MULTIVARIATE STUDY OF ACHEIS MOLECULES
Mapping Pharmacophoric Profile of AChEIs Via PCA
Érica Cristina Moreno Nascimento and João Batista Lopes Martins
Laboratório de Química Computacional, Universidade de Brasília, Campus Universitário Darcy Ribeiro, Brasília, Brazil
Keywords: Alzheimer disease, AChE Inhibitors, DFT, Multivariate analysis, PCA.
Abstract: Alzheimer's disease (AD) is a degenerative dementia. The causes of AD are not well determined, and the
most popular strategy for AD treatment is the cholinergic hypothesis, that consists in the use of drugs with
an inhibitory effect on acetylcholinesterase (AChE) enzyme, to prevent the decrease on the neurotransmitter
(acetylcholine) concentration in synaptic clefts. Structural, electronic and spatial parameters of 10 drugs
with known inhibitory effect on AChE (AChEI) were determined. The parameter values were obtained by
means of calculations at B3LYP/6-31+G(d,p) level. The multivariate analysis of principal components
(PCA) method was applied to 18 parameters to determine the pharmacophoric profile. PCA study was
performed to reduce the sample space of properties and get the ones that are major AChEI components.
1 INTRODUCTION
Alzheimer's disease (AD) is the most common type
of dementia in population over 60 years old (Pivetta,
2008; Sugimoto, 2002; Sippl, 2001). It is a
progressive and degenerative disease (Alcaro, 2002).
In AD patients, the concentration of
acetylcholine (ACh) neurotransmitter is markedly
reduced in the transmission of neural impulses. This
occurs due to the small production of ACh in
neurons, causing the cholinergic deficit, where the
cognitive function remains severely impaired
(Francis, 1999). Based on this information, it was
suggested the cholinergic hypothesis. The
hypothesis consists of applying drugs that can bring
benefits and significant improvement on AD patient
cognitive functions. (Camps, 2002; Sugimoto,
2002).
The acetylcholinesterase inhibitors (AChEI) are
currently the main strategy for the treatment of AD
patients in a cholinergic therapy (Sugimoto, 2002).
Some AChE inhibitors act by competing with ACh,
others inhibit the OH group acylation of amino-acid
residue Ser200, forming a carbamoyl ester, more
stable than the acetate and less able to leave the
enzyme active site (GORGE) (Alcaro, 2002).
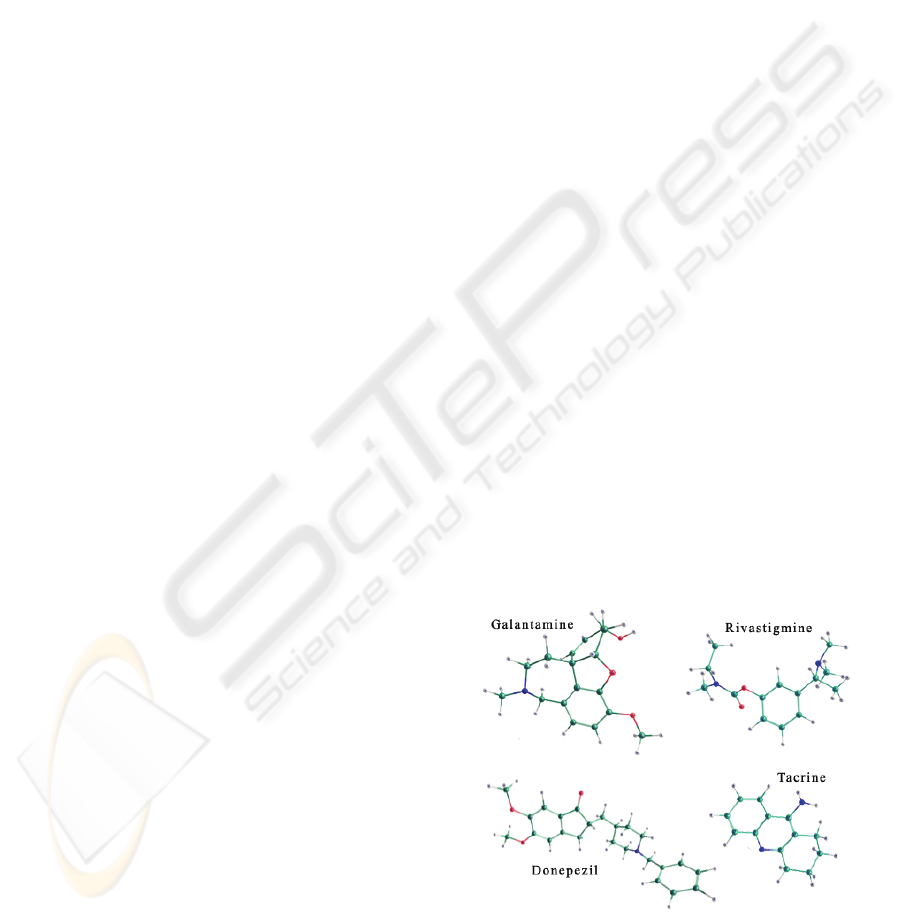
Some drugs act as inhibitors of AChE, shown in
Figure 1, among them tacrine (THA), first drug
approved by FDA for the AD treatment (Proctor,
2000; Sugimoto, 2002), followed by donepezil
(E2020), rivastigmine (RIVA) and galantamine
(GALA) (Racchi, 2004). Some have been studied
and tested clinically for AD treatment, such as
