
SIGNAL QUALITY ASSESSMENT FOR CAPACITIVE ECG
MONITORING SYSTEMS USING BODY-SENSOR-IMPEDANCE
Stephan Heuer, Sebastian Chiriac, Malte Kirst
FZI Research Center for Information Technology, Haid-und-Neu-Str. 10-14, 76131 Karlsruhe, Germany
Adnene Gharbi, Wilhelm Stork
Institute for Information Processing Technology, Karlsruhe Institute of Technology, Karlsruhe, Germany
Keywords:
Capacitive ECG measurement, Unobtrusive ECG measurement, Non-contact ECG, Active electrodes, Artifact
detection, Signal quality, Context signals, QRS detection, Adaptive filtering.
Abstract:
Contactless capacitive ECG measurement is an unobtrusive way of acquiring cardiovascular data. However,
movement artifacts present a common problem with this technique. A means of assessing signal quality and
confidence is therefore desirable. In this paper we present a capacitive ECG measurement system with an
integrated module that constantly monitors the electrode-body-impedance. Moreover, we present a method
to derive an artifact level signal from this electrode-body-impedance that can be used to estimate the signal
quality of the capacitive ECG measurement. First results of measurements with this system are shown.
1 INTRODUCTION
Continuous cardiovascular health monitoring systems
enable a wide range of applications both in the do-
main of physiological long-term monitoring and in
psychophysiological monitoring. Both fields of appli-
cation require unobtrusive system concepts, that are
for the most part realized as wearable devices and
smart clothing or as ambient unobtrusive sensor sys-
tems. Examples for such systems can be found in
(Park et al., 2006; Lamparth et al., 2009; Lim et al.,
2006).
The amount of data acquired with said systems is
often large, especially in long-term applications, de-
manding automatic (pre-)analysis. Reliable accurate
analysis is yet difficult to achieve due to artifacts re-
sulting from motion during daily routine activities.
Additionally, there is often a trade-off between sig-
nal quality and sensor integration aspects. Taking this
into consideration, system design must not only con-
sider sensor development for the respective applica-
tion but also requires tight integration of well adapted
algorithms for automatic signal analysis.
In ECG monitoring tasks, artifacts from exter-
nal noise and motion are the most common fac-
tors that impair signal quality. Reliable detection of
R-peaks, for example as a necessary step in heart
rhythm analysis, is not given during intervals with
artifacts, leading to wrong detection results. This
is especially a problem with contactless, capacitively
coupled ECG systems, specifically when coupling is
weak and body-sensor-distance is not constant, as for
example in chair-integrated solutions (Aleksandrow-
icz et al., 2007). It is therefore desirable to improve
the detection accuracy in automatic ECG analysis for
contactless ECG measurement systems.
2 RELATED WORK
Using a mobile ECG measurement system with gal-
vanic dry electrodes, (Ottenbacher et al., 2008) simul-
taneously acquire ECG and electrode-skin-impedance
data (as well as acceleration data). In order to validate
the results a reference ECG signal with wet electrodes
is recorded in parallel.
They propose a method to improve an automatic
QRS detector by calculating an artifact level from the
recorded electrode-skin-impedance using adaptive fil-
tering and post-processing. Thus they are able to
mark artifact regions in the ECG signal and exclude
them from automatic detection, considerably decreas-
ing false positive and false negative QRS detections.
Adapting parts of this method to a contactless
454
Heuer S., Chiriac S., Kirst M., Gharbi A. and Stork W..
SIGNAL QUALITY ASSESSMENT FOR CAPACITIVE ECG MONITORING SYSTEMS USING BODY-SENSOR-IMPEDANCE.
