
TOWARDS A NEW HOMOGENEOUS IMMUNOASSAY
FOR GONADOTROPIN-RELEASING HORMONE BASED ON
TIME-RESOLVED FLUORESCENCE ANISOTROPY
Peter D. Dowd
Department of Bioengineering, University of Strathclyde, Wolfson Centre, 106 Rottenrow, Glasgow, U.K.
Jan Karolin, Carol Trager-Cowan, David J. S. Birch
Department of Physics, Centre for Molecular Nanometrology, University of Strathclyde, SUPA
John Anderson Building, 107 Rottenrow, Glasgow, G4 0NG, U.K.
William H. Stimson
Strathclyde Institute of Pharmacy and Biomedical Science, University of Strathclyde
Royal College, 204 George Street, Glasgow, U.K.
Keywords: Homogeneous immunoassay, Time-resolved fluorescence depolarisation, Gonadotropin-releasing hormone.
Abstract: A new method for detecting gonadotropin-releasing hormone type 1 (GnRH-1) is proposed. The method is
based on a fluorescence polarisation immunoassay where the GnRH-1 vies for the binding site on the anti-
GnRH (antibody 7B10.1D10) with a labelled peptide consisting of a fragment of GnRH labelled with
fluorescein. Time-resolved anisotropy decays indicate increased correlation times for the labelled fragment
in the presence of 7B10.1D10 compared with the correlation times for the labelled peptide fragment alone.
When GnRH-1 is added to the solution of the labelled peptide in the presence of 7B10.1D10 it has the effect
of reducing the correlation times. The results demonstrate the possibility of introducing the labelled
fragment as a competitor to the antigen in the fluorescence polarisation immunoassay for GnRH-1 and,
more generally, in the case where there is a disadvantage in labelling the antigen, such as GnRH-1, with a
fluorescent probe.
1 INTRODUCTION
In this report we describe a potential method for
detecting gonadotropin-releasing hormone type-1
(GnRH-1) using a fluorescence polarisation
immunoassay (FPI). Conventionally in FPI’s, an
antigen labelled with a fluorescent probe is displaced
from a specific antibody binding site by an
unlabelled antigen. In this method we have replaced
the labelled antigen with a labelled ‘fragment’ of the
antigen thus eliminating the problematic issues
surrounding GnRH-1.
GnRH-1 (the mammalian form of GnRH) is a
hypothalamic hormone and it plays a fundamental
role in the events leading to the synthesis and release
of leuteinising hormone (LH) and follicule
stimulating hormone (FSH) into the circulatory
system (Conn & Crowley, 1991). GnRH is essential
in reproductive function and expression of GnRH
has even been reported in tissues such as the ovary,
breast and prostate (Chen et al, 2002). Although
GnRH influences conditions such as GnRH-
dependent precocious puberty, the expression of
GnRH in tissue areas outside the hypothalamic
region and in the immune system is not fully
understood. Nevertheless, due to the suggestion that
GnRH expression maybe a possible marker for
certain tumours and the success of GnRH agonists
used in a variety of disorders, monitoring the levels
and effects of GnRH are of significant clinical
interest (Tanriverdi et al, 2004).
FPI’S are appealling for medical diagnostic and
point of care testing (POC) applications because
they can be performed in a homogeneous format and
184
Dowd P., Karolin J., Trager-Cowan C., S. Birch D. and Stimson W..
TOWARDS A NEW HOMOGENEOUS IMMUNOASSAY FOR GONADOTROPIN-RELEASING HORMONE BASED ON TIME-RESOLVED FLUORES-
CENCE ANISOTROPY.
DOI: 10.5220/0003152001840188
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 184-188
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

with relatively few preparation steps (Price, 2001).
Using antigens labelled with fluorescent markers,
FPI’s rely on the difference in anisotropy (or
polarisation) measurements of a labelled antigen
compared with the anisotropy of the labelled antigen
in association with an antibody (Terpetsching et al,
1994). More specifically, in FPI’s the emission
from the free labelled antigen must be depolarised.
Thus the rotational correlation time for the antigen
must be much shorter than that of the probe.
