
SYNCHRONISATION OF BIOLOGICAL CLOCK SIGNALS
Capturing Coupled Repressilators from a Control Systems Perspective
Thomas Hinze, Mathias Schumann and Stefan Schuster
Department of Bioinformatics, Friedrich Schiller University Jena, E.-Abbe-Platz 1-4, D-07743 Jena, Germany
Keywords:
Chronobiology, Coupled repressilators, Reaction-diffusion kinetics, Internal/external clock synchronisation,
Control system, Phase-locked loop.
Abstract:
Exploration of chronobiological systems emerges as a growing research field within bioinformatics focusing
on various applications in medicine, agriculture, and material sciences. From a systems biological perspective,
the question arises whether biological control systems for regulation of oscillative signals and their technical
counterparts utilise similar mechanisms. If so, modelling approaches and parameterisation adopted from build-
ing blocks can help to identify general components for clock synchronisation. Phase-locked loops could be an
interesting candidate in this context. Both, biology and engineering, can benefit from a unified view. In a first
experimental study, we analyse a model of coupled repressilators. We demonstrate its ability to synchronise
clock signals in a monofrequential manner. Several oscillators initially deviate in phase difference and fre-
quency with respect to explicit reaction and diffusion rates. Accordingly, the duration of the synchronisation
process depends on dedicated reaction and diffusion parameters whose settings still lack to be sufficiently
captured by comprehensive tools like the Kuramoto approach.
1 INTRODUCTION
In both spheres, biological and technical systems, os-
cillatory signals play a major role in order to trigger
and control time-dependent processes. Core oscilla-
tors are the simplest devices for generation of contin-
uously running clock signals. To this end, signal pro-
cessing units consisting of at least one feedback loop
can suffice (Russo and di Bernardo, 2009). So, it is
no surprise that probably numerous evolutionary ori-
gins led to oscillative reaction networks while inde-
pendently technical attempts succeeded in construc-
tion of single clocks or clock generators.
The situation becomes more complicated if sev-
eral of those core oscillators start to interact. Re-
sulting biological systems are commonly driven to
achieve a synchronous behaviour towards an evolu-
tionary advantage. Correspondingly, clock synchro-
nisation in technical systems is frequently inspired by
the need to follow a global time. Interestingly, the
formalisation of clock synchronisation processes is
quite distant from each other. While in distributed
computer systems, stepwise algorithmic approaches
(like Berkeley or Christian’s method, (Tanenbaum
and van Steen, 2001)) predominate, biological sys-
tems adjust their clock signals more gradually. Its
formalisation is either based on reaction-diffusion ki-
netics or employs the more abstract Kuramoto method
(Kuramoto, 1984), an analytic signal coherence mea-
sure restricted to sinusoidal signal shape to counteract
phase shift between each pair of core oscillators.
We define different temporally oscillating signals
to be synchronous to each other if and only if they
meet three conditions: (1) The oscillatory signal must
run undamped to avoid signal weakening. (2) Asymp-
totical or total harmonisation of the oscillatory sig-
nals meaning that after a finite amount of time called
t
sync
(time to synchronisation), both temporal sig-
nal courses converge within an arbitrarily small ε-
neighbourhood. (3) The resulting oscillatory sig-
nal after t
sync
has to be monofrequential to ensure
chronoscopy (constant progression of time measure).
The central prerequisite of a core oscillator to
be capable of synchronisation to others is its abil-
ity to vary its oscillation frequency within a speci-
fied range (Granada and Herzel, 2009). This variation
can be achieved by forcing, by resetting, or by spe-
cific selective perturbations affecting the oscillating
signal. Without any external influences, core oscilla-
tors resume their individual free-running oscillatory
behaviour, mostly by loosing their synchronicity.
Topologically, clock synchronisation can be ac-
101
Hinze T., Schumann M. and Schuster S..
