapist logs in on the web-based Myotel portal,
selects Lisa from the patient list, and zooms in/out
on the (historical) muscle activation patterns of the
data available. The therapists seeks for differences in
left and right side of the trapezius muscle, tries to
find patterns in muscle relaxation over the day
and/or week, identifies tasks which accompany
elevated levels of muscle activation of the trapezius
etcetera. In addition, screenshots of deviating or
remarkable muscle activation patterns can be send to
Lisa for more detailed feedback and discussion.
After four weeks of treatment, Lisa visits the
myofeedback therapist (in vivo) for the final
counseling session and to collect the myofeedback
equipment. After four weeks of treatment the pain
intensity in the neck-shoulder has been reduced and
Lisa is able to recognize symptoms of insufficient
levels of muscle relaxation even in the absence of
the service.
Refinements of the scenario towards use cases
which specify detailed actor’s interactions with the
intended system, for example by incorporating
detailed FICS elements, are beyond the scope of this
early phase requirements elicitation paper.
3.2 Complementary Elicitation Inputs
To augment the elicitation process which is based on
the developed scenario, other elicitation process
inputs have been used.
3.2.1 Cross Disciplinary Study
ICT developers investigate literature on pain
treatment and partially study trial design. The
benefits of such study for these ICT developers are
for example:
• understand medical vocabulary better; this is a
necessity in a collaboration with trial designers
who co-shape the intended system (see for
similar experience also in Cysneiros (2002));
SCENARIO-BASED REQUIREMENTS ELICITATION IN A PAIN-TELETREATMENT APPLICATION
409

• get a better understanding in the clinical case,
including the treatment protocol, to enable
alternative solutions (cf. Cysneiros (2002)). The
inclusion and exclusion criteria e.g. provide the
characteristics of patients that are useful for the
design of the human machine interfaces;
• get a better insight in the required quality of the
clinical data. This helps the estimation of the
required Quality of Service of data transport
channels.
3.2.2 Stakeholder Analysis
Telemedicine typically requires holistic solutions.
Demands or constraints imposed by the many
different telemedicine stakeholders have to be taken
into account in case that the teletreatment protocol
has to be medically approved or deployed in large
scale. For example, treatment protocols have to
conform to medical regulations such as the
regulations and guidelines of the governments, the
Medical EThical Committees (METCs) or the
specific association of the specialty.
In our case, the METCs in the four European
countries have to approve the trial design. To
anticipate to their demands, the trial design includes
patient data privacy and security aspects, because
regulatory stakeholders typically do not participate
in the participatory design where requirements
elicitation takes place. In medicine therefore, we
often deal with stakeholders who are not users of the
intended system. We identify and analyze the
interest of these stakeholders, thereby augmenting
the requirements elicitation process which is based
on the scenario.
A special MyoTel stakeholder is the European
eTEN Programme Commission who sponsors the
trial (Section 2). This stakeholder constrains the
system software adaptation, because the eTEN
programme only grants minor software adaptation.
Consequently, this stakeholder reduces the set of
elicited requirements to those that are feasible for
implementations, not only from a cost/benefit
perspective but also from a software development
effort perspective.
Due to the scope of the project, i.e. collecting
medical evidence by trials, the identified
stakeholders are the relevant ones for preparation
and running the trials. Other stakeholders, for
example, relevant for large scale roll out of the neck-
shoulder pain treatment service are not considered in
this requirements elicitation process.
Stakeholders that have been identified or inferred
from the scenario or the trial design are: the users
stakeholder (i.e. representing both the patients and
therapists), the telemedicine system provider, the
communication system provider who provides the
wired or wireless data transport connections, the
MyoTel Centre of Excellence (CoE) who hosts the
trial, the employer of the patient who provides the
computing and internet access facility, the METC
and to some extent the project sponsor.
3.2.3 Role of Goals
We adopt the scenario definition of Van Helvert &
Fowler (2003), because scenarios described in terms
of tasks and activities are in accordance with
working practices in medicine. We analyze and
decompose the treatment tasks or activities,
including their objectives (i.e. goals), to collect or
infer requirements. Objectives of the treatment
protocol (e.g. specified in the trial design),
corresponding tasks and the associated activities
(e.g. identified or inferred from the scenario) play an
important role in inferring new requirements or in
identifying alternative requirements. Our approach is
therefore goal-oriented (Letier and Lamsweerde,
2004; Mylopoulos et al., 1991; Yu, 1997) and
accordingly, we decompose goals of treatment tasks
in a similar but informal way as in the previously
referred papers (
Figure 2).
