
A SENSORIZED PLATFORM TO MONITOR AND REGULATE VAD
FUNCTIONALITIES
Wearable and Implantable Devices to Improve VAD Performances and Extend its
Field of Application
N. Taccini, M. Nannizzi, C. Ciaponi, P. Valdastri and P. Dario
CRIM-Lab, Scuola Superiore Sant’Anna, Pisa, Italy
Keywords:
Biomedical instrumentation, Implantable electronics, Electrical bio-impedance, Hemodynamics monitoring,
Health monitoring devices.
Abstract:
A novel system to control Ventricular Assist Device (VAD) is under study in the frame of the project SEN-
SORART (FP7-ICT-248763, funded by the European Commission). The SensorART platform will be open,
interoperable, extendable and VAD-independent and it will incorporate different hardware and software com-
ponents in order to improve both the quality of the patients’ treatment and the workflow of the specialists.
In the frame of this project a set of implantable sensors will be identified, developed and/or adapted for long
term implant, in order to gather all the necessary information to control the VAD functionalities by a wearable
hardware unit. Wearable sensors will be studied to assist the decision algorithms by gathering physiological
parameters using non invasive approach. Purpose of this paper is to give a brief description of the system under
study and describe the preliminary activity performed during the developing of the implantable and wearable
sensors. In particular, the evaluation and calibration of a novel catheter pressure sensor is reported and the
results of the feasibility of a tonometry wearable system is described.
1 INTRODUCTION
Heart failure (HF) is the most increasing cause of
death in Western Countries. For that reason, together
with the difficulty of having a sufficient number of
donor organs, it is recognized that the device-based
therapeutic approaches will assume an increasingly
important role in treating the growing number of pa-
tients with advanced heart failure, not only as bridge
to transplant, but also as destination therapy. In this
scenario the project SENSORART (FP7-ICT-248763,
funded by the European Commission) leads to the de-
velopment of a complete system composed by hard-
ware and software devices able to control the Ventric-
ular Assist Device. Scope of this paper is to describe
the performed activity in the study of the sensor mod-
ule.
2 MATERIALS AND METHODS
2.1 System Overview
The proposed platform will be able to vary the func-
tional parameter of the VAD to support patients with
chronic heart failure. It will also provide informa-
tion to healthcare professionals, allowing patients to
be treated at home without renouncing to accessing
high medical expertise. The main research and de-
velopment key modules of the integrated platform
will be: Sensor Module, Signal Acquisition Mod-
ule, Hardware Controller and Remote Control Frame-
work. Figure 1 depicts the described platform in prin-
ciple.
Most VAD controllers derive now all their infor-
mation by the pump’s power consumption, using this
information predominantly as monitoring (to detect
suction, low flow conditions, pump stoppage) and
to trigger alarms. The SensorART platform would
take advantage also from physiological signals ac-
quired by a mix of implantable and wearable sensors.
All the implantable and wearable sensors will wire-
lessly communicate with a wearable device. This unit
315
Taccini N., Nannizzi M., Ciaponi C., Valdastri P. and Dario P..
A SENSORIZED PLATFORM TO MONITOR AND REGULATE VAD FUNCTIONALITIES - Wearable and Implantable Devices to Improve VAD
Performances and Extend its Field of Application.