SYNCHRONISATION OF BIOLOGICAL CLOCK SIGNALS - Capturing Coupled Repressilators from a Control Systems Perspective.
DOI: 10.5220/0003121301010106
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2011), pages 101-106
ISBN: 978-989-8425-35-5
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
complished by two different strategies called exter-
nal and internal (Pikovsky et al., 2001). External
strategies comprise a central leading clock that propa-
gates its time signal throughout the whole network of
downstream core oscillators which adjust their indi-
vidual signals by accelerating or slowing down their
frequency for a certain amount of time. Here, we ob-
serve an unidirectional coupling from the leading cen-
tral clock to all others. In contrast, internal strategies
aim at a mutual clock exchange between the network
members. The coupling topology is mostly bidirec-
tional, and each involved core oscillator is going to
adjust its signal based on a weighted sum of the sig-
nals released by its adjacent clocks.
Within a case study, we exemplify internal syn-
chronisation by a biological system composed of
bidirectionally coupled repressilators. To this end,
we model the entire gene regulatory networks using
reaction-diffusion kinetics. Afterwards, we conduct
two comprehensive simulation studies. The first one
discloses the time to synchronisation subject to ini-
tial phase shift between the elementary repressilators.
Its balanced diffusion rate acts as coupling strength.
It appears that synchronisation of initially antipha-
sic signals is most time-consuming for weak coupling
while it has a negligible effect for strong coupling.
A second simulation study investigates the synchro-
nisation behaviour with respect to different initial fre-
quencies of the single repressilators. The obtained
numerical results are envisioned to identify building
blocks and their parameterisation towards composi-
tion of a control system following the concept of
phase-locked loops.
2 INTERNAL
SYNCHRONISATION:
COUPLED REPRESSILATORS
2.1 Reaction Network and Kinetics
We identified a network of bidirectionally coupled re-
pressilators to be an appropriate candidate to explore
internal synchronisation within a biological system.
A repressilator is a gene regulatory network consist-
ing of three focal proteins (LacI, TetR, cI) that mu-