physostigmine (PHYSO), and others are in testing
phase and are promising candidates to approval,
including huperzine A (HUPE), metrifonate
(METRI), dichlorvos (DDVP), phenserine (PHEN)
and the tacrine dimer (DIMTHA) (Racchi, 2004;
Camps, 2002; Kaur, 2000). These drugs, are
indicated for the treatment in mild to moderate
stages, when the patient still has independent
cognitive activity.
Figure 1: Some AChEI molecules structures.
Biochemically, these AChEI molecules have in
common the inhibitory action on the AChE.
245
Cristina Moreno Nascimento É. and Batista Lopes Martins J. (2010).
MULTIVARIATE STUDY OF ACHEIS MOLECULES - Mapping Pharmacophoric Profile of AChEIs Via PCA.
In Proceedings of the First International Conference on Bioinformatics, pages 245-249
DOI: 10.5220/0002745602450249
Copyright
c
SciTePress
However, the chemical class (pharmacophoric
groups) and structures involved in such molecules
have non similar chemical characteristics. Therefore,
quantum mechanical calculations to find electronic
structure properties were needed to determinate the
common and relevant properties that these
molecules are sharing.
In this study the structural and electronic
properties of acetylcholinesterase enzyme inhibitors
were theoretically obtained and related to their
activity by multivariate analysis by seeking the
principal components (PCA), which were used to
establish the pharmacophoric profile of those drugs.
Multivariate analysis is a powerful tool to
investigate candidates to AChE inhibitors. PCA was
used to determinate significant properties in classical
acetylcholinesterase inhibitors (Nascimento, 2008).
PCA was also applied to propose promising new
candidates, in order to produce potential active
AChEI molecules for the DA treatment (de Paula et
al., 2009).
This study aims to obtain pharmacophoric
profile of AChEI molecules and to contribute to the
development of new inhibitors of AChE. We are
interested in provide insights for new descriptors of
known AChE inhibitor molecules in order to
contribute to the understanding how these drugs are
correlated to each other using PCA chemometrics
method.
2 COMPUTATIONAL DETAILS
Density functional theory (DFT) at B3LYP hybrid
functional level was applied in this study. The 6-
31+G(d,p) basis set was used. The geometries of
target drugs were optimized using internal
coordinates. The theoretical calculations were
performed using Gaussian03 (Frisch, 2003)
program, in order to determine the best electronic
and geometrical parameters. OSIRIS (Sander, 2001)
program was used to determine the logP and logS
parameters. PCA study was carried out using
MATLAB
®
(Rahman, 2009) program.