DOI: 10.5220/0003132103150318
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 315-318
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
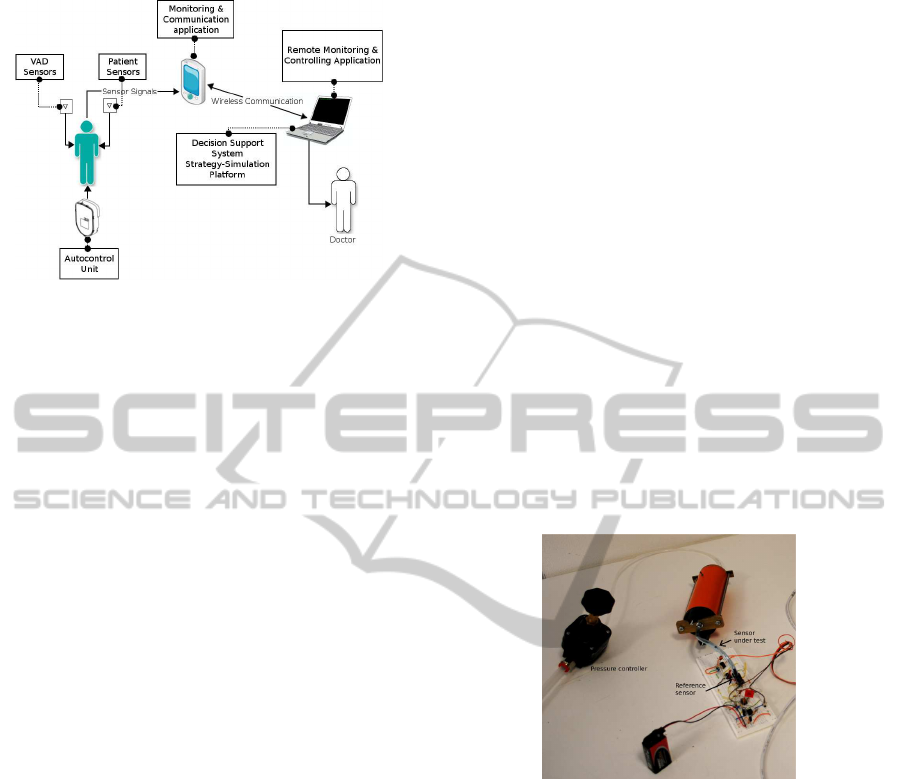
Figure 1: SensorART platform.
will allow to auto-adjust the blood flow provided by
the VAD to the patient’s heart according to signals
coming from physical and physiological sensors, by
means of a wireless link to the VAD actuators. More-
over, this unit will monitor the energy consumption,
as well as the VAD functionality, thus generating the
appropriate crucial and vital alert messages. Real-
time adjustable thresholds and control parameters will
be a peculiar feature of the proposed platform. The
auto-regulation control algorithm will be fully ad-
justable to the patient’s condition and will incorpo-
rate context-aware techniques in order to meet the pa-
tient’s needs according to his/her status. Physiologi-
cal sensors are usually invasive, with a complex pack-
age, and not reliable in long term implants. Within
the SensorART project, wearable approach will be ex-
ploited together with the implantable ones in order to
minimize risk of failures. This paper will describe
the actual stage of the project regarding the develop-
ment of implantable (see 2.2) and wearable sensors
(see 2.3).
2.2 Implantable Sensors
Main physiological sensors described in literature as
suitable for LVADs monitoring are: oxygen satura-
tion sensor (Nakamura et al., 2000), pressure sensor
(Bullister et al., 2001), flow rate sensor (Waters et al.,
1999), and acceleration (Maeda et al., 1988) sensors.
The first stage of the development of the implantable
devices consists in a screening of the cited parame-
ters during tests on laboratory mock-up and in-vivo,
in implantable or wearable version. After these trials,
the long term implant issue will be faced and a proper
package will be studied. Thanks to the collaboration
with clinical experts it has been possible to determine
a set of variables to be monitored by implantable sen-
sors in order to monitor the residual heart functional-
ities, the VAD function and the patient’s reaction to
the treatment. These parameters are: left atrial pres-
sure, aortic blood flow rate, aortic pressure, blood
pressure and flow in the VAD cannula, pulmonary
artery or right ventricular pressure. A previous work
of the authors (Valdastri et al., 2008) describes a wire-
less implantable platform for in vivo monitoring. A
similar approach has been used to integrate an inno-
vative pressure sensor made by STMicroelectronics,
that is still in evaluation phase (P30PCB). After the
necessary characterization phase, this sensor will be
encapsulated in a custom made catheter to perform
the first session of tests. An electronic front-end has
been developed in order to acquire the signal derived
by the sensor inserted in a controlled pressure cham-
ber. The output of the sensor needs a differential read-
ing that is performed by an instrumentation amplifier
(AD623N). The pre-amplified signal is then low pass
filtered by an RC filter (band pass at 20 Hz) and am-
plified by a non inverting amplifier, realized with a
CMOS operational amplifier (LMC6482).