tually inhibit their expression from genes (lacI, tetR,
cI) (Elowitz and Leibler, 2000). We employ a system
composed of two coupled repressilators located in
two adjacent cells inspired by Garcia-Ojalvo (Garcia-
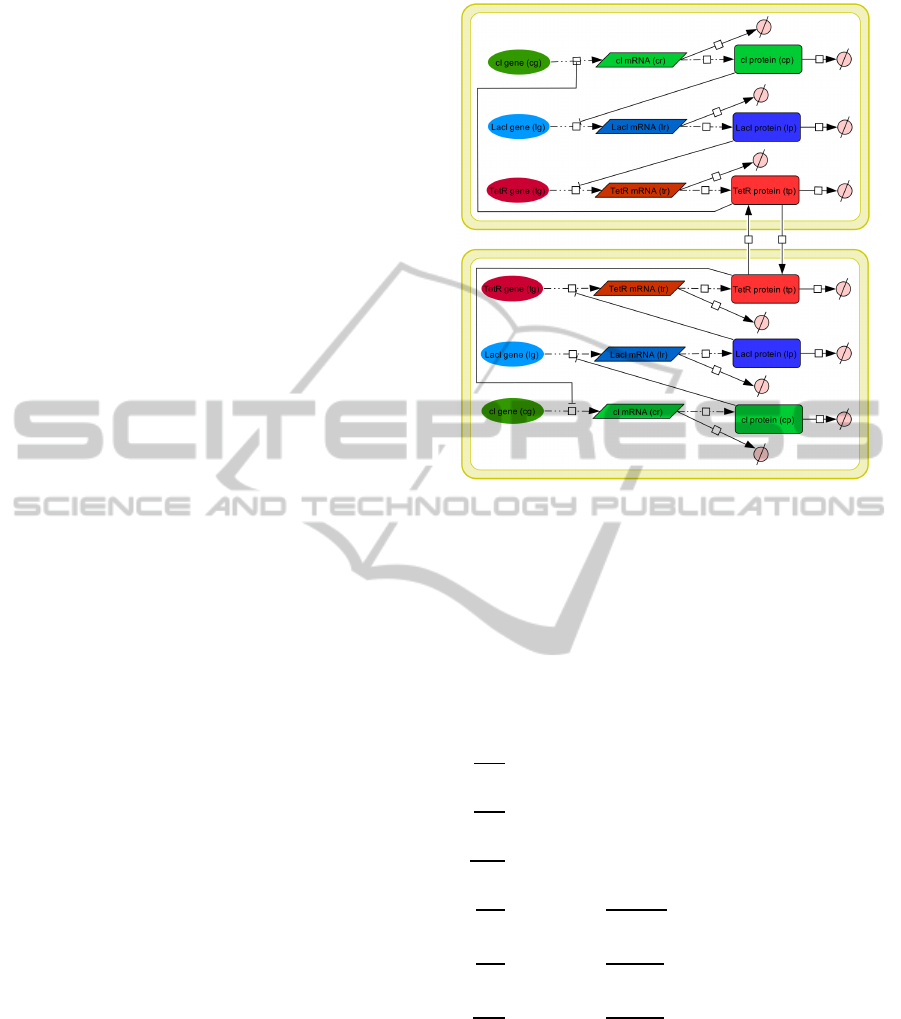
Ojalvo et al., 2004), see Fig. 1.
Let TetR be a protein able to migrate between the
cells, it acts as coupling element. Its diffusion rate diff
specifies the variable bidirectional coupling strength.
Figure 1: Network topology of the TetR-coupled repressi-
lator model with diffusion between both core oscillators.
The dynamical behaviour of the network can be spec-
ified by reaction-diffusion kinetics based on corre-
sponding ordinary differential equations (ODEs). For
species names in the ODEs, we abbreviate (LacI,
TetR, cI) = (lp, tp, cp) for the proteins and (lacI, tetR,
cI) = (lr, tr, cr) for the mRNA. The set of equations
for each single repressilator reads:
d lp
d t
= k
lr
· lr− k
lp
· lp
d tp
d t
= k
tr
· tr− k
tp
· tp− diff·tp+ diff· tp
external
d cp
d t
= k
cr
· cr− k
cp
· cp
d lr
d t
= α
0
+
α· k
n
m
k
n
m
+ cp
− k
lr
· lr− k
lr2
· lr
d tr
d t
= α
0
+
α· k
n
m
k
n
m
+ lp
− k
tr
· tr− k
tr2
· tr
d cr
d t
= α
0
+
α· k
n
m
k
n
m
+ tp
− k
cr
· cr− k
cr2
· cr
We utilise the parameter setting α
0
= 0.03, α =
29.97, k
m
= 40, n = 3, k
{lp,tp,cp}
= 0.069, k
{lr,tr,cr}
=
6.93, k
{lr2,tr2,cr2}
= 0.347 resulted from a parame-
ter fitting based on the available experimental data
(Garcia-Ojalvo et al., 2004). Additionally, the ini-
tial species concentrations in case of no phase shift
are chosen at the limit cycle, e.g. lr = 0.819, tr =
2.388, cr = 0.068, lp = 36.263, tp = 166.685, cp =
64.26.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
102

The repressilator’s oscillation frequency mainly
depends on the degradation reaction rates. Diffusion
of TetR proteins from one repressilator to its adja-
cent counterpart causes the same effect. This allows
to control the frequency just by forcing using a sus-
tained dissipation of diffusing TetR proteins. Fig. 2
illustrates a typical synchronisation run.
time steps
TetR abundance
Figure 2: Typical synchronisation run of two coupled re-
pressilators, coupling strength diff= 0.04, initial phase shift
182
◦
(arbitrarily chosen). Simulation carried out with Co-
pasi using ODEs and parameter settings given in Sec. 2.1.