Therefore, when this method is applied to antigens
with a low molecular weight, fluorescein with a
lifetime near 4ns is typically chosen as the
fluorescent probe (Spencer et al, 1973). In
comparison with the free labelled antigen the
polarisation will increase when the labelled antigen
binds to the antibody.
In the case of GnRH-1, labelling demands either
the addition or removal of an amino acid. The
addition of an amino acid can lead to an alteration in
conformation due to the increase in size and change
in charge. This is less likely to occur if an amino
acid is removed. Therefore, in an attempt to preserve
the antibody binding site which is in the centre of
the peptide (Khan et al, 2003), we describe a method
that incorporates the 9-amino acid fragment of
GnRH-1 labelled with fluorescein to act as a
replacement for the conventional labelled antigen in
the assay. Furthermore, we use time-resolved
anisotropy techniques in an effort to reveal any
underlying issues that may arise from using the
labelled fragment in place of the labelled antigen.
Fluorescence spectroscopy is a technique that offers
sensitivity down to a single-molecule level, with a
signal-to-niose ratio that improves with
measurement duration, and is therefore well placed
for the development of ultrasensitive biosensors
such as fluorescence-based immunoassays.
2 THEORY
The timed-resolved fluorescence anisotropy, r(t), is
an angular correlation function that conveniently is
defined as (Lakowicz, 2006)
() ()
()
() 2 ()
vv vh
vv vh
I
tIt
rt
I
tIt
−
=
+
(1)
where I
vv
(t) and I
vh
(t) are the recorded fluorescence
intensities at time t following an excitation event at t
= 0, and where the first and second subscript refers
to the orientation of the excitation and emission
polariser respectively. In the simplest case, with a
fluorophore rigidly attached to a macromolecule that
undergoes free isotropic rotational diffusion, the
anisotropy is described by a mono-exponential
decay function
0
() exp
g
lobal
t
rt r
φ
⎛⎞
=−
⎜⎟
⎜⎟
⎝⎠
(2)
The time-zero ansiotrpoy r
0
can take the maximum
value 0.4 for one-photon excitation (assuming the
transition dipoles for absorption and emision are
parallel). The correlation time
φ
global
is related to the
hydrodynamic volume, V, of the macromoleule and
can be calculated form the Stokes-Einstein Debye
relationship
global
b
V
kT
η
φ
= (3)
wher
η
is the viscosity of the solvent, k
b
Boltzmann
constant and T the absolute temperature.
It is often observed when analysing the
fluorescence anisotropy recorded from fluorescently
labelled macromolecules, for example proteins, that
the anisotropy expression is given by a bi-
exponential decay law
12
() exp exp
local global
tt
rt b b
φφ
⎛⎞
⎛⎞
=−+−
⎜⎟
⎜⎟
⎜⎟
⎝⎠
⎝⎠
(4)
The correlation times are then often interpreted to
reflect on a local wobbling motion of the
fluorophore in its binding site,
φ
local
, and an overall
rotational diffusion of the macromolecule,
φ
global
. If
φ
global
>>
τ
, it will not be resolved in a time
correlated single-photon counting experiment, and
will only be evident by a plateau value of value r
∞
. If
there is also a fraction of free fluorophore that is not
bound to the macromolecule, as in our case, there
might be a fraction of the antigen that is not bound
to the antibody and then the anisotropy expression
reads
12
() exp exp
free local
tt
rt b b r
φφ
∞
⎛⎞
⎛⎞
=
−+ − +
⎜⎟
⎜⎟
⎜⎟
⎝⎠
⎝⎠
(5)
In the present work anisotropy data was analysed
according to equation (5).
TOWARDS A NEW HOMOGENEOUS IMMUNOASSAY FOR GONADOTROPIN-RELEASING HORMONE BASED
ON TIME-RESOLVED FLUORESCENCE ANISOTROPY
185

Figure 1: The pyroGlu on GnRH-1 is replaced by a fluorescein that is attached to the peptide backbone of the peptide
fragment by a caproic acid linker.
