
COMPUTATIONAL PREDICTIONS FOR THE NUCLEATION
MASS AND LAG TIMES INVOLVED IN A42 PEPTIDE
AGGREGATION
Preetam Ghosh
1
, Bhaswati Datta
2
and Vijayaraghavan Rangachari
3
1
Department of Computer Science, Virginia Commonwealth University, Richmond, VA, U.S.A.
2
School of Computing,
3
Department of Chemistry & Biochemistry, University of Southern Mississippi
Hattiesburg, MS, U.S.A.
Keywords: Alzheimer’s Disease, Protein self-assembly, Ordinary differential equation, Mass-kinetics.
Abstract: The aggregates of amyloid-β (Aβ) peptide are the primary neurotoxic species in the brains of Alzheimer’s
patients. We study the molecular-level dynamics of this process employing chemical kinetic simulations by
dissecting the aggregation pathway into pre-nucleation, post-nucleation and protofibril elongation stages.
Here, we discuss how our earlier identified rate constants for protofibril elongation were incorporated into a
simplified simulation of the complete aggregation process to understand the lag-times in the sigmoidal fibril
growth curves of fibril formation. We also present some initial findings on the rate constants and possible
hypotheses on the nucleation mass involved in the pre-nucleation stage.
1 INTRODUCTION
In Alzheimer’s disease (AD), the aggregates of a
protein called, Aβ are strongly believed to be the
cause for neuronal death and cognitive decline
(Selkoe, 2003). Aβ aggregates to form large fibrillar
deposits that follows a sigmoidal growth pattern
involving a ‘lag-phase’ prior to fibril growth. The
lag-phase is generated due to an initial rate-limiting
step of nucleation (Jarrett, 1993); (Harper, 1997).
However, the precise mechanism of nucleation and
size of the nucleus are not known. Accurate in vitro
analyses of the process is difficult as the
intermediate oligomers are difficult to isolate and
characterize. However, one intermediate from the
post-nucleation phase, called protofibrils were
identified (Walsh, 1997) that show propensities to
both elongate as well as laterally associate to grow
into mature fibrils. However, many previous works
on Aβ aggregation kinetics have not incorporated the
pre-nucleation events that constitute a critical step of
the aggregation process, more likely due to the
difficulty in doing so for stochastic processes.
It is important to identify the nucleation mass
and the kinetic rate constants involved in all the
different phases of Aβ aggregation: pre-nucleation,
post-nucleation and fibril elongation. Various
models on Aβ aggregation reviewed in (Morris,
2009), use curve fitting without considering the pre-
nucleation events. Recently, (Lee, 2007) reported a
molecular-level model of insulin aggregation that
forms the basis for the model presented here. Earlier,
we modelled the protofibril elongation and lateral
association stages to report the kinetic rate constants
involved (Ghosh, 2010). Here, our contributions are
summarized as follows: (i) use the rate constants
from protofibril elongation into the biophysically
similar post-nucleation phase; (ii) create a model to
estimate the lag-times and nucleation mass of Aβ42;
(iii) report in vitro Aβ42 aggregation experiments
that motivate our nucleation mass estimates; (iv)
discuss the problems in directly comparing the
simulated lag-times to those from experiments.
2 A PROCESS SIMULATION
2.1 In Vitro Results on Aβ42
Aggregation
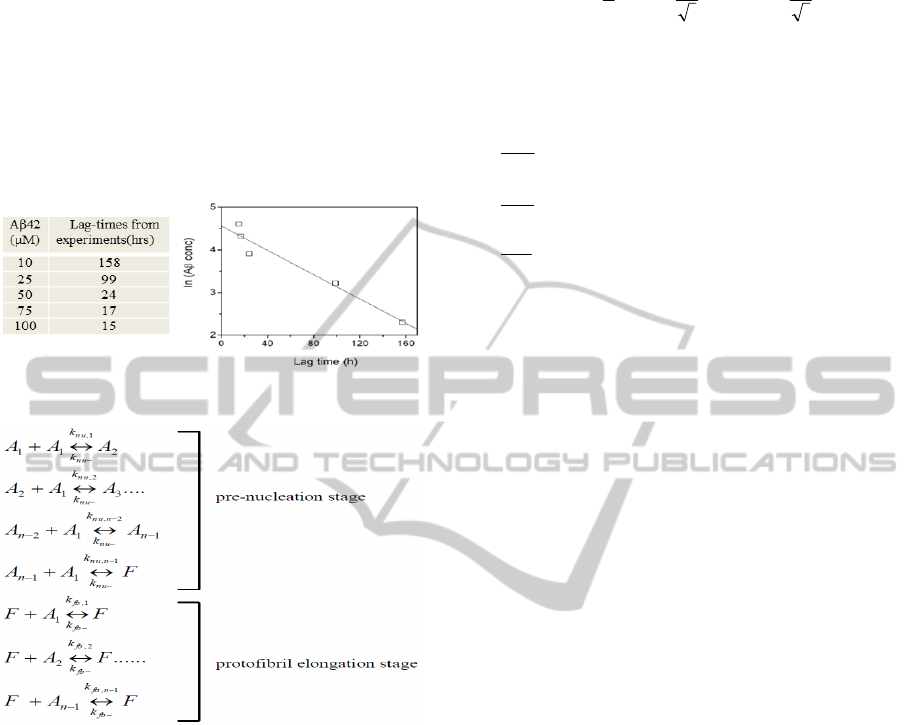
We monitored Aβ42 aggregation in five different
concentrations, 10, 25, 50, 75 and 100
M by
thioflavin-T (ThT) fluorescence (lag-times shown in
Fig 1). Since Aβ aggregation is nucleation-
312
Ghosh P., Datta B. and Rangachari V..