2.2 Synchronising Initial Phase Shifts
For the synchronisation study, we set up both repres-
silator’s initial concentrations at the individual limit
cycle in order to avoid effects occurring within the
transient phase (stabilisation phase). Afterwards, a
two-dimensional parameter scan was conducted vary-
ing the initial phase shift of both repressilators be-
tween 0
◦
and 360
◦
and simultaneously varying the
coupling strength within the relevant range diff= 0.01
to 0.13 (weak to strong coupling). The time to syn-
chronisation was obtained assuming a signal conver-
gence of one minute per day (ε-neighbourhood’s in-
terval length =
1
1440
of oscillation period), see Fig. 3.
The simulation study exhibits a correlation be-
tween coupling strength (diff) and time to synchro-
nisation. Since a strong coupling (diff = 0.13) has
a more significant effect on the adjacent repressila-
tor’s behaviour, synchronisation is achieved fast. In
this case, even the influence of different initial phase
shifts can be widely neglected. The situation becomes
different when considering a weak coupling. Here,
the initial phase shift predominantly determines the
time to synchronisation. Initial antiphase rhythmicity
(phase shift 180
◦
) between both repressilators causes
0 50 100 150 200 250 300 350
2000 4000 6000 8000 10000 12000 14000
diff = 0.01
diff = 0.04
diff = 0.07
diff = 0.1
diff = 0.13
180°
phase shift φ
time to synchronize
initial phase shift
time to synchronisation
diff = 0.01
diff = 0.04
diff = 0.07
diff = 0.10
diff = 0.13
Figure 3: Time to synchronisation subject to various initial
phase shifts. Parameter diff= 0.01, . . . , 0.13 denotes cou-
pling strength from weak to strong coupling. Initial an-
tiphase rhythmicity (phase shift 180
◦
) between both repres-
silators causes the highest effort to synchronise both oscil-
latory signals by mutual forcing.
the highest effort to synchronise both oscillatory sig-
nals by mutual forcing. In this context, it is inter-
esting to mention that the ability of the repressilator
coupling to synchronise initial antiphase rhythmicity
is a direct consequence of the (slight) asymmetric os-
cillatory signal shape. While symmetric oscillation
curves (like sinusoidal signals) persist in antiphase
when coupled, hence unable to synchronise, asym-
metric curves (like spiking signals) entail a kind of
unbalanced response to forcing. There is no equi-
librium between forcing effects shortening and those
advancing the oscillatory period. The remaining ef-
fect is sufficient to initiate synchronisation. The slight
asymmetry of the diagram in Fig. 3 also results from
the asymmetric shape of the repressilator’s oscilla-
tory signal. Interestingly, a medium coupling strength
(diff = 0.07) generates a behaviour in which time
to synchronisation for increasing initial phase shift
can be compensated within a range of approximately
50
◦
. . . 100
◦
and 260
◦
. . . 310
◦
, respectively.
2.3 Synchronisation of Different Initial
Frequencies
We demonstrate the ability of the repressilator cou-
pling to synchronise different initial frequencies in the
elementary repressilators. To this end, individual pro-
SYNCHRONISATION OF BIOLOGICAL CLOCK SIGNALS - Capturing Coupled Repressilators from a Control Systems
Perspective
103

tein degradation rates k
lp
, k
tp
, k
cp
had been modified in
conjunction with setting up all initial concentrations
at the individual limit cycle. From this, we conducted
a parameter scan taking into account the ratios of ini-
tial frequencies.
The purpose of this case study is to answer four
questions: (1) Is there any synchronisation window, a
continuous range of parameter settings, that runs the
entire system into synchronisation? In other words,
can we detect a variant of a so-called Arnold tongue?