3 MATERIALS AND METHODS
GnRH-1 is a decapeptide with the amino acid
sequence pyroGlu
1
-His
2
-Trp-
3
-Ser
4
-Tyr
5
-Gly
6
-Leu
7
-
Arg
8
-Pro
9
-Gly
10
-NH
2
. GnRH-1 (cat. no. L7134) and
[des-pGlu
1
]-RH-LH (cat. no. L8762) were purchased
from Sigma-Aldrich, Inc. The labelled fragment
([des-pGlu
1
]-RH-LH-Acp-FITC) was purchased
from GL Biochem (Shanghai) Ltd (Figure 1).
Antibody specific to GnRH-1 was produced from
the monoclonal antibody secreting cell line,
7B10.1D10, by using standard cell culture
techniques (Khan et al, 2003). From experiment, the
antibody was found to have an IgG
1
isotype and was
shown to bind to both GnRH-1 and [des-pGlu
1
]-RH-
LH by ELISA. The antibody was also shown to bind
to Protein L agarose resin purchased from Pierce
(cat. no. 20510) thus implying that the antibody has
kappa light chains and, more specifically, VκI light
chains. The Protein L agarose resin was used in the
antibody purification procedure following the Pierce
protocol. All solutions for fluorescence
measurements were prepared using a 20 mM sodium
phosphate buffer, pH 7.4.
Time-resolved fluorescence and anisotropy
decay time measurements were performed using the
time-correlated single photon counting technique
(TCSPC) on a FluoroCube (Horiba Jobin Yvon IBH
Ltd, Glasgow), (Apperson et al, 2009). The
excitation source was a 482 nm PicoBrite running at
10 MHz repetition rate and with an instrumental full
width half maximum of 50 ps. The emitted
fluorescence was observed on a TBX-04 detector
and data was analysed in IBH DAS6 software suite.
In the first instance the purpose of the
experiment was to compare the time-resolved
fluorescence measurements of the labelled fragment
with those from the labelled fragment in the
presence of the antibody 7B10.1D10 to confirm that
the altered GnRH structure could be captured by the
antibody. Secondly, it was to assess that the effect
could be reversed by adding GnRH-1 to the mixture
(Figure 2).
Figure 2: Schematic illustrating the initial bulk solution
condition where it is most likely that one labelled
fragment is bound to the antibody and the end bulk
solution condition where the antibody binding sites are
most likely to be occupied by GnRH-1.
The time-resolved fluorescence, recorded in the
magic angle condition, and anisotropy decays of six
different mixtures (Table 1) were measured.
Solution A contained the labelled fragment and
solution B contained the labelled fragment in the
presence of antibody where the number of potential