Figure 2 shows the system realized for the cali-
bration using a reference sensor (MPX5050 GP by
Freescale semiconductor). The acquisition circuit,
Figure 2: Calibration system for the pressure system.
based on a wireless microcontroller (CC2430 by
Texas Instruments) can communicate wirelessly (zig-
bee ready) or by USB connection to a PC. A graphi-
cal interface, acquires and stores data with a sampling
rate of 25 Hz by both test and reference sensors. The
pressure in the chamber has been varied step by step
in the range from 8 kPa to 35 kPa. The range has been
chosen taking into account the final field of applica-
tion, that is the radial artery pressure measurement.
The measures have been performed on both growing
and decreasing pressures, to evaluate the hysteresis of
the signal. Tests have been performed on 3 sensors
to assure the reproducibility. Three complete cycles
have been acquired for each sensor with pressure set
as following: 8 kPa, 16 kPa, 24 kPa, 32 kPa and 35
kPa. The calibration curves have been approximated
by polynomial fitting by using Matlab software. Also
the stability of each sample has been tested, by means
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
316
of long acquisition (30 min) at stable pressure (8 kPa,
24 kPa and 35 kPa).
2.3 Wearable Monitoring Devices
One of the most relevant physiological signals to be
monitored is arterial blood pressure. This can be mon-
itored by either implantable or wearable sensors. In
particular, radial tonometry (Chemla et al., 2008), has
been explored to derive the central arterial pressure
by means of non-invasive technique. An example of
such type of device is the BPro by HealthSTATS
1
. In
the frame of the project a wearable system with wire-
less communication will be developed. In order to
monitor the central pressure 24/7 and pulse by pulse,
the system will be designed to assure the stability of
the signal in case of patient’s arm movements. Wear-
ability, ease of use and ergonomics will be carefully
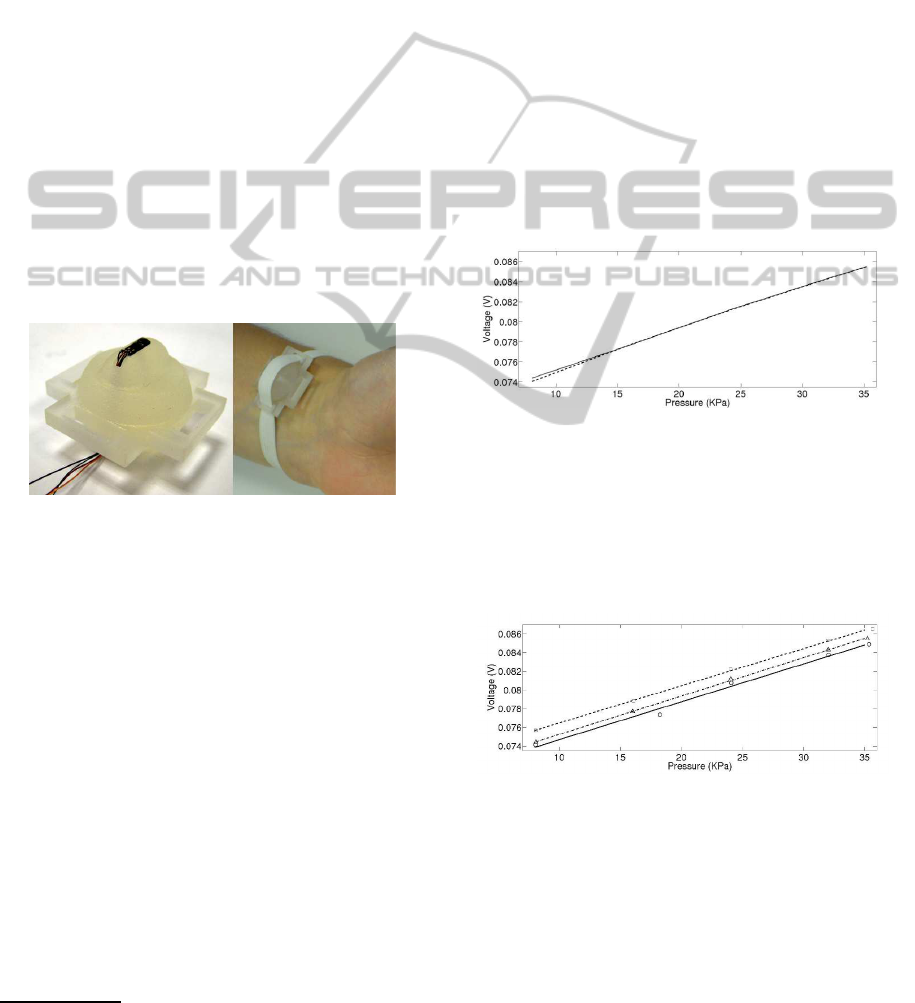
considered. An ad hoc support has been produced by
fast prototyping with a 3D printer (Invision SI2 by
Inition), to investigate this technique. Figure 3 shows
the support and its placement on the wrist for the pre-