Pharmacophoric profile of AChEI molecules was
acquired using the PCA multivariate statistical
method. This method was used to correlate the
studied AChEI molecule properties and their
inhibitory activity, as well as to reduce the initial set
of parameters (electronic and structural properties),
generating the most relevant ones. The eighteen
parameters used in the PCA analysis were: dipole,
HOMO, HOMO-1, LUMO and LUMO+1 energies,
heteroatom charge, hydrogen charge of most acid
atom, molecular volume, H-H distance, partition
coefficient logP, logS, number of hydrogen
receptors and donors (H
recp
e H
don
), number of
aromatic rings, (LUMO-HOMO) GAP, molecular
size (largest intramolecular distance), rotation
degrees freedom and Polar Surface Area (PSA),
using the optimized geometries.
2.1 PCA Method
PCA is a major technique employed in chemometric
analysis to study a set of multivariate data (Mizutani,
2004). PCA method has two aims: the decrease of
variable sets, and the selection of the best properties
linearly independent to represent a system (principal
components).
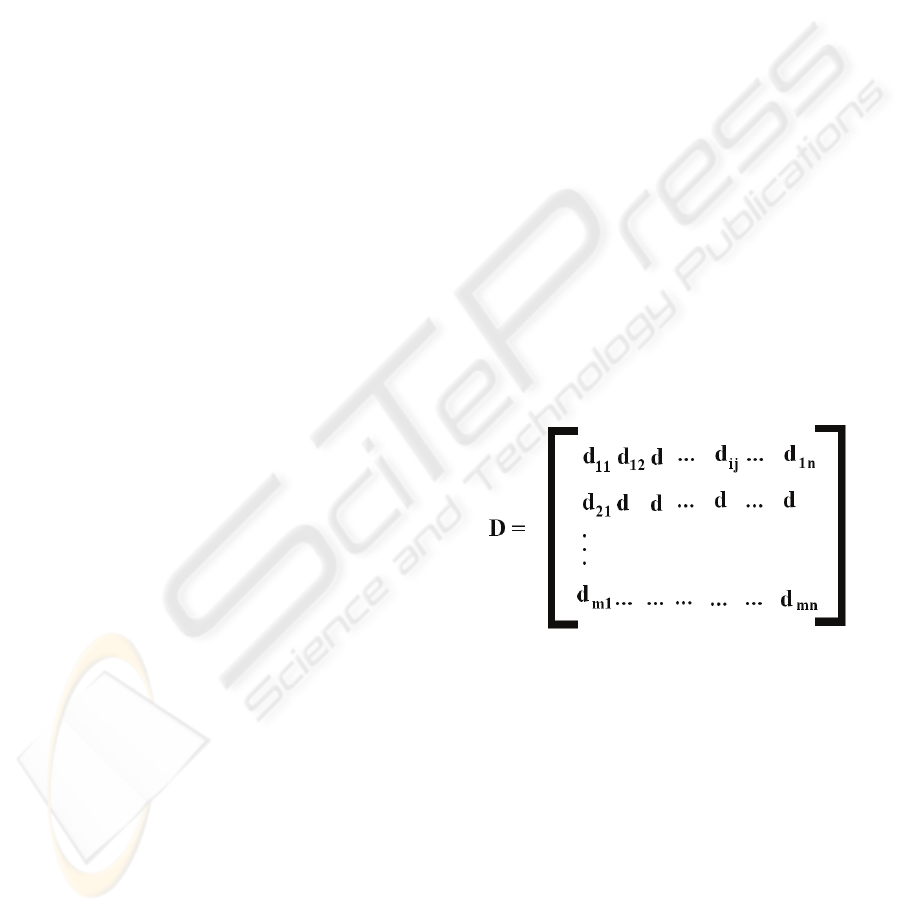
As a general example, we have a chemical
experiment performed with m numbers of molecules
resulting in n numbers of properties. Studies using
PCA data processing (D) is performed by
considering the n numbers of variables (properties)
system on m numbers of objects (molecules). Then,
the generated matrix D (Figure 2) consists of mxn
elements. The j-th variable is represented by a
column vector and the i-th object is represented by a
row vector, also called the response vector, and can
be described by a point in n-dimensional space
(Anderson, 1984).