(2) If a synchronisation window could be identified,
which of the three conditions necessary for synchro-
nised oscillations become violated by leaving the de-
limiting parameter settings? (3) How is the time
to synchronisation distributed within the synchroni-
sation window? (4) Which synchronous frequency
does result from the initially different frequencies af-
ter synchronisation?
While question (1) seems suitable to be answered
in part using the Kuramoto method (Kuramoto, 1984),
an analytical ODE-based technique, a sufficient clar-
ification of questions (2), (3), and (4) requires an ex-
plorative simulation study. An essential part of this
study is the calculation of the frequencies governed
by an oscillatory signal. To this end, we utilise the
discrete Fast Fourier Transformation (FFT) for long-
term data accompanied by sampling and counting of
local oscillatory signals maxima or minima for short-
time data series. Time to synchronisation is again
measured by the number of elapsed time steps up to
convergence of one minute per day (cf. Sec. 2.2).
If synchronisation is obtained, we can distinguish
two qualitative scenarios characterised by the result-
ing synchronous frequency in relation to either initial
frequencies.
Fig. 4 depicts a typical temporal course towards
synchronisation of two marginally different initial
frequencies (solid lines). During the synchronisa-
tion process, both frequencies converge to a common
value (dashed curves). This value deviates from both
initial frequenciesbut arises in between. The synchro-
nisation itself runs rather fast.
In contrast, a stronger – however slight – deviance
of initial frequencies turns the synchronisation into a
final frequencyasymptotically convergingto the max-
imum initial frequency, see Fig. 5 for an example.
Here, the synchronisation process takes more time.
The latter case coincides with arrival at the limits
of the synchronisation window marking the maximal
deviance of initial frequencies leading to synchroni-
sation. Inside the synchronisation window, the syn-
chronous frequency becomes adjusted in between of
both initial frequencies, and the more we approach to-
wards the boundaries of the synchronisation window,
0.0015 0.0016 0.0017
time steps
synchronous frequency
0
2000 4000 6000 8000
initial frequency cell 1 (0.001645)
initial frequency cell 2 (0.001578)
frequencies cell 1 towards synchronisation
frequencies cell 2 towards synchronisation
Figure 4: Typical temporal course towards synchronisation
of two marginally different initial frequencies (solid lines)
converging to a common value (dashed curves). Coupling
strength: diff = 0.01, ratio of initial frequencies:
0.001645
0.001578
≈
1.042. Synchronous frequency: 0.001616.
0.0015 0.0016 0.0017
time steps
synchronous frequency
0
2000 4000 6000 8000
initial frequency cell 1 (0.001691)
initial frequency cell 2 (0.001578)
frequencies cell 1 towards synchronisation
frequencies cell 2 towards synchronisation
Figure 5: Typical temporal course towards synchronisa-
tion at the boundary of the synchronisation window. Syn-
chronous frequency asymptotically reaches the maximum
of either initial frequencies (dashed curves). Initial frequen-
cies marked by solid lines. Coupling strength: diff = 0.01,
ratio of initial frequencies:
0.001691
0.001578
≈ 1.072. Synchronous
frequency: 0.001690.
the synchronous frequency converges to the maxi-
mum of both initial frequencies.
We obtain a synchronisation window delimited by
polyfrequential oscillations with respect to the ratios
of initial frequencies and loss of undamped oscillation
with respect to the coupling strength, see Fig. 6. We
checked whether an oscillatory signal is undamped or
not by evaluating the eigenvalues of the Jacobian ma-
trix derived from the ODEs specifying the reaction-
diffusion kinetics.