liminar tests.
Figure 3: P30PCB STM pressure sensor and the support for
the tonometry device (left), placement of the system on the
radial artery (right).
The support is designed in order to assure the arte-
rial applanation, while reducing the discomfort. Ap-
propriate slots allow to use an elastic ribbon to fix
the support on the wrist. It is possible to regulate
the applied force on the skin by the protrusion with
the inserted sensor simply by varying the tension of
the elastic band. The pressure sensor is the STM
P30PCB described in 2.2. An additional sensing sys-
tem, that will be developed, regards impedance car-
diography (ICG). Also in this case either implanted
or wearable strategy can be pursued. ICG can moni-
tor the fluid content of the trunk, thus acting as heart
failure detector (Bour and Kellett, 2008), or hemody-
namics variables such as: stroke volume (SV), stroke
index (SI), thoracic fluid content (TFC), systemic vas-
cular resistance (SVR), systemic vascular resistance
index (SVRI), acceleration index (ACI), velocity in-
1
www.healthstats.com/en/bpro.html
dex (VI), pre-ejection period (PEP), left ventricular
ejection time (LVET) and systolic time ratio (STR).
The under study wearable system performs a tho-
racic bioimpedance measurement by means of four
electrodes placed on the thorax at the diaphragm level.
The development is in an early stage and in vivo tests
are scheduled to evaluate innovative approaches.
3 RESULTS AND DISCUSSION
3.1 Characterization of the Pressure
Sensor
Tests have been performed on 3 samples of the
P30PCB by using the system described in 2.2, in or-
der to calibrate and evaluate the pressure sensor. The
figures 4 and 5 show the results of the tests in term of
hysteresis and reproducibility.
Figure 4: Typical hysteresis plot (dotted line for decreasing
pressure, full line for increasing pressure).
The average hysteresis has been evaluated by cal-
culating the maximum difference, in term of percent-
age of the full scale of the test, between the curves rel-
ative to growing and decreasing pressure. The mean
among all tests of so calculated hysteresis is 0.33%.
Figure 5: Experimental values (triangles, circles and
squares) and fitting lines for the three tested pressure sen-
sors.