Figure 2: mxn matrix of data set.
The aim of PCA study is to explain the variance
and covariance structure of an aleatory vector of
variables, using a linear combination of original set
yielding the principal components (PCs) (Anderson,
1984).
The PCs can be seen as axes of maximum
distribution of objects. We can visualize the layout
of objects in these new sets of axes. The layout
formed by the projection of these objects in the main
components is called the graph of scores. Its
coordinates are derived from the product of the data
matrix by the matrix of eigenvectors. If the first two
or three eigenvectors explain a significant amount of
the total variance, a plot of scores are the
BIOINFORMATICS 2010 - International Conference on Bioinformatics
246

coordinates that are accurate in many dimensions of
the larger original space (Gnanadesikan, 1997;
Marriot, 1974).
PCA study was conducted using the auto-
stepping method, since the structural and electronic
properties have different dimensions.
3 RESULTS AND DISCUSSIONS
Table 1 shows the most relevant properties of the
AChEI optimized geometries at B3LYP/6-
31+G(d,p) level. PCA cumulative variance using
four principal components, PC1, PC2, PC3 and PC4,
are 38.3, 59.2, 73.2 and 83.3%, respectively, which
are significant for the whole set.
Table 1: Most relevant properties of AChEI molecules at
B3LYP/6-31+G(d,) level.
AChEI
V
olume
(Å
3
)
Size
(Å)
H-H
(Å)
HOMO-1
(eV)
logP Aromatic
rings
DDVP
185 7.849 1.796 -8.57 1.66 0
DIMTHA
606 19.386 1.998 -6.71 3.88 4
E2020
454 12.254 2.342 -6.07 4.14 3
GALA
329 10.29 2.359 -6.03 1.39 2
HUPE
286 9.051 1.625 -6.75 2.60 1
METRI
207 7.068 2.307 -8.30 0.80 0
PHEN
391 14.808 2.491 -6.07 2.99 1
PHYSO
321 12.927 2.319 -6.08 1.94 2
RIVA
312 11.242 2.460 -6.53 2.86 1
THA
236 9.516 1.683 -6.56 3.13 2
To increase accuracy and to determine the most
relevant properties a systematic study was carried
out for all possible combinations of 18 properties for
all 10 drugs.
The variables number was reduced to 6
(described below), nevertheless keeping the sample
space of 10 objects (AChE): molecular volume,
molecular size, H-H distance, HOMO-1 energy,
partition coefficient logP and number of aromatic
rings. The criterion for reducing the variables
number to six was to improve the cumulative
variances percent using combinatorial analysis of the
18 properties of 10 drugs.
The information that describes the drugs as
AChEI molecules may be represented by three
principal components. Figure 3 depicts PC1xPC2
scores. PC1 (with 63.5% of variance) x PC2
(accounting for 19.1% of variance) x PC3 (9.1% of
variance), satisfactorily account for more than 90%
of the variance of the entire data set.
Equations 01, 02 and 03 show the calculated
PC1, PC2 and PC3 coefficients, respectively. The
PC1 was represented essentially by the drug volume
and size (structural parameters). For PC2 the most
relevant are the H-H distance and HOMO-1 orbital
energy (structural and electronic parameters), while
PC3 is mainly represented by the H-H distance.
