
CONSTRUCTION AND ANALYSIS OF AN ARTIFICIAL
NEURONAL NETWORK USING A NEURON-COLLECTING,
MICRO-PATTERNING METHOD BASED ON
A MULTI-ELECTRODE ARRAY SYSTEM
Hideyuki Terazono
1
, Hyonchol Kim
1
, Masahito Hayashi
1
, Akihiro Hattori
2
,
Hiroyuki Takei
1,3
and Kenji Yasuda
1,2
1
Yasuda"On-chip Molecular Cell" Project, Kanagawa Academy of Science and Technology, Kanagawa, Japan
2
Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Tokyo, Japan
3
Department of Life Sciences, Faculty of Life Sciences, Toyo University, Tokyo, Japan
Keywords: Artificial neuronal networks, Hippocampal neurons, Micro-chip, Agarose, Alginate, Micro-patterning,
Multi-electrode.
Abstract: We developed three techniques to make artificial neuronal networks constructed from rat hippocampal
neurons. 1) a method of non-invasively collecting primary cultured neurons and their deposition, 2) a
technique for microprocessing agarose for the purpose of assembling artificial neuronal networks, 3) a
multi-electrode array system for measurement of the multi-point extracellular potential of neurons. The
three techniques allow us to assemble and evaluate artificial neuronal networks constructed from particular
cells. We can manipulate neuro-transmission pathways and investigate roles played by the innate period or
stability information for each individual cell in the framework of physiological mechanism. It is thus
possible to construct and demonstrated the actual neuronal networks simulated by the computed neural
networks.
1 INTRODUCTION
Neuronal networks in the brain form acceptable
patterns for external information such as long term
potentiation (LTP) or long term depression (LTD)
(
Pelletier JG, 2008). Therefore, it is thought that cells
in the neuronal networks form acceptable or
resistible spatial patterns for external information.
So far, neuronal networks have been analysed
both in vivo and in vitro, but it was very difficult to
analyse informational hysteresis because neurons in
the brain and a culture dish make a self-formation of
synapse that we can’t manipulate. If an artificial
neuronal network can be constructed with desired
neuron types and synapse direction, the
informational hysteresis of neurons in neuronal
networks can be analysed very easily.
With this aim, we developed three techniques for
making artificial neuronal networks, 1) a technique
for picking cells from a group of primary cultured
neurons in a non-invasive fashion using a digestible
thin sheet and depositing the selected cells in a
micropattern, 2) a technique for microprocessing
agarose for assembling artificial neuronal networks
through manipulation of the direction of
neurotransmission on a culture dish, 3) a technique
for measuring the multi-point extracellular potential
of neurons with a multi-electrode array system, these
cells can be also stimulated during measuring if
necessary.
The three techniques allow us to assemble and
evaluate artificial neuronal networks constructed
from particular cells. We can manipulate neuro-
transmission and investigate the physiological
mechanism such as the innate period or stability
information for each individual cell.
2 METHOD
2.1 Neuron Preparation and
Cultivation
Dispersed cultures of hippocampal cells were
304
Terazono H., Kim H., Hayashi M., Hattori A., Takei H. and Yasuda K..
CONSTRUCTION AND ANALYSIS OF AN ARTIFICIAL NEURONAL NETWORK USING A NEURON-COLLECTING, MICRO-PATTERNING METHOD
BASED ON A MULTI-ELECTRODE ARRAY SYSTEM.
DOI: 10.5220/0003641203040307
In Proceedings of the International Conference on Neural Computation Theory and Applications (NCTA-2011), pages 304-307
ISBN: 978-989-8425-84-3
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
prepared from E18 of Wistar rats according to the
National Institutes of Health guidelines for
laboratory animal care and safety. The hippocampal
formation was dissected out from anesthetized
animals in ice-cold Hanks balanced salt solution.