The table 1 collects the slope, the zero value and
the coefficient of determination (R
2
) for each calibra-
tion curve, calculated by linear polynomial fitting us-
ing Matlab software.
The coefficient of determination is very close to
one, so the sensors can be considered linear. The
differences in the zero values among the calibration
A SENSORIZED PLATFORM TO MONITOR AND REGULATE VAD FUNCTIONALITIES - Wearable and
Implantable Devices to Improve VAD Performances and Extend its Field of Application
317
Table 1: Coefficients for the calibration curve of the three
sensors.
Slope Zero value R
2
S1 0,0004 0,0706 0,9987
S2 0,0004 0,0725 0,9989
S3 0,0004 0,0711 0,9874
curves of the samples make necessary the calibration
of each implantable device. This is not a problem for
the field of application of the SensoART platform.
As example of the stability test, figure 6 represents
a typical sensor output at 24 kPa for 30 minutes. All
the sensors showed a high stability.
Figure 6: Typical sensor output at 24 kPa.
3.2 Tonometry
Some preliminary acquisitions have been performed
with the wireless tonometry device described in 2.3,
placed as in figure 3. The gathered signal depicts the
pressure wave of the radial artery of the wrist, as de-
picted in figure 7.
3.3 Conclusions
SensorART project aims to develop a disrupting sys-
tem that will change substantially the application of
VAD in heart failure treatment. The activity presented
in this paper is a preliminary step towards this chal-
lenging result. The evaluation of the P30PCB sensor
as candidate for the pressure implantable catheter and
the tonometry system gave positive results. The lin-
ear calibration curve and the reproducibility of this
sensor, together with its small dimensions allow to in-
tegrate it in a catheter to start the evaluation in labora-
tory mock-up and in-vivo test. Next steps will be the
design and production of a custom catheter and the
study for the package for a long term implant. The
preliminary tonometric signal, acquired by the proto-
type system, demonstrated the feasibility of this ap-
proach. Next development of this system will lead to
the improvement of the reliability during movement
and to make easier the wearing process.
Figure 7: Pressure signal of the radial artery gathered by the
tonometry device.
ACKNOWLEDGEMENTS
The research leading to these results has received
funding from the European Community’s Seventh
Framework Programme (FP7/2007-2013) under grant
agreement num. 248763 (SensorART Project).
The pressure sensors under test have been kindly
provided ahead of commercialization by STMicro-
electronics.
REFERENCES
Bour, J. and Kellett, J. (2008). Impedance cardiography:
a rapid and cost-effective screening tool for cardiac
disease. In Eur J Intern Med., volume Epub 2008 Feb
11.
Bullister, E., Reich, S., and Entremont, P. (2001). A blood
pressure sensor for long-term implantation. artificial
organs. In Artificial Organs, volume 25(5), pages
376–379.
Chemla, D., Teboul, J., and Richard, C. (2008). Non inva-
sive assessment of arterial pressure. In Curr Opin Crit
Care, volume 14(3), pages 317–321.
Maeda, K., Chinzei, T., Imachi, K., and et al. (1988). Pre-
dictive control by physical activity rate of a total ar-
tificial heart during exercise. In ASAIO Transaction,
volume 34, pages 480–484.
Nakamura, M., Homma, A., Tatsumi, E., and et al. (2000).
Mixed venous oxygen saturation as a promising pa-
rameter for the physiologic control of the artificial
heart. In ASAIO J, pages 761–766.
Valdastri, P., Rossi, S., Menciassi, A., Lionetti, V., Bernini,
F., Recchia, F., and Dario, P. (2008). An implantable
zigbee ready telemetric platform for in vivo monitor-
ing of physiological parameters. Sensors and Actua-
tors, Volume 142:369–378.
Waters, T., Allaire, P., Tao, G., and et al. (1999). Motor
feedback physiological control for a continuous flow
ventricular assist device. In Artificial Organs, volume
23(6), pages 480–487.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
318