COMPUTATIONAL PREDICTIONS FOR THE NUCLEATION MASS AND LAG TIMES INVOLVED IN Aβ42 PEPTIDE AGGREGATION.
DOI: 10.5220/0003782603120316
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2012), pages 312-316
ISBN: 978-989-8425-90-4
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
3
1,,
3
1,,
1
);
1
1(
2
1
i
kk
i
kk
fbifbnuinu
dependent, increase in concentration decreases the
lag-time besides increasing the rate of aggregation.
Hence, we observed the least lag-time for 100
M
followed by 75, 50, 25 and 10
M concentrations
respectively. In addition, there was an inverse linear
correlation between the logarithm of Aβ42
concentration and the corresponding lag-time as
shown in Fig 1. This observation was later used to
accurately characterize the Aβ42 nucleation mass
based on the simulated lag-times.
Figure 1: Lag-times from in vitro experiments.
Figure 2: Reactions towards fibril formation.
2.2 Modified Model on Aβ Aggregation
Here, we adapt the insulin aggregation model in
(Lee, 2007) for the Aβ42 system. We characterize
the pathway using biochemical reactions, compute
the reaction fluxes and formulate the differential
equations for each oligomer concentration as a
function of time. Solving the set of homogeneous
ODEs allow us to study the temporal dynamics of
each oligomer. Fig 2 shows the modified set of
reactions considered in our simulation.
Here, A
i
’s denote i-mers, n is the nucleation mass
and F is a fibril. The following assumptions were
made: a) monomer adds to i-mers until fibril
formation; b) nucleation involves monomer addition
as well as a structural change in the oligomer A
n
(this conformational change is implicit); c) post-
nucleation events are faster, as the forward rate
constants for post-nucleation are much higher than
those in pre-
nucleation (i.e., k
nu,n+i
>>k
nu,i
) (a ~10
8
fold difference
;
1,...,2;
2
1,...,1;
1,...,1;
111,
,,1,
1
2
1,,1,
1
,,
11,,
FkAAk
dt
dF
niJJJ
dt
dA
JJJ
dt
dA
niFkFAkJ
niAkAAkJ
nunnnu
ifbinuinu
i
n
i
fbinunu
fbiifbifb
inuiinuinu
was reported in (Lee, 2007)); d) the reverse reaction
rate constants are assumed to be independent of size
i, and abbreviated as k
nu-
and k
fb-
. e) since agitation
drastically shortens the lag-times, k
nu,i
and k
fb,i
are
assumed to be diffusion-limited; using the Stokes-
Einstein equation, the diffusivity is proportional to
the inverted cubic root of i, resulting in:
Hence, the reaction fluxes and differential
equations can be derived as follows:
2.3 Integrating Protofibril Elongation
A complete simulation of the Aβ system requires an
estimate of the following six parameters: k
nu,1
, k
fb,1
,
k
nu-
, k
fb-
, n and b, where, b is the constant that maps
ThT fluorescence to concentration estimates. It is
impossible to try out different values for each of
these variables to properly match the experimental
plots due to the huge solution space. Hence we
dissected the sigmoidal fibril-growth curve in
(Ghosh, 2010) into: (i) pre-nucleation stage (ii) post-
nucleation stage and (iii) protofibril elongation
stage. The pre- and post-nucleation stages are well-
approximated by the set of reactions shown in Fig 2.