DOI: 10.5220/0003160004540458
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2011), pages 454-458
ISBN: 978-989-8425-35-5
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

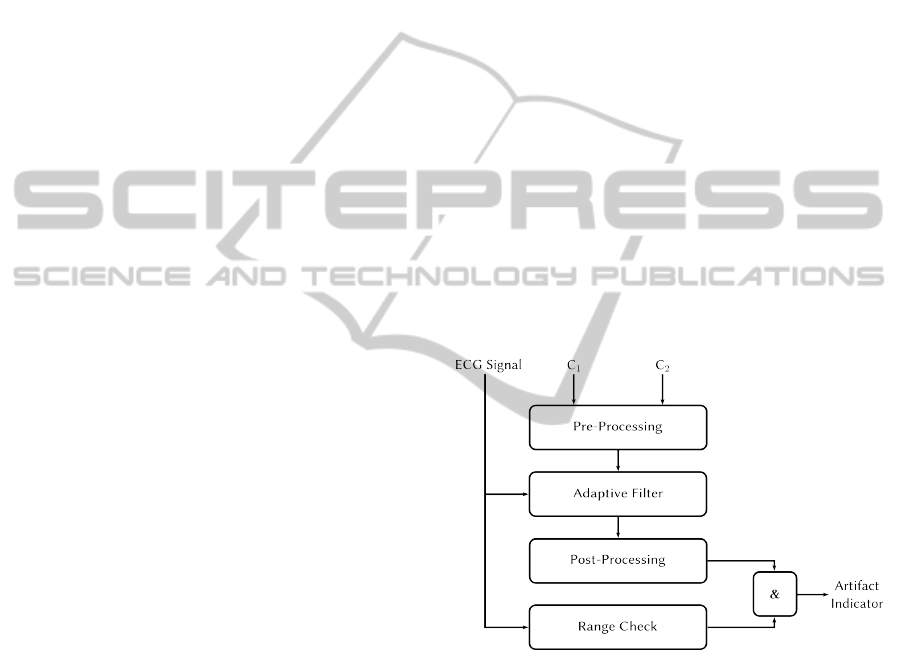
Figure 1: Block diagram of the developed contactless ECG
system with integrated capacitance measurement module.
The textile electrode C
ex
carries the excitation signal gen-
erated by the AD7152. Body movements generate capaci-
tance variations that are sensed by C
1
and C
2
.
ECG-system in an air-plane seat, (Schumm et al.,
2009) propose a method to predict the signal quality
of a contactless ECG recording. A number of sta-
tistical measures derived from the ECG signal itself
as well as additional pressure sensors attached to the
back of the capacitive electrodes are used as features
to derive a quality signal for the measurement.
3 METHOD
The most prominent source of artifacts in capacitive
ECG measurement is the relative movement between
the body and the capacitive electrodes. This changes
the capacitive coupling between sensor and body, re-
sulting in a voltage peak in the signal if a voltage
difference between the subject and the measurement
system exists. Additionally, CMRR of the differen-
tial stage is reduced, because a mismatch in source
impedances occurs.
As impedance between sensor and body changes
with movement, we propose a method to monitor this
impedance and use it as an indicator for the quality of
the sensor-body-contact. We therefore present an un-
obtrusive measurement system with capacitive ECG
electrodes that records the sensor-body-capacitance
of each electrode in addition to a differential capa-
citive ECG lead.
With these signals we derive an artifact indicator
signal similar to (Ottenbacher et al., 2008) that can
be used in conjunction with automatic QRS detec-
tion algorithms to reduce false detections and improve
the overall detection quality in unobtrusive ECG mea-
surement applications.
4 INSTRUMENTATION
4.1 Measurement System
For the experiment a contactless capacitive ECG
measurement system has been developed and inte-
grated into the backrest of a chair. Additionally, we
have integrated a capacitance measurement module
in order to continuously monitor the CCE-to-body
capacitance.
The overall system structure is shown in Figure 1
and consists of
• a battery-powered, wireless 16-bit data acquisi-
tion platform with an analog front-end for capa-
citive coupled ECG electrodes,
• a capacitive driven-seat electrode (as described by
(Keun Kim et al., 2005)) that can be disabled, de-
pending on common-mode noise level,
• active capacitively coupled ECG electrodes
(CCEs) with a dedicated sensor area for sensor-
to-body capacitance measurement (C
1,2
) and
• a two channel capacitance measurement module
featuring the AD7152 capacitance-to-digital con-
verter by Analog Devices (Analog Devices Inc.,
2008).
The two combined CCE/Capacitance-monitoring
electrodes are realized as multi layer PCBs with iso-
lated sensor areas for ECG and capacitance measure-
ment, details of such an electrode are shown in Fig-
ure 2.
Figure 2: Contactless ECG chair with combined electrodes
for capacitance and ECG measurement. Measurements can
be performed with or without backrest cover.