Then hippocampal formation was treated with
0.25% trypsin (Wako) and 0.01% DNase I (Sigma)
at 37°C for 30 min. After adding Fatal bovine serum,
cells were centrifuged at 1,000 rpm for 5 min. The
remaining cells were dispersed in 2 mL Neurobasal
(Invitrogen Neurobasal medium) supplemented with
2% B-27 (Invitrogen) and 1% penicillin-
streptomysin at 37°C. For primary cultures and
recultures, neurons and glias were plated onto a 35-
mm culture dish coated with poly-L-lysine (Iwaki) at
a cell density of 1.0 × 10
5
cells / cm
2
at 37°C in a
humidified 5% CO
2
and 95% air atmosphere.
2.2 Formation of a Cell Collection Dish
for Primary Neurons and Neurons
Network
80 µL of 1.5% sodium alginate was put on a 35-mm
culture dish and sodium alginate was spin-coated at
3,000 rpm for 10 sec, and dried. Subsequently, the
sodium alginate was gelled by applying 1.5% CaCl
2
,
and a calcium alginate thin layer was made on the
culture dish. Next, 200mM poly-l-lysine hydro-
borate (PLL) and 4% polyethyleneimine(PEI)was
coated.
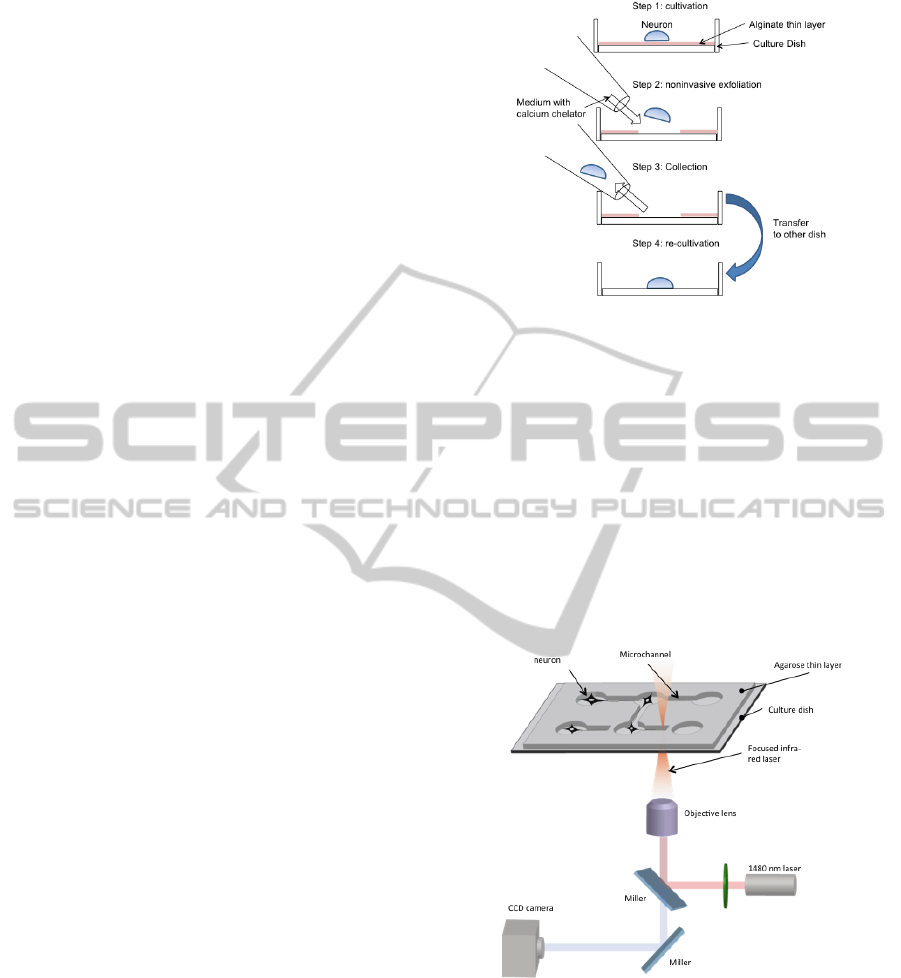
2.3 Detaching and Transferring the
Cells
For detaching and transferring cells, a glass capillary
whose internal diameter was 0.6 mm was heated,
pulled, and fire polished to make the internal
diameter around 80 µm using a puller (Narishige)
and a micro forge (Narishige). The micro-capillary
was fire polished and siliconized by sigmacote
(Sigma). To detach the cells, the capillary was filled
with a culture medium with 5mM EDTA ・ 2Na
(Dojindo Laboratories)
2.4 Re-culture of the Collected Cells
Releasing of the medium containing EDTA from the
microcapillary and retrieving cells was controlled by
adjusting the air pressure in the microcapillary using
a pneumatic manual micro-injector (Eppendorf). The
retrieved cells were put onto another culture dish
and cultivated. A second cultivation dish for neuron
was coated with PLL.