Example. A therapist who supervises the training of
a patient strictly (a task), needs amongst others to
check the training discipline of the patient (a
subtask, which goal is e.g. represented by
SupervisedCompliance in Figure 2). Figure 2
presents a partial goal model expressed using the
notation discussed in Letier and Lamsweerde (2004).
The goal of managing the training compliance of a
patient is represented by PatientCompliance. This
goal can be achieved if amongst others
SupervisedCompliance is achieved. The figure also
shows that the preparation of consultation material
(whose goal is represented by ConsultationMaterial)
is achieved when the Activity Relaxation Patterns
(ARPs) of the patient is analyzed and annotated. For
this, a minimum set of measured data (mARPset)
has to be available. The arrow with multiple tails in
Figure 2 symbolizes an AND-refinement of the goal
at the head of the arrow by the goals at the tails of
the arrow (Letier and Lamsweerde, 2004), see also
(Mylopoulos et al., 1991). In the goal model
furthermore, the therapist may retrieve the patient’s
activity relaxation pattern data (PatientARPs) by
operationally logging in onto the MyoTel portal
(RoleBasedLogin), listing and fetching the patient’s
files that contain the RMS and RRT of the measured
ACT4SOC-EHST 2009 - 4th International Conference on Software and Data Technologies
410
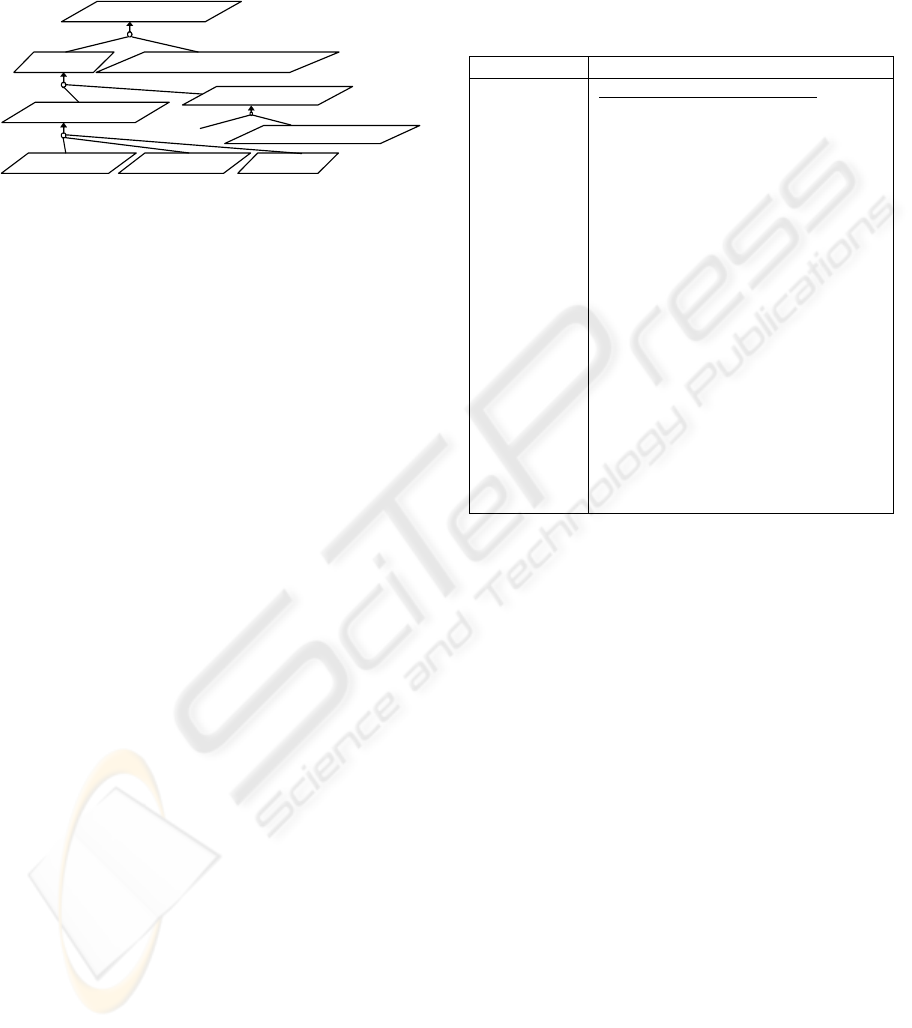
right and left shoulders s-EMG signals (which goals
are represented by ListPatientARPs and
CollectARPs, respectively; see also “tha_10” and
“tha 23” in Table 1).
ConsultationMaterial
mARPset Analysis&AnnotationsOfARPset
(private)PatientARPs
RoleBasedLogin ListPatientARPs Col lectARPs
PatientCom
p
liance
Super visedCompliance
…
Figure 2: Consultation Material Preparation Goal Model.