All 6 properties listed are positive in PC1, i.e.,
the 6 properties contributes in the first principal
component. 62% of the variance is represented by
the PC1 (Equation 01).
PC1(%)= 24
Volume
+ 22
Size
+ 2
H-H
+ 14
HOMO-1
+ 17
lo
g
P
(1)
PC2(%)=
0.1
Volume
+ 0.1
Size
+ 78
H-H
+ 8
HOMO-1
- 11
lo
g
P
(2)
PC1(%)=
14
Volume
- 17
Size
- 3
H-H
+ 63
HOMO-1
+ 3
logP
(3)
Figure 3 shows that PC1 tends to cluster the
AChEI molecules by the six selected properties. It
follows the formation of groups of well-defined
AChE molecules, four of the AChEI molecules,
already approved by FDA for AD group treatment,
form a cluster, GALA, RIVA, PHYSO and PHEN.
The distribution along PC1 is satisfactory, since the
AChE with similar molecular volume are
considerable closer to each other: GALA/ RIVA/
PHYSO/ PHEN and HUPE/ THA. AChEI molecules
with smaller molecular volumes are in negative
scores region, the AChEI with molecular volumes
between 236 and 606 Å
3
are in regions of positive
scores.
On the other hand, PC2 is dominated by the H-H
distance (+0.88224), which separates compounds
into two groups according to the distance between
the two most acid hydrogens: the first group is found
within values of H-H less than 2.0 Å . The second
group is within values higher than 2.0 Å distances.
There is a clear separation between the groups,
Table 1 shows that the E2020 has intermediate
values for all properties, except for logP, which has
negative coefficient and is the dispersion element of
PC2.
Figure 3: PC1 versus PC3 scores at level B3LYP/6-
31+G(d,p).
MULTIVARIATE STUDY OF ACHEIS MOLECULES - Mapping Pharmacophoric Profile of AChEIs Via PCA
247
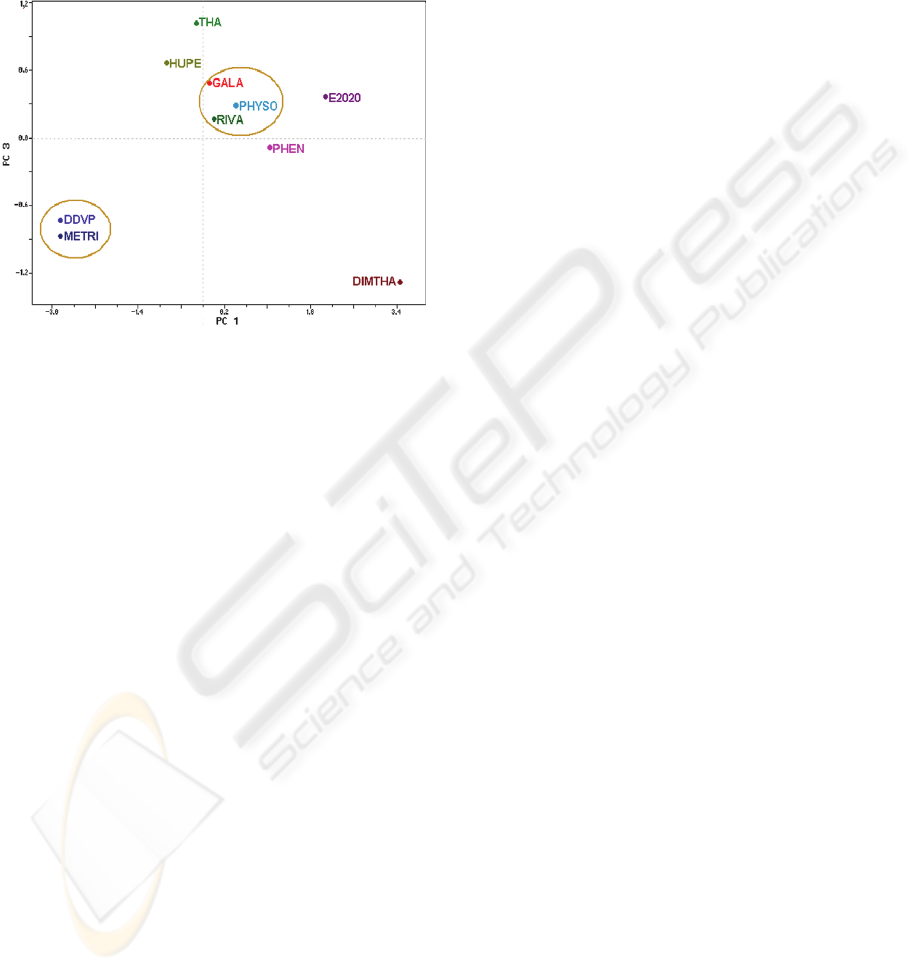
Figure 4 shows PC1 versus PC3 scores plot.