However, protofibril elongation stage needs to
combine reactions from both post-nucleation and
lateral association. This requires the estimation of
two more rate constants: the forward and backward
rate constants for the lateral association stage
denoted by k
la
and k
la-
respectively. In our previous
report (Ghosh et al., 2010), we estimated the post-
nucleation rate constants (k
fb,1
, k
fb-
, k
la
and k
la-
)
separately and verified them with in vitro
experiments as follows: k
fb,1
=9.0 × 10
3
(h
-1
mM
-1
), k
fb-
=4.5 × 10
2
(h
-1
), k
la
=9.0 × 10
-1
(h
-1
mM
-1
), k
la-
=6.0 ×
10
-3
(h
-1
). We next directly substitute the fibril
elongation rate constants into our modified model to
predict the lag-times.
COMPUTATIONAL PREDICTIONS FOR THE NUCLEATION MASS AND LAG TIMES INVOLVED IN Aβ42
PEPTIDE AGGREGATION
313

3 RESULTS AND ANALYSIS
Our model makes all possible oligomers in the pre-
nucleation stage mathematically tractable due to the
abstraction that any post-nucleation stage aggregate
(starting from the nucleation mass itself, i.e., A
n
) is
treated as a fibril (i.e., F). However, this model does
not consider the length of the fibrils as variables and
hence cannot match the plateaus of the ThT
fluorescence curves generated by experiments. This
is because the fibrils of differing length will have
different contributions on ThT intensity which
cannot be directly captured using this model. Indeed,
in Fig 4, we have mapped the concentration of F to
ThT intensity for different initial Aβ concentrations,
and each curve saturates at the same peak. This
problem was circumvented by assuming different
mapping constants in (Lee, 2007) to separate the
peaks for different Aβ initial concentrations, which
is not a biophysically correct assumption (as
discussed in (Ghosh, 2010)). Thus, this model
cannot implement an entirely accurate simulation of
the pathway. In this paper, however, our main goal
is to study the lag times in the pathway, and hence
predict a working range for the nucleation mass. As
our model in Fig 2 can accurately study the pre-
nucleation stage oligomers, we will henceforth use it
to study only the lag times in the aggregation
pathway generated for different values of the
nucleation mass (n).
Table 1: Lag-times (in hrs) from our simulation for
various estimates of nucleation mass.
3.1 Lag Time Predictions
In Table 1, we show the simulated lag times for
different nucleation mass and initial Aβ
concentration. In order to find the pre-nucleation
rate constants along with the nucleation mass, we
use the following scheme: estimate the rate constants
that give the maximum lag times for each value of
the nucleation mass. Note that, changing the rate
constants further to achieve higher lag-times render
the system of differential equations unstable.
Interestingly, the simulation shows 4 distinctly
different regimes of lag times corresponding to 4
different pairs of rate constants in pre-nucleation
(highlighted using different colors in Table 1). At
the same time, this also characterizes four different
regimes of nucleation masses associated with Aβ
aggregation summarized as follows: Regime 1:
n=7,8,9,10,11; Regime 2: n=12,13,14; Regime 3: n=
15,16,17; Regime 4: n= 18,19,20,21.
The rate constants for each of these regimes are
shown in Table 2. Note that the forward rate
constants were fixed for each nucleation mass, while
the backward rate constant were varied to achieve
the highest lag times as reported in Table 1. The
problem here is that each of the nucleation masses
does allow us to find a pair of rate constants for the
pre-nucleation stage. It is however, not possible to
match the simulated lag times to that observed
experimentally (as reported in Fig 1). We will
discuss this problem in the next section.
Table 2: Rate constants for prenucleation stage for various
estimates of nucleation mass.
Figure 3: Close to linear semi-log plots.
However, as seen in Fig 1 (and also from other
experiments in our lab consistently), the semi-log
plot of the lag-times against initial concentration of
Aβ is linear. So we used this property to figure out
what values of nucleation mass are most feasible for
the Aβ42 pathway. Note that n=10, 11 (in Regime 1)
and n=12 (in Regime 2) are close to linear and
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
314

hence may serve as good approximations of the
nucleation mass (Fig 3). It was also observed in
course of the simulations that an initial concentration
of 10 µM made the simulation erratic for a wide
range of rate constants (because of increased
dynamism and stochasticity in the system with lower
molecular count of the species rendering the ODEs
unstable). So, we generated these semi-log plots for
the different regimes (data not shown) by removing
the data points for 10 µM. Indeed, these curves show
a more stable relationship between the lag times and
the initial concentrations, and we find close to linear
behavior for n=10,11 (in Regime 1), n=12,13 (in
Regime 2) and n=15,16,17 (in Regime 3).