Figure 1: The procedure of collecting a cultured single
neuron.
2.5 Agarose Microprocessing System
for the Single Cultivation and
Regulating the Direction of Neurite
Microstructures (microchamber and microchannel)
to fix cell positions, guide neurites and form the
network patterns were created using a photothermal
etching method. A 1480-nm infrared laser beam was
focused on the agar thin layer through the objective
lens of the microscope on the culture dish, causing
the agar at the focal point to melt (Fig.2).
Figure 2: Device configuration of an agarose
microprocessing system.
2.6 An agarose Microprocessing and
Multi-electrode Array System for
the Measurement of an Action
Potential of an Artificial Neuronal
Network
A multi-electrode array (MEA) chip was formed on
a glass slide consisting of either a 8×8 or 16×4
CONSTRUCTION AND ANALYSIS OF AN ARTIFICIAL NEURONAL NETWORK USING A
NEURON-COLLECTING, MICRO-PATTERNING METHOD BASED ON A MULTI-ELECTRODE ARRAY SYSTEM
305

electrode array. This system enables measurement of
extracellular action potentials of single neurons and
a sampling rate of 100 kHz per channel can be used.
The MEA chip was made of indium tin oxide (ITO)
whose transparency facilitate subsequent
microfabrication of microchamber and
microchannel, so that this system allow us to
fabricate agarose microchambers and microchannels
on the MEAs chip. To measure action potential of
artificial neuronal network of neurons, agarose
microstructures was made on the MEAs chip.
3 RESULTS
3.1 Preparation of a Collection Dish for
Primary Neurons
Sodium alginate was put on a culture dish, and
coated by spin-coater, and then dried in air.
Subsequently, sodium alginate was gelled by
applying CaCl
2
solution. A thin calcium alginate
sheet was dried and washed. Subsequently, PLL and
PEI were coated.
Primary neurons adhered onto the PLL-micro-
contact-printed alginate dish and extended the
neurites and axons very well.
3.2 Collection of Cultured Primary
Hippocampal Neurons and
Re-cultivation
Primary hippocampal neurons were initially cultured
on the detaching-culture dish for neurons. After a
few minutes, neurons started to adhere on the
detaching-culture dish. After 1 day, hippocampal
neurons extended their neurites. After confirming
that neurites of cells were extended, the medium
containing EDTA was loaded by a micro-capillary.
Then calcium alginate around the target cell was
immediately solated, and target cells were released
from the layer. The released neurons were collected
with a pipette very easily. They were cultured on
another dish coated with PLL. All steps from cell
collecting to re-culturing required less than 2 min.
The collected neuron retained their shapes and did
not shrink, and re-cultivated neurons are extended
their neurites immediately (Fig3).
Figure 3: Micrographs of each step for collecting cultured
neuron.
3.3 Neuron Cultivation on the
Agarose-micropatterned Chamber
Primary neurons dissected from hippocampal
formation were initially cultured in an agarose
microchamber. Neurons transferred by a micro-
pipette adhered onto the microchamber. After 1 day,
neurite of neurons extended along the
microchamber. Neuron didn’t adhere onto the
bottom of the small chamber whose diameter was
20µm. Furthermore, neurons didn’t extend neurites
along the microchannel whose width was less than
7µm.