3.2.4 Requirements Elicitation Results
A close inspection of the trial design and scenario
yields the following treatment objectives:
• strictly supervised training based treatment to
reduce neck-shoulder pain: from the perspective
of educational science, the intended system has
to facilitate remote collection and processing of
training material, which are Root Mean Squares
(RMS) and Relative Rest Time (RRT) of
patient’s left and right shoulder’s s-EMGs and
the three feedback mode (Section 2). Learning
objective of this training is that the patient is
able to recognize symptoms of insufficient
muscle relaxation (see e.g. the last sentence in
the scenario). A therapist supervises the training
strictly, meaning that the therapist needs to
check training compliance regularly and at
arbitrary but convenient moments for the
therapist. This moreover ensures that measured
data collected in amounts and at quality needed
for data analysis.
• assessment of the ICT and the diary based
treatment: an objective which originates from
the trial design.
Actors are users of the intended system; each of
them has specific responsibility and represents an
identified stakeholder. Actors that can be identified
from the scenario, in particular the PACT elements,
are the patients and the therapists. A close inspection
of the trial design and the stakeholder analysis
additionally identifies two system administrator
actors. One of them represents the CoE trial
programme owners of the participating institutes and
has the responsibility to organize the trial and to
administrate logistic, privacy and security aspects
(as required by the METC of the CoE country). The
other represents the telemedicine system provider
stakeholder and has the responsibility to initialize
the setting of the intended system, e.g. to register the
CoE system administrators and to set-up the MyoTel
m-Health portal (Figure 1).
Table 1: Example of Actor, Task, Activity and Priority.
Actors Tasks, Activity & (Priority)
myofeedback
therapist
Supervised tele-treatment task:
tha_10 activity: password protected
login to MyoTel Portal as a
myofeedback therapist; (need to
have);
tha_11 activity: list patient’s list and
selects a patient; (need to have);
…
tha_12 activity: view selected patient
data with the options to zoom in/out,
scroll (or provide start and end
date/time) to view, view the
differences between left and right
muscle activation and relaxation
patterns. Zoom values are e.g. ... ;
(need to have);
…
tha_23 activity: check the training
discipline of patients by checking the
data on the MyoTel portal any time (cf.
tha_11); (need to have);
Functional needs that have to be supported by the
intended system can be identified from the PACT
activities of identified actors. From these functions,
in turn, requirements can be derived. If the scenario
contains FICS elements, the interactions induce the
requirements more straightforwardly. Table 1
illustrates some of the elicited requirements for the
intended system.
From the elicited requirements, ICT services that
are able to support the teletreatment tasks, or
associated activities, can be identified and
dimensioned (cf. Van Helvert and Fowler (2003)),
for example, internet data transport services to bring
measured RMS or RRT data to the therapist for
analysis, web services to register patients or
therapists and role based login services to control
data access to ensure privacy and security of data.
4 OBSERVATION & DISCUSSION
Requirements elicitation in telemedicine is a
challenging research topic because to some extent it
has to be in alignment with the evidence based way
of working in medicine. An approach which
combines a holistic and a detailed in depth way of
SCENARIO-BASED REQUIREMENTS ELICITATION IN A PAIN-TELETREATMENT APPLICATION
411

requirements elicitation is needed. In this work, we
make use of a scenario and parts of the trial design
in combination with a task and goal analysis and
also a stakeholders’ analysis taken from a business
model perspective. This perspective has led us to the
sponsoring stakeholder, which imposes additional
constraints on the permitted software development
effort. This stakeholder would have been missed if
we had not considered the set of service provider,
technology provider, organisational including
regulatory and financial stakeholders. We also view
treatment training as an educational science
workform, which has a well defined structure in
respect of objectives, roles, tasks and input/output
resources in the planning, doing and evaluating
phases of training. For example, patient’s learning
goals and needed learning material can therefore be
made explicit. We consider this way of combining
methods and techniques fruitful and expect that it
also is typical for requirements elicitation in the
domain of (diagnostic-treatment) telemedicine.
Requirements elicitation in telemedicine
moreover requires an intensive collaboration
between trial designers and ICT developers, for
example to make topics of discussions explicit and
mutually understandable. Thereby, the ICT
developers need a good level of understanding of the
medical vocabulary. Mutual understanding can also
be improved by applying a handshake mechanism
(i.e. protocol) during information exchange between
trial designers and ICT developers in the iteration to
strive for a common universe of discourse. In the
discussed scenario based requirements elicitation
process, the trial designers took the lead in the
development of the scenario, which acts as the
common universe of discourse in the collaboration.