Moreover, the simulation results indicate that a
medium coupling strength (diff = 0.07) enables syn-
chronisation within the largest ratio of initial frequen-
cies ranging from 0.697 to 1.294. This means in terms
of systems application for clock synchronisation that
a clock signal can be temporarily slowed down (post-
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
104
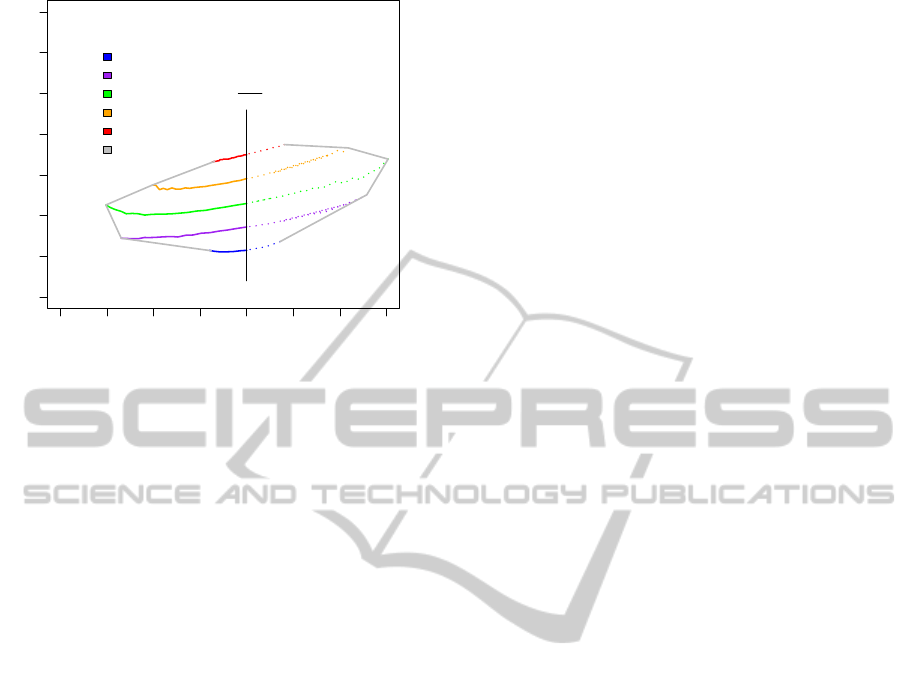
0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3
0.0010 0.0020 0.0030 0.0040
diff = 0.01
diff = 0.04
diff = 0.07
diff = 0.1
diff = 0.13
min/max Quotient
Freq 1
Freq 2
== 1
frequency parameter ratio
synchronized frequency
ratio of initial frequencies
synchronous frequency
Figure 6: Synchronisation window: ratios of initial fre-
quencies subject to synchronous frequency considering a
variety of relevant coupling strengths diff = 0.01, . . . , 0.13
(variant of an Arnold tongue, a circle map disclosing de-
pendencies of system parameters within a range of stable
oscillation). Due to the bidirectionally balanced coupling
strength, an almost symmetric synchronisation window can
be obtained which is delimited by polyfrequential oscilla-
tions with respect to the ratios of initial frequencies and loss
of undamped oscillation with respect to coupling strength.
pone the clock) and speeded up (put the clock for-
ward) with up to approximately 30% of its velocity.
The knowledge about parameterisation, capabilities
and limits of an oscillatory system envisioned to act
as a biological clock is essential for subsequent inte-
grative modelling, synthesis, and implementation of a
corresponding frequency control system.
Bidirectionally coupled repressilators exhibit the
ability to synchronise their oscillatory signals by forc-
ing. It has been observed that arbitrary initial phase
shifts become compensated while an adaption of the
entire system to different initial frequencies of the sin-
gle oscillators spans a synchronisation window.
3 EXTERNAL
SYNCHRONISATION:
REPRESSILATOR AS CORE
OSCILLATOR
The repressilator can be seen as an advantageous tool
to conduct external synchronisation when embedded
as core oscillator into a frequency control system
based on the concept of phase-locked loop (Stensby,
1997), PLL for short. These systems adapt their oscil-
latory output signal to an external stimulus acting as
reference. In contrast to internal synchronisation, the
external stimulus is not affected. A biological exam-
ple is given by circadian clocks that harmonise their
oscillatory behaviour with the daily light-dark rhyth-
micity (Bell-Pedersen, 2005). Here, the light acts
as external stimulus. Fig. 7 illustrates the general
scheme of PLL. One or several coupled core oscil-
lators constitute its central part. The signal compara-
tor as downstream module determines the difference
between core oscillator output and external stimulus.