3.4 Measurement of the Extracellular
Action Potential of Micropatterned
Single Neurons
A microchamber was made on top of each electrode
and these microchambers were interconnected via
micro-channels. The Primary hippocampal neurons
were initially cultured on the detaching culture dish
for neuron. After a few minutes, neurons started to
adhere onto the detaching culture dish. After 1 day,
hippocampal neurons extended their neurites. After
7days, the extracellular action potential was
recorded (Fig.4).
Figure 4: An extracellular action potential recording from
single neuron on the micropatterned structure.
20µV
0.1 sec
NCTA 2011 - International Conference on Neural Computation Theory and Applications
306

4 DISCUSSION
Alginic acid is a viscous gum derived from algae
and is composed of β-D-mannuronate and α-L-
guluronate. Calcium alginate, which is a salt of
alginic acid, is harmless to cells and used as a
scaffold in tissue transplantation (Heise, 2005).
However, cells cannot adhere to intact calcium
alginate. In this study, alginate sheet that have both
properties that transform sol/gel state and
adhesiveness of cells could be made. Using this
sheet, a specific cell can be collected without
exfoliating surrounding cells.
Recently, Okano et al. have developed
techniques, which allow us to detach cells from
culture dishes without using digestive reagents9.
Temperature dependent polymer, poly (N-
isopropylacrylamide) (PIPAAm) changes the
hydrophilic/ hydrophobic property in a temperature-
dependent manner (Masuda, 2008). PIPAAm is
hydrophobic at 37°C and hydrophilic at 20°C, so
that cells on the PIPAAm coated culture dish can be
detached from the culture dish without perturbing
the extracellular matrix and intercellular connection
such as tight junctions. Such a method gives us cell
sheets that retain intercellular connections. Using
this technique, the stick cardiac tissue stacked mono-
layered cardiac cell sheet can be made.
However, individual cells that have specific
property cannot be collected with this method
because temperature cannot be controlled on the
scale of micrometer. In fact, dispersed cultured cells
have heterogeneous properties while if averaging the
physiologically property, dispersed culture cells are
apparently homogeneous. So that, to align the
physiological properties homogeneously, it must be
necessary to develop a method to collect each single
cell from culture dishes non-invasively.
On the other hand, our method is suitable for
collecting single cells or small clusters of cells.
Therefore, for example, if there are several types of
differentiated or undifferentiated cells derived from
ES or iPS cells in the culture dish, our method can
allow us to collect only targeted cells.
Moreover, primary neurons were cultured on the
agarose-micropatterned chamber. The cultured
neurons extended neurite along the microchannel.
Furthermore, the extracellular action potential of
single neuron can be measured by an agarose-
micropatterned multielectrode array.
The results of three techniques; the noninvasive
collection method of neuron, agarose
microproceesing method and multielectrode array,
allow us to make artificial neuronal networks using
neurons regulating direction of neurotransmission,
and to measure the activity of artificial neuronal
networks. The next stage of the study is to construct
basic components working in the actual brain.
5 CONCLUSIONS
We developed three techniques 1) a non-invasive
neuron collection method, 2) an agarose micro-
processing technique, 3) a multielectrode array
system. These techniques allow us to construct and
demonstrated the actual neuronal networks
simulated by the computed neural networks.
ACKNOWLEDGEMENTS
We greatly thank Ms. Misa Sasajima, Ms. Hiromi
Mikami, and Ms. Tamae Takato for their technical
assistance. This work was financially supported by
the Kanagawa Academy of Science of Technology.
REFERENCES
Pelletier J. G., Lacaille J. C., 2008, O. Prog Brain Res.
169, 241-250.
Heise, M., Koepsel, R., Russell, A. J. & McGee, E. A.
2005, Reprod Biol Endocrinol 3, 47.
Masuda, S., Shimizu, T., Yamato, M. & Okano, T. 2008,
Adv. Drug Del. Rev. 60, 277-285.
CONSTRUCTION AND ANALYSIS OF AN ARTIFICIAL NEURONAL NETWORK USING A
NEURON-COLLECTING, MICRO-PATTERNING METHOD BASED ON A MULTI-ELECTRODE ARRAY SYSTEM
307