SIGNAL QUALITY ASSESSMENT FOR CAPACITIVE ECG MONITORING SYSTEMS USING
BODY-SENSOR-IMPEDANCE
455
The inner area of each electrode (A
CCE
≈ 28 cm
2
)
was connected to the capacitive ECG front-end
whereas the outer ring (A
C
1,2
≈ 19 cm
2
) was con-
nected to the input of the capacitance-to-digital mod-
ule (compare Figure 1). The ECG sampling rate was
set to 500 Hz while the capacitance measurement was
running with 200 Hz per channel, the maximum sam-
pling rate possible with the AD7152.
4.2 Capacitance Measurement
Configuration
In order for the AD7152 to perform the capacitance
measurement, a square wave excitation signal with
f
ex
≈ 32 kHz and V
ex
= 3.2 V is generated on-chip.
The value of a capacitance connected between the ex-
citation signal output and the measurement input of
the chip is directly converted to a 12-bit digital value
and can be read via an I
2
C-compatible interface. The
device offers two channels that can either be operated
in single-ended mode or in differential mode (Analog
Devices Inc., 2008).
Using one-channel differential mode in our setup,
we have connected the excitation signal to a large
sheet of conductive textile (A
ex
≈ 300 cm
2
) that was
located on the seat area, as indicated in Figure 2.
As A
ex
A
C
1,2
, this setup can be considered a “Hu-
man Transmitter” (Zimmerman et al., 1995). The ex-
citation signal becomes a common-mode signal on
the body and movements of the upper body result
in capacitance changes that can be registered by the
AD7152.
The device’s maximum input range is ±2 pF in
differential mode. Common-mode capacitances of
up to 5 pF can be compensated on-chip. In our ex-
periment, we intended to monitor movements within
a range of ∆x = 0.5 mm. . . 5 mm. This results in a
coupling capacitance range between body and ring-
electrode of ∆C
c
= 47 pF. . . 4.7 pF when the body-
electrode contact is considered a plate capacitor with
cotton (ε
r
= 1.4) as dielectric.
In order to adjust the coupling capacitance ∆C
c
to the
chip’s input range, series capacitors of 4.7 pF were
inserted between the electrodes and the inputs of the
AD7152. This results in an effective capacitance
range of ∆C
e f f
= 1.9 pF per electrode with an offset
of C
o
= 2.4 pF.
5 ARTIFACT DETECTION
ALGORITHM
The motion artifact detection algorithm we have im-
plemented consists basically of four steps: At first an
artifact level representing the intensity of artifacts in
the ECG signal is computed. The generated artifact
level is post-processed and in a third step it is con-
verted by means of a threshold detector into a binary
artifact indicator signal. As a last step, this artifact in-
dicator signal is logically ANDed with an additional
parameter derived directly from the ECG signal wave-
form. This signal is finally used to mark artifact re-
gions in the original ECG signal.
Artifact Level from Capacitance Data. The dif-
ferential capacitance signal from the AD7152 was
high pass filtered to remove offsets with a 4
th
order
Butterworth filter, cutting off signals below 1 Hz, and
the absolute value was taken. Then adaptive filtering
(Haykin, 2001) was applied in order to estimate arti-
facts in the ECG signal from the capacitance signal.
A filter with LMS algorithm has been used, the filter
length was set to 0.2 s.
Figure 3: Signal processing chain: the artifact indicator sig-
nal is derived from ECG and impedance data.
Post-processing & Threshold Filtering. As a post-
processing step the signal containing the artifact level
from the previous adaptive filter step was squared and
filtered with a moving average filter. The filter length
was set to 0.5 s.
This was the input to the threshold detection that
transforms the continuous artifact level signal into a
binary artifact indicator signal.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
456

0 . 0 0 5 0 . 0 1 0 . 0 1 5 0 . 0 2
0
2
4
Time [s]
V
e x
[V]
Figure 4: The 32 kHz excitation signal shows gaps every
7 ms, resulting in 140 Hz common-mode noise in the ECG
signal if no further measures are taken.
Final Artifact Marker Signal. Supplementary to
the artifact indicator, we have taken an additional pa-
rameter into account: using the original ECG data, we
verify that the signal lies within the range of [10, 90]%
LSB
ADC
of the analog-digital-converter, in order to
eliminate regions where the signal is near saturation.
Then the artifact indicator and the in-range indica-
tor were logically ANDed to generate the final artifact
marker signal. When this signal is high, the corre-
sponding region in the ECG signal is marked as arti-
fact region. Furthermore, artifact regions that do not
have a minimum distance of 1 s are joined.