Thereafter, the ICT developers took the lead in the
requirements elicitation process which is based on
the developed and to some extent commonly
understood scenario. In this way, intentions or
objectives can be preserved better because the right
experts manage the development.
Despite our effort to address requirements
elicitation thoroughly in a multidisciplinary
collaboration, we observe a limitation. Lack of cross
disciplinary knowledge and homonyms of terms
have caused misinterpretations. In the developed
scenario, the terms “feedback on results” and
feedback on performance were not recognized by the
ICT developers during the requirements elicitation
process as specific concepts known in the area of
augmented feedback strategies. Feedback on
performance (also called Knowledge of Performance
(KP)) is in some cases better than feedback on
results (Knowledge of Results (KR)). Although the
developed system in this perspective happens to
work correctly, the ICT developers have not
identified a requirement which explicitly excludes
solutions that do not make sense, for example in
which the ICT support for tactile KR feedback is
more complex, therefore more sensitive to resource
failures, than the support for visual KP feedback.
5 CONCLUSIONS
This paper presents a scenario based approach to
elicit requirements in the domain of telemedicine
that is in alignment with the evidence based working
practices in medicine. Such an alignment is
considered one of the success factor conditions for a
usable medical system.
The scenario is developed in a multidisciplinary
collaboration between medical professionals and
ICT developers. Medical professionals take first the
lead in the scenario development to ensure
conformity to the treatment protocol and to preserve
treatment objectives when the actor-activity oriented
scenario is iterated and extended with actor-system
interactions. Then, the ICT developers take the lead
in the subsequent requirements elicitation process.
Besides scenarios, the elicitation approach makes
also use of the trial design, a stakeholder’s analysis
and a task and goal analysis to complement the
incompleteness of scenarios. The mix of methods
and techniques known in the area of requirements
engineering and trial design is considered the
contribution of this paper.
The discussed requirements elicitation addresses
the early phase of requirements engineering, later
phases are considered future work for which
scenarios as discussed in this paper have to be
refined to use cases to detail further the actor-system
interactions.
A challenging future research topic is to
investigate requirements engineering for more
complex treatment protocols which involve several
professional caregivers who jointly manage the
disease of patients, for example the treatment
management of Chronic Obstructive Pulmonary
Diseases (COPD).
ACKNOWLEDGEMENTS
This work has been sponsored by the EU under the
eTEN programme in project MyoTel – 046230.
ACT4SOC-EHST 2009 - 4th International Conference on Software and Data Technologies
412

REFERENCES
Benyon, D. and Macaulay, C., 2002. “Scenarios and the
HCI-SE design problem”, Interacting with Computers,
14, pp. 397-405;
Cysneiros, L.M., 2002. “Requirements Engineering in the
Health Care Domain”, in Proc. of the IEEE Joint Int.
Conf. on Requirements Engineering (RE’02);
Go, K. and Carroll, J.M., 2004. “The Blind Men and the
Elephant: Views of Scenario-Based System Design”,
Interactions, pp. 45 – 53;
Letier, E. and Lamsweerde, A. van, 2004. “Reasoning
about Partial Goal Satisfaction for Requirements and
Design Engineering”, SIGSOFT’04/FSE-12, Newport
Beach, CA, Oct. 31 – Nov. 6, 2004;
Meinert, C.L., 1986. “Clinical Trial: Design, Conduct and
Analysis”, Oxford University Press, New York;
Mylopoulos, J., Chung, L. and Yu, E., 1991. “From
Object-Oriented to Goal-Oriented Requirements
Analysis”, Communications of the ACM, 42, 1, Jan.
1991, pp. 31-37;
MyoTel, “MYOfeedback based TELetreatment Service”,
EU/eTEN programme - 046230,
http://www.myotel.eu, visited Jun. 2009;
Sackett, D.L., and Rosenberg, W.M.C., 1995. “The need
for evidence-based medicine”, Journal of the Royal
Society of Medicine, 88, pp. 620-624;
Van Helvert, J. and Fowler, C., 2003. “Scenario-based
User Needs Analysis”, Chimera Working Paper 2003-
02, Colchester, University of Essex;
Yu, E., 1997. “Towards Modelling and Reasoning Support
for Early-Phase Requirements Engineering”, in
Proceedings of the 3rd IEEE Int. Symp. on
Requirements Engineering (Washington D.C., USA,
Jan. 6-8, 1997), RE'97, pp. 226-235;
Wootton, R., Craig, J., Patterson, V., 2006. “Introduction
to Telemedicine”, the Royal Society of Medicine
Press.
SCENARIO-BASED REQUIREMENTS ELICITATION IN A PAIN-TELETREATMENT APPLICATION
413