There are two patterns in PC3, i.e., DDVP / METRI
and GALA / PHYSO / RIVA. Which can be
explained, since PC3 is dominated by the orbital
energy of HOMO-1 (+0.79272) (see Equation 3).
These molecules have values close to the HOMO-1
and the molecular volume (Table 1).
Figure 4: PC1 versus PC3 scores at level B3LYP/6-
31+G(d,p).
As can be seen in Figures 3 and 4, the equations
generated in PCs indicate that the electronic
properties are the most significant in the AChEI
molecules study, such as energy of HOMO-1. The
structural parameters that also contribute are:
molecular volume, size of the drug and H-H
distance.
4 CONCLUSIONS
The PCA study showed that electronic property, –
HOMO-1 orbital energy, logP, numbers of aromatic
ring, and structural parameters - volume, drug size
and H-H - are the most significant properties, i.e.,
the principal components of the AChEI drugs
pharmacophforic profile.
Thus, it is estimated that a good candidate to
inhibit the acetylcholinesterase enzyme must
includes: partition coefficient values between 0.8
and 4.9; logS between -5.0 and -1.5; polar surface
area between 30.0 and 60.0 Å
2
. The torsional
degrees number of freedom sufficient to be able to
rearrange itself adequately inside the AChE active
site is also important. Other desirable features for the
AChEI molecules are: preferably aromatic systems
or groups that simulate surface electron density of
aromatic systems; sufficient amount of hydrogen
acceptors and few donors of hydrogen. Furthermore,
according to B3LYP/6-31+G(d,p) level results the
inhibitor should have: HOMO-1 orbital energy
between -8.60 and -6.00 eV; and the distance among
the two more acidic hydrogens molecule between
1.600 – 2.500 Å. Together, all these properties
participate in the pharmacophoric profile of the
studied AChEIs molecules.
ACKNOWLEDGEMENTS
This study is supported by CNPq, and Funpe/UnB.
We also acknowledge the computational resource of
CENAPAD/SP.
REFERENCES
Alcaro, S., Scipione, L., Ortuso, F., Posca, S., Rispoli, V.
& Rotiroti, D. (2002). Molecular modeling and
enzymatic studies of the interaction of a choline
analogue and acetylcholinesterase. Bioorganic &
Medicinal Chemistry Letters, 12, 2899-2905.
Anderson, T. W. 1984. An Introduction to Multivariate
Statistical Analysis. John Wiley & Sons. New York, 2nd
edition.
Camps, P. (2002). Cholinergic Drugs in Pharmacotherapy of
Alzheimer’s Disease. Mini Reviews in Medicinal
Chemistry, 2, 11-25.
de Paula A.A.N., Martins J.B.L., dos Santos, M.L.,
Nascente, L. de C., Romeiro, L.A.S., Áreas, T.F.M.A.,
Vieira, K.S.T., Gambôa, N.F., Castro, N.G., Gargano,
R. (2009). New potential AChE inhibitor candidates.
European Journal of Medicinal Chemistry, 44, 3754–
3759.