antibody binding sites is much greater than the
number of labelled fragment molecules in the
solution. GnRH-1 is added to the mixture giving
solutions C-F.
Table 1: The concentrations of antibody, labelled fragment
and GnRH-1 for each mixture.
sample [7B10.1D10]
μM
[labelled fragment]
nM
[GnRH-1]
M
A - 4.94 -
B 0.33 1.97 -
C 0.33 1.10 0.56×10
-6
D 0.11 0.66 1.13×10
-3
E 0.11 0.66 1.69×10
-3
F 0.11 0.65 5.61×10
-3
Fl
+
U
+
Fl
Labelled fragment
bound to antibody
GnRH-1
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
186
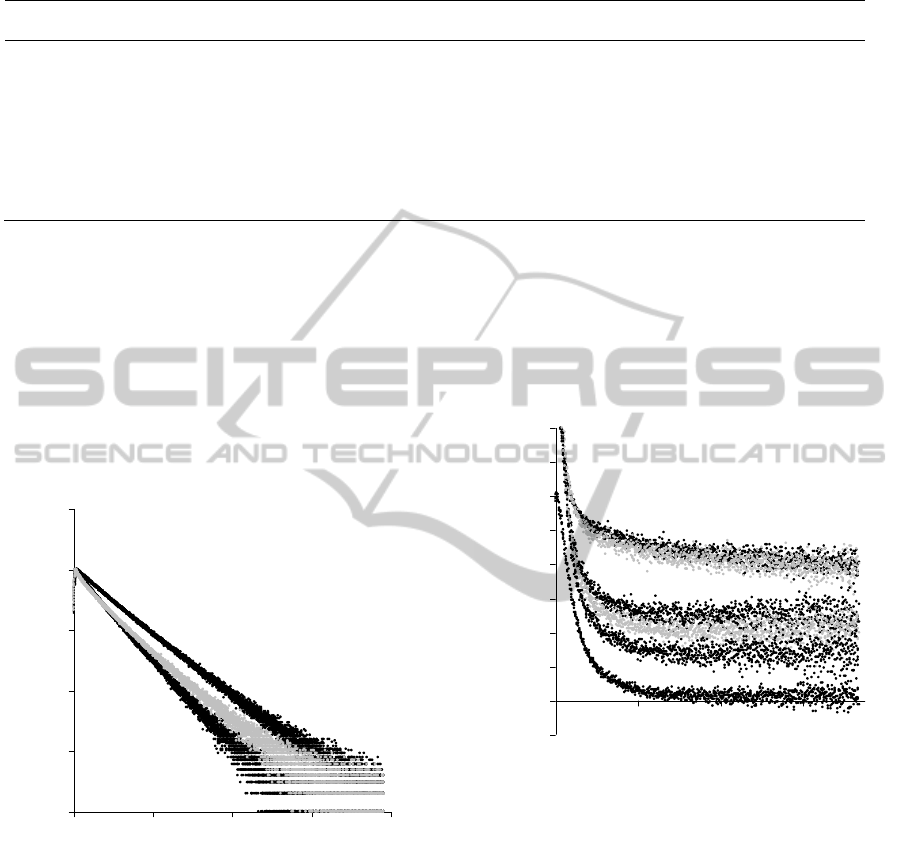
Table 2: Lifetimes resolved into two components
τ
1
and
τ
2
and also anisotropies at r
0
, r
∞
and correlation times
φ
1
,
φ
2
for all
solutions.
sample
τ
1
/ ns
τ
2
/ ns χ
2
r
0
φ
1
/ ns
φ
2
/ ns r
∞
χ
2
A 0.76 (8.94%) 2.88 (91.06%) 1.03 0.34 0.22 (29.7%) 0.59 (70.3%) 0.00 1.00
B 1.17 (4.82%) 4.09 (95.18%) 1.03 0.49 1.70 (78.1%) 0.16 (21.9%) 0.20 1.06
C 0.93 (5.46%) 3.99 (94.54%) 1.17 0.37 0.96 (67.7%) 0.24 (32.3%) 0.21 1.10
D 0.61 (10.19%) 3.25 (89.71%) 1.43 0.31 0.00 (0.0%) 0.41 (100.0%) 0.13 1.18
E 0.68 (10.55%) 3.26 (89.45%) 1.43 0.36 0.14 (9.4%) 0.43 (90.6%) 0.10 0.94
F 0.90 (13.98%) 3.21 (86.02%) 1.21 0.37 0.12 (10.8%) 0.47 (89.2%) 0.07 1.03
4 RESULTS
On examining the time-resolved intensity decay
times of each mixture it is evident that the decay
time must be resolved into two or more components.
Analysis for two components is shown in Table 2.
The trend is more clearly shown qualitatively in the
decay curves.
1
10
100
1000
10000
100000
0 10203040
Time /ns
Counts
Figure 3: Time-resolved decays showing qualitatively the
difference between decay times of the labelled fragment
(A), the labelled fragment in the presence of antibody (B)
and when an excess of GnRH-1 is added to the solution
(D).
The most dramatic changes are shown in Figure
3 where the greatest difference in the decay time is
seen between the labelled fragment (A) and the
labelled fragment in the presence of antibody
(B).When an excess of GnRH-1 is added to the
solution (D) the mean decay time is reduced and the
photophysics is also altered.
A similar pattern is repeated in the time-resolved
anisotropy measurements and the greatest change in
anisotropy occurs between the labelled fragment (A)
and the labelled fragment in the presence of
antibody (B) (Figure 4).
-0.05
0
0.05
0.1
0.15
0.2
0.25
0.3
0.35
0.4
0246
Time /ns
Anisotropy
Figure 4: Time resolved anisotropy decays for all
solutions. The anisotropy reduces as more GnRH-1 is
added to the solution (C-F).