The phase shift between either signals is an ideal can-
didate to form an error signal able to adjust the core
oscillator. The error signal passes a global feedback
path along with damping and delay by dedicated low-
pass filters. Finally, the resulting smoothened signal
influences the core oscillator(s) by increasing or de-
creasing its frequency.
We expect to demonstrate that all functional mod-
ules required for a PLL control system can be imple-
mented as interacting reaction networks. Both mod-
ules, signal comparator and global feedback path, ef-
ficiently employ low-pass filters. Signal transduc-
tion cascades found in cell signalling networks are
a common biological motif to cover the functional-
ity of low-pass filters (Marhl et al., 2005). Here, a
focal protein alters its chemical state according to a
trigger signal. Here, a chemical state is specified by
addition or removal of phosphate groups to/from the
focal protein. In case of low-frequency triggers, the
subsequent modification of the chemical state can fol-
low. Along with increasing frequency of the trigger,
a threshold exists denoting that the reaction system is
now too slow to follow the trigger and ends up in a
steady state by means of a chemical equilibrium.
Having a chemical low-pass filter at hand, the
functionality of the global feedback path is com-
pletely covered. The signal comparator benefits from
low-pass filters to obtain the fundamental frequency
of both signals, core oscillator output and external
stimulus. Then, the phase shift between both signals
or the signal difference, respectively, can be extracted
by performing arithmetic operations. Reaction net-
works to this task are effectively feasible assuming
that substrate species concentrations encode operands
while product species concentrations (in steady state)
constitute the operational output (Hinze et al., 2009).
For example, the set of two reactions X
1
+ X
2
→ Y
and degradation Y →
/
0 in conjunction with mass-
action kinetics conducts a multiplication of the form
Y = X
1
(0) · X
2
(0) with initial concentrations X
1
(0)
and X
2
(0) as multipliers. Addition, non-negative sub-
traction, and division can be expressed in a similar
way. Altogether, this allows construction of a PLL
explicitly composed of reaction-diffusion networks.
SYNCHRONISATION OF BIOLOGICAL CLOCK SIGNALS - Capturing Coupled Repressilators from a Control Systems
Perspective
105
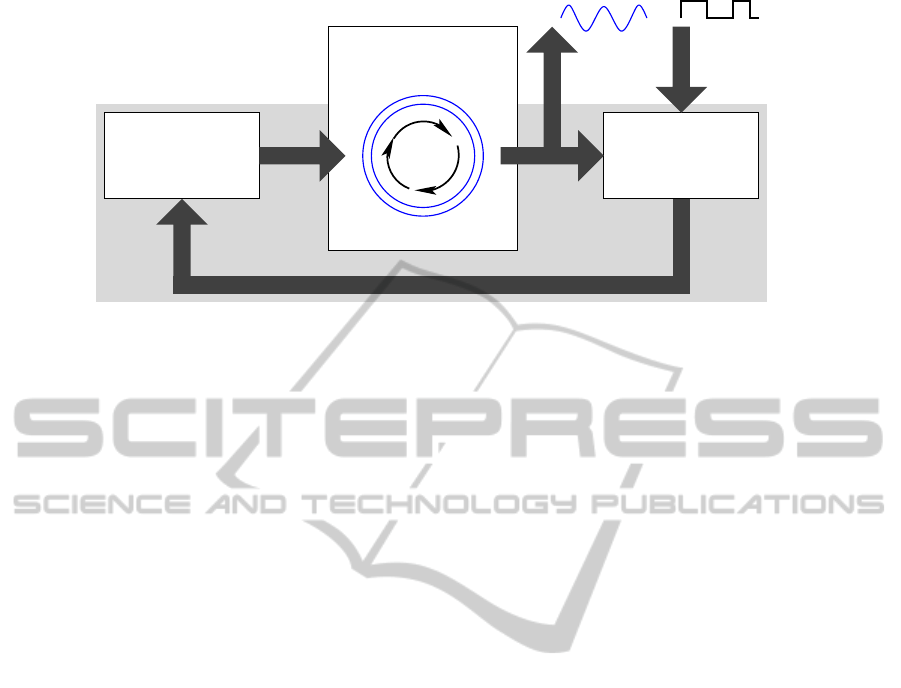
coupled
one or several
core oscillator(s)
local feedback(s)
global feedback path
damping and delay)
(loop filter for
affects
frequency
signal
tuning
signal comparator
frequency deviation)
(phase difference or
signal
output (reference)
stimuli
external
error
signal
Figure 7: General scheme of a frequency control system based on the concept of phase-locked loop (PLL). The system adapts
its oscillatory output signal to an external stimulus acting as reference for external synchronisation.