The next question is whether 10,11,…,17 is the
right range for the nucleation mass, or can we
further reduce it? Fig 4 shows the concentration
curve for F against time and different initial
concentrations. One requirement for the rate
constants reported above is that these curves must
saturate to the same peak as expected
mathematically. So we considered this to be another
constraint that reduced the range of feasible
nucleation masses to n=10, 11…,14. Note that n=15,
16, 17 did not allow the concentration curves to
saturate (data not shown), and hence were ruled out
as possible candidates for the nucleation mass.
Figure 4: Simulated fluorescence change curves for
different initial concentrations with n=12.
3.2 Can we Compare Simulated and
Experimentally Observed Lag
Times?
The experimental ThT fluorescence plots show the
cumulative effect of all oligomers of a certain size
(and beyond). The results shown above plot the
concentration of F which model the cumulative
effect of all the nucleated oligomers in the pathway.
However, it is assumed that all nucleated oligomers
show up on the ThT curves (this is generally not the
case from actual experiments). Hence, the lag times
estimated from our model are lower than that seen
experimentally. Also, it is not yet known what size
of oligomers actually show up ThT positive and
hence the experimental estimates are at best the
maximum limits of the lag times for each initial Aβ
concentration. To get around this problem, we varied
the rate constants to estimate the maximum possible
lag times for each value of the nucleation mass. This
is still an approximation of the actual system and
needs further study. Ideally, we need to know what
sizes of oligomers are considered ThT positive such
that the experimental curves can be meaningfully
compared to the simulated plots. The present paper,
however, gives us a feasible range of nucleation
masses to work with in order to build a complete
simulation of the on-pathway. The rate constants
estimated in this exercise can serve as a guidance for
the complete simulation where we will need a more
detailed model (with separate parameters for each
post-nucleation oligomer) to properly model their
effects on the system.
4 CONCLUSIONS
In this paper, we have studied the lag times in the
sigmoidal Aβ fibril formation pathway. We also
reported that the nucleation mass can potentially be
in the range 10,11,…, 14 mers. In order to reduce
the complexity of the entire fibril formation
pathway, we used the rate constants that we have
earlier estimated for the post-nucleation stage into a
modified model that can approximately characterize
the complete pathway. These estimates will serve as
the basis for implementing a complete and accurate
simulation of the pathway wherein we have
approximately estimated all the 6 variables involved.
Such a simulation will pave the path to study the
complete system dynamics of Aβ aggregation
leading to a better understanding of AD in general.
ACKNOWLEDGEMENTS
This work was supported by NSF-1158608.
REFERENCES
Selkoe, D. J., Schenk, D. (2003). Alzheimer's disease:
molecular understanding predicts amyloid-based
therapeutics, Annu Rev Pharmacol Toxicol 43, 545-
584.
Harper, J. D., Lansbury, P. T., Jr. (1997). Models of
amyloid seeding in Alzheimer's disease and scrapie:
mechanistic truths and physiological consequences of
COMPUTATIONAL PREDICTIONS FOR THE NUCLEATION MASS AND LAG TIMES INVOLVED IN Aβ42
PEPTIDE AGGREGATION
315

the time-dependent solubility of amyloid proteins,
Annu Rev Biochem 66, 385-407.
Jarrett, J. T., Berger, E. P., Lansbury, P. T., Jr. (1993). The
C-terminus of the beta protein is critical in
amyloidogenesis, Ann N Y Acad Sci 695, 144-148.
Walsh, D. M., Lomakin, A., Benedek, G. B., Condron, M.
M., Teplow, D. B. (1997). Amyloid beta-protein
fibrillogenesis. Detection of a protofibrillar
intermediate, J Biol Chem 272, 22364-22372.
Morris, A. M., Watzky, M. A., Finke, R. G. (2009).
Protein aggregation kinetics, mechanism, and curve-
fitting: a review of the literature, Biochim Biophys
Acta 1794, 375-397.
Ghosh, P, Kumar, A, Datta, B, Rangachari, V (2010).
Dynamics of protofibril elongation and association
involved in Aβ42 peptide aggregation in Alzheimer’s
disease, BMC Bioinformatics 2010, 11(Suppl 6):S24,
pp. 1-19.
Lee, C. C., Nayak, A., Sethuraman, A., Belfort, G.,
McRae, G. J. (2007). A three-stage kinetic model of
amyloid fibrillation, Biophys J 92, 3448-3458.
BIOINFORMATICS 2012 - International Conference on Bioinformatics Models, Methods and Algorithms
316