6 EVALUATION & RESULTS
With the system presented above, we have conducted
measurements with several subjects. The driven-
seat circuit was not necessary because the systems’
coupling to earth ground was very weak due to the
battery-powered, wireless concept. Yet with the ca-
pacitance measurement circuit enabled, we observed
a large amount of common-mode noise on the ECG
signal with a frequency of approximately 140 Hz, the
source of which was not obvious at first. A closer
look at the excitation signal of the AD7152 revealed
that even though the device was used in one-channel
differential mode with continuous conversion, gaps in
the excitation signal occurred with a distance of ap-
proximately 7 ms (see Figure 4). This was visible as
common-mode noise in the ECG signal.
Enabling the driven-seat circuit to remove this
noise was not an option, as a 180
◦
phase shift is neces-
sary to destructively interfere with the undesired fre-
quencies. In the case of 140 Hz, this would result in a
rather high bandwidth of the driven-seat stage. Then,
the excitation signal of 32 kHz would have been at-
tenuated too, resulting in incorrect capacitance mea-
surement values. The solution for us was to simply
narrow the bandwidth of the CCEs by adding 50 Hz
low-pass filters.
6.1 Artifact Signal
The proposed algorithm generates an artifact signal
with very high dynamics. For slight capacitance
changes generated by breathing and light movements,
the signal amplitudes stay very low. Higher move-
ment amplitudes can increase the artifact amplitudes
up to 4 orders of magnitude. Hence, a threshold could
be identified that separates regular ECG episodes
from the ones with artifacts. First results showed, that
no adaptation of the threshold was necessary for dif-
ferent subjects.
6.2 Sample Measurement
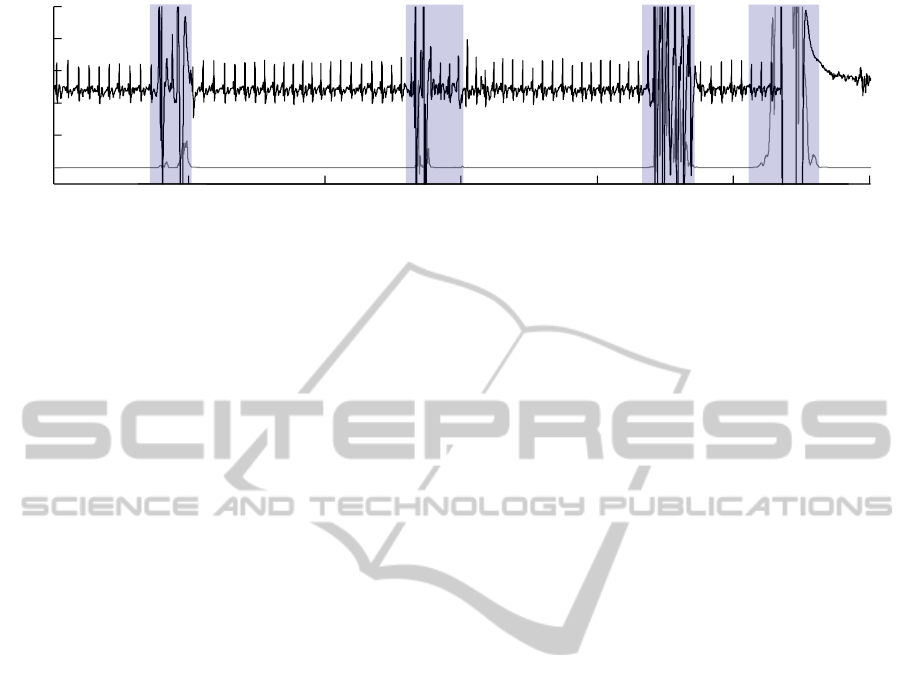
Figure 5 finally shows an example of the computa-
tion of artifact regions for a capacitive ECG signal
recorded with the system proposed in this paper. The
gray trace represents the artifact level generated by
adaptive filtering of the differential capacitance values
captured by the AD7152. Above a certain threshold,
the artifact indicator goes high. The artifact region
can be marked in the ECG signal.
7 SUMMARY & OUTLOOK
Non-contact capacitive ECG systems represent an un-
obtrusive way to acquire cardiovascular data. Yet, due
to the measurement principle, these systems are sensi-
tive to motion artifacts. For automatic signal analysis,
a means of estimating signal quality is therefore de-
sired.