4 CONCLUSIONS
Bidirectionally coupled repressilators synchronise
their oscillatory signals by forcing. Arbitrary initial
phase shifts become compensated while adaption to
different initial frequencies spans a synchronisation
window. Coupled repressilators can be seen as a part
of a biological control system based on the concept
of phase-locked loops. Further research has been
directed to finalise the entire frequency control sys-
tem by integration of additional components for sig-
nal comparison and damping, demonstrated by low-
pass filters biologically implemented as specific sig-
nal transduction cascades. The simulations described
in this paper were carried out using Copasi (Hoops,
2006), statistical evaluation using [R].
ACKNOWLEDGEMENTS
We gratefully acknowledge funding from the German
Federal Ministry of Education and Research (project
no. 0315260A, Research Initiative in Systems Biol-
ogy).
REFERENCES
Bell-Pedersen, D. (2005). Circadian rhythms from multiple
oscillators: lessons from diverse organisms. Nat. Rev.
Genet., 6:544–556.
Elowitz, M. B. and Leibler, S. (2000). A synthetic oscil-
latory network of transcriptional regulators. Nature,
403:335–338.
Garcia-Ojalvo, J., Elowitz, M., and Strogatz, S. (2004).
Modeling a synthetic multicellular clock: repressila-
tors coupled by quorum sensing. Proc. Natl. Acad.
Sci. U.S.A., 101:10955–10960.
Granada, A. and Herzel, H. (2009). How to achieve fast
entrainment? the timescale to synchronization. PLoS
ONE 4, e7057.
Hinze, T., Fassler, R., Lenser, T., and Dittrich, P. (2009).
Register machine computations on binary numbers by
oscillating and catalytic chemical reactions modelled
using mass-action kinetics. International Journal of
Foundations of Computer Science, 20(3):411–426.
Hoops, S. (2006). COPASI – a COmplex PAthway SImula-
tor. Bioinformatics, 22:3067–3074.
Kuramoto, Y. (1984). Chemical oscillations, waves, and
turbulences. Springer.
Marhl, M., Perc, M., and Schuster, S. (2005). Selective reg-
ulation of cellular processes via protein cascades act-
ing as band-pass filters for time-limited oscillations.
FEBS Letters, 579(25):5461–5465.
Pikovsky, A., Rosenblum, M., and Kurths, J. (2001). Syn-
chronization: A universal con- cept in nonlinear sci-
ences. Cambridge University Press.
Russo, G. and di Bernardo, M. (2009). How to synchronize
biological clocks. Journal of Computational Biology,
16:379–393.
Stensby, J. (1997). Phase-locked loops. CRC Press.
Tanenbaum, A. S. and van Steen, M. (2001). Distributed
Systems: Principles and Paradigms. Prentice Hall In-
ternational.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
106