Francis, P. T. (1999) The cholinergic hypothesis of
Alzheimer’s disease: a review of progress. J Neurosurg
Psychiatry, 66, 137-147.
Frisch, M. J., G.W. Trucks, H.B.S., G.E. Scuseria, M.A. Robb,
J.R.C., Zakrzewski, V.G., Montgomery, Jr. J.A., Stratmann,
R.E., Dapprich, J.C.B.S., Millam, J.M., Daniels, A.D., Strain,
K.N.K.M.C., Farkas, O., Tomasi, J., Barone, V., Cammi,
M.C.R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S.,
Petersson, J.O.G.A., Ayala, P.Y., Cui, Q., Morokuma, K.,
Rabuck, D.K.M.A.D., Raghavachari, K., Foresman, J.B.,
Ortiz, J.C.J.V., Stefanov, B.B., Liu, G., Liashenko, A.,
Komaromi, P.P.I., Gomperts, R., Martin, R.L., Fox D.J., Al-
Laham, T.K.M.A., Peng, C.Y., Nanayakkara, A., Gonzalez,
C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W.,
Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M.,
Replogle E.S. And Pople, J.A. (2003). Gaussian03 (Rev.
A11) Pittsburgh, PA, Gaussian Inc.
Gnanadesikan, R. 1997. Methods for statistical data analysis of
multivariate observations. John Wiley & Sons. New York,
2nd edition.
BIOINFORMATICS 2010 - International Conference on Bioinformatics
248

Kaur, J. & Zhang, M. Q. (2000). Molecular modelling and
QSAR of reversible acetylcholinesterase inhibitors.
Current Medicinal Chemistry, 7, 273-294.
Marriott, F. H. C. 1974. Interpretation of multiple observations.
Academic Press. London, 1st edition.
Nascimento, É. C. M.; Martins, J. B. L.; Santos, M. L.;
Gargano, R. (2008). Theoretical study of classical
acetylcholinesterase inhibitors. Chemical Physics
Letters, 458, 285-289.
Pivetta, M. (2008). Na raiz do Alzheimer. Pesquisa Fapesp.
21, 16-21.
Racchi, M., Mazzucchelli, M., Porrello, E., Lanni, C. & Govoni,
S. (2004). Acetylcholinesterase inhibitors: novel
activities of old molecules. Pharmacological Research.,
50, 441-451.
Rahman, O. M., J., Foster, A. & Gertler, P. (1994-2009)
MATLAB. U.S. Patent Nos. 6,857,118; 6,973,644;
6,993,772; 7,010,364; 7,051,333; 7,051,338; 7,096,154;
7,139,686; 7,165,253; 7,181,745; 7,228,239; 7,231,631;
7,237,237; 7,340,441; 7,353,502; 7,359,805; 7,365,311;
7,369,127; 7,400,997; 7,428,737; 7,454,659; 7,454,746;
7,460,123. USA.
Sander, T. (2001). OSIRIS Property Explorer. Actelion
Pharmaceuticals Ltd, Gewerbestrasse 16, 4123 Allschwil,
Switzerland.
Sippl, W., Contreras, J. M., Parrot, I., Rival, Y. M. & Wermuth,
C. G. (2001). Structure-based 3D QSAR and design of
novel acetylcholinesterase inhibitors. Journal of
Computer-Aided Molecular Design. 15, 395-410.
Sugimoto, H., Ogura, H., Arai, Y., Iimura, Y. & Yamanishi, Y.
(2002). Research and development of donepezil
hydrochloride, a new type of acetylcholinesterase
inhibitor. Japanese Journal of Pharmacology, 89, 7-20.
MULTIVARIATE STUDY OF ACHEIS MOLECULES - Mapping Pharmacophoric Profile of AChEIs Via PCA
249