Combining a capacitive ECG measurement sys-
tem with a capacitance-to-digital conversion de-
vice, we have presented an unobtrusive ECG sys-
tem that continuously monitors the electrode-body-
capacitance. This capacitance value represents a con-
text signal that can be used to derive a quality signal
for the capacitive ECG measurement.
Using adaptive filtering and post-processing we
were able to show first results of the system perfor-
mance. With the system, it is possible to mark re-
gions with artifacts in the capacitive ECG signal, and
exclude them from further processing thus improving
automatic analyzability.
Currently we are building a database with mea-
surements recorded with our system. With the data
we will be able to quantify the improvement of au-
tomatic analysis of unobtrusively acquired ECG data
due to our method. For automatic QRS detection, a
considerable decrease in false (positive and negative)
detections due to the proposed method should be pos-
sible, yielding an increase in sensitivity and positive
SIGNAL QUALITY ASSESSMENT FOR CAPACITIVE ECG MONITORING SYSTEMS USING
BODY-SENSOR-IMPEDANCE
457
10 20 30 40 50 60
−2
−1
0
1
2
3
Time [s]
Amplitude [a.u.]
Figure 5: Using the adaptively filtered capacitance signal (lower trace) it is possible to generate an indicator signal that can
be used to mark artifact regions (shaded) in a capacitive ECG measurement.
predictivity. Excluding artifact-contaminated parts of
the signal also prevents the internal thresholds of the
QRS-detector from assuming suboptimal values. The
evaluation will also help to understand the applica-
tion limits of the system and the applicability of the
method.
Further work will comprise the improvement of
the artifact detection algorithm by optimizing the
adaptive filter parameters and timing parameters, as
well as investigations whether the system can also be
used for the correction of artifacts.
REFERENCES
Aleksandrowicz, A., Walter, M., and Leonhardt, S. (2007).
Wireless ECG measurement system with capacitive
coupling. Biomedizinische Technik. Biomedical engi-
neering, 52(2):185–92.
Analog Devices Inc. (2008). AD7152 Datasheet:12-Bit
Capacitance-to-Digital Converter.
Haykin, S. (2001). Adaptive Filter Theory. Prentice Hall,
4th edition.
Keun Kim, K., Kyu Lim, Y., and Suk Park, K. (2005).
Common mode noise cancellation for electrically non-
contact ECG measurement system on a chair. In Engi-
neering in Medicine and Biology Society, Annual In-
ternational Conference of the IEEE, volume 6, pages
5881–3.
Lamparth, S., Fuhrhop, S., Kirst, M., Wagner, G., and Ot-
tenbacher, J. (2009). A Mobile Device for Textile-
integrated Long-term ECG Monitoring. In Dössel, O.
and Schlegel, W. C., editors, World Congress on Med-
ical Physics and Biomedical Engineering, Septem-
ber 7-12, 2009, Munich, Germany, volume 25/5 of
IFMBE Proceedings, pages 278–281, Berlin, Heidel-
berg. Springer Berlin Heidelberg.
Lim, Y. G., Kim, K. K., and Park, K. S. (2006). ECG mea-
surement on a chair without conductive contact. IEEE
transactions on bio-medical engineering, 53(5):956–
9.
Ottenbacher, J., Kirst, M., Jatobá, L., Huflejt, M., Gross-
mann, U., and Stork, W. (2008). Reliable motion ar-
tifact detection for ECG monitoring systems with dry
electrodes. In Engineering in Medicine and Biology
Society, Annual International Conference of the IEEE,
pages 1695 –1698.
Park, C., Chou, P. H., Bai, Y., Matthews, R., and Hibbs, A.
(2006). An ultra-wearable, wireless, low power ECG
monitoring system. 2006 IEEE Biomedical Circuits
and Systems Conference, pages 241–244.
Schumm, J., Axmann, S., Arnrich, B., and Tröster, G.
(2009). Automatic Signal Appraisal for Unobtrusive
ECG Measurements. In Proceedings of the Biosignal
Interpretation Conference.
Zimmerman, T. G., Smith, J. R., Paradiso, J. A., Allport, D.,
and Gershenfeld, N. (1995). Applying electric field
sensing to human-computer interfaces. In Proceed-
ings of the SIGCHI conference on Human factors in
computing systems - CHI ’95, pages 280–287, New
York, New York, USA. ACM Press.
BIOSIGNALS 2011 - International Conference on Bio-inspired Systems and Signal Processing
458