As more GnRH-1 is added to the solution the
anisotropy falls suggesting that more of the labelled
fragment is spending more time free in solution than
bound to the antibody thus revealing the competition
between the labelled fragment and the GnRH-1 for
the 7B10.1D10 binding sites. Time-resolved
anisotropy decay measurements reveal information
about the rotational motion of the labelled peptide
molecules. Again, in almost all cases there are two
significant contributions to the rotational motion but
no attempt has been made as yet to explain the
results quantitatively.
A
B
D
A
B
D
E
F
C
TOWARDS A NEW HOMOGENEOUS IMMUNOASSAY FOR GONADOTROPIN-RELEASING HORMONE BASED
ON TIME-RESOLVED FLUORESCENCE ANISOTROPY
187

5 CONCLUSIONS
As a first step in designing an FPI for GnRH-1 we
used time-resolved fluorescence techniques (where
the antigen GnRH-1 competes with a synthetic
labelled fragment for the binding sites on the
specific GnRH-1 antibody). We demonstrated
qualitatively that the labelled fragment can bind to
the antibody and that competition between the
labelled fragment and the GnRH-1 for the binding
sites on the antibody 7B10.1D10 does occur.
ACKNOWLEDGEMENTS
This work was supported by a grant from the
EPSRC to the University of Strathclyde Medical
Devices Doctoral Training Centre.
REFERENCES
Apperson, K., Karolin, J., Martin, R. W., Birch, D. J. S.,
2009 Nanoparticle Metrology Standards Based on
Time-resolved Fluorescence Anisotropy of Silica
Colloids. Meas. Sci. Technol. 20, 025310
Chen, A., Kaganovsky, E., Rahimipour, S., Ben-Aroya,
N., Okon, E., Koch, Y., 2002 Two Forms of
Gonadotropin-releasing Hormone (GnRH) are
Expressed in Human Breast Tissue and Over
expressed in Breast Cancer: A Putative Mechanism for
the Antiproliferative Effect of GnRH by Down-
Regulation of Acidic Ribosomal Phosphoproteins P1
and P21. Cancer Research. 62, 1036–1044
Conn, P. M., Crowley, W. F., Jr. 1991 Gonadotropin
releasing Hormone and its Analogues. N. Engl. J.
Med. 324, 93-103
Khan M. A. H., Ferro V. A., Stimson W. H. 2003 Use of a
Highly Specific Monoclonal Antibody Against the
Central Variable Amino Acid Sequence of
Mammalian Gonadotropin Releasing Hormone to
Evaluate GnRH-1 Tissue Distribution Compared with
GnRH-1 Binding Sites in Adult Male Rats. AJRI. 49,
239-248
Lakowicz J. R., 2006 Principles of Fluorescence
Spectroscopy, 3rd ed. Kluwer Academic/Plenum
Publishers, New York City, New York
Price, C. P., 2001 Clinical Review: Point of Care Testing.
BMJ, 322, 1285-1288
Spencer, R. D., Toledo, F.B., Williams, B.T., and Yoss,
N.L 1973 Design, Construction, and 2 Applications
for an Automated Flow-cell Polarization Fluorometer
with Digital Read Out-Enzyme-Inhibitor (Antitrypsin)
Assay and Antigen-antibody (Insulin-insulin
Aantiserum) Assay. Clin. Chem. 19, 838-844
Tanriverdi, F., Silveira, L., Ganzalez-Martinez, D., Hu,Y.,
Bouloux, P., Kelestimur, F. 2004 Gonadotropin-
releasing Hormone Type-I (GnRH-1) Expression in
Peripheral Lymphocytes Aand Possible Immune
Action. Erciyes Tıp Dergisi (Erciyes Medical
Journal). 26 (3), 105-112
Terpetsching, E., Szmacinski, H., Lakowicz J. R., 1995
Fluorescence Polarization Immunoassay of a High-
Molecular-Weight Antigen Based on a Long-Lifetime
Ru-Ligand Complex. Anal. Biochem. 227, 140-147
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
188
