
NONINVASIVE MEASUREMENT OF BLOOD ACID-BASE (pH)
USING CONCENTRATIONS OF EXHALED GASES
A. S. Altaan
1
, O. Abdallah
1
, Mohammad T. Othman
2
, Nasser Musaab
3
and A. Bolz
1
1
Biomedical Engineering Institute, Karlsruhe Institute for Technology, Karlsruhe, Germany
2
Department of Physical Education, College of Basic Education, Mosul University, Mosul, Iraq
3
Department of Internal Medicine and Cardiovascular, Ibn Sina Teaching Hospital, Mosul, Iraq
Keywords: Exhaled Breath, Partial Pressure of Oxygen, Concentration of Carbon Dioxide, Non-invasive Blood Acid-
base (pH).
Abstract: An important property of blood is its degree of acidity and alkalinity which is referred to as acid-base
balance. The acidity or alkalinity of the blood is indicated on the pH scale. The blood pH has a serious
effect on all of the body’s systems and the body uses different mechanisms to control the blood’s acid-base
balance. Acid-base imbalances result primarily from metabolic or respiratory failures, both imbalances
cause changing in the normal range of CO2 in the blood. The concentrations of oxygen and carbon dioxide
from the exhaled breath were used to evaluate the pH of the blood. The results show the relation between
concentration of the exhaled CO2 and the blood acid-base pH; decreasing CO2 causes the blood to be
alkaline, while increasing CO2 leads the blood to become acidic.
1 INTRODUCTION
During exercise the muscles are working harder than
normal and, as a result, they require more energy
than normal. Since the ATP energy used by the
muscles is generated with the aid of oxygen, it
follows that an increase in exercise intensity will
result in an increase in muscular oxygen demands.
Therefore, increased exercise intensity ultimately
corresponds to an increased in the volume of the
consumed oxygen. As the muscles working harder
they release more CO2 this will affect the balance of
O2 and CO2 in the blood, this is the reason that
breathing gets progressively faster and deeper as
exercise intensity increases, the body is trying to
provide more oxygen to the working muscles and
release the resulting carbon dioxide so that they can
generate enough ATP energy to keep the athlete
moving. Homeostasis is the overall process of
maintaining stability of the body’s internal physical
and chemical systems. These processes involve rapid
correction of disturbances that may arise, as well as
instance by instance adjustments to prevent gross
disturbance from arising. A simple example is that if
the heart rate and the respiratory rate did not
increase during physical exertion, body chemistry
would be significantly altered by the resulting deficit
in oxygen and accumulation of carbon dioxide. The
amount of carbon dioxide in the blood has an
immediate and direct effect on the body’s acid-base
balance, a key aspect of the internal chemical state.
1.1 Effect of the O2 and CO2 on the pH
Hydrogen ion activity can significantly affect the
metabolic function of the cells. Bicarbonate ion
(HCO
3
) is the most important form of CO2, both
HCO
3
and H
+
are carriage by blood. CO2 combines
with water to form carbonic acid, and this
dissociates to HCO
3
and H
+
. The conversion of CO2
to H
+
and HCO
3
-
ions has tremendous implications
for acid–base physiology. Every day, resting
metabolism produces more than 15,000 mmol of
CO2, or 15,000 mmol/L of carbonic acid, and this
acid leaves the body through the lungs. By
comparison, the kidneys typically excrete only 100
mmol/L of acid per day. The ability to change blood
pCO2 levels rapidly by changing ventilation has a
powerful effect on blood pH, so acid–base balance
depends on the integrated function of respiratory and
renal systems. Regulation of hydrogen ion (H
+
)
balance is similar in some ways to the regulation of
other ions in the body. For instance, to achieve
264
S. Altaan A., Abdallah O., T. Othman M., Musaab N. and Bolz A..
NONINVASIVE MEASUREMENT OF BLOOD ACID-BASE (pH) USING CONCENTRATIONS OF EXHALED GASES.
DOI: 10.5220/0003789502640269
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing (BIOSIGNALS-2012), pages 264-269
ISBN: 978-989-8425-89-8
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
homeostasis, there must be a balance between the
intake or production of H+ and the net removal of
H+ from the body.
1.2 Chemical Relations of CO2 in the
Blood
Hydration (combination with water) of dissolved
carbon dioxide sets up an equilibrium with carbonic
acid, which plays a key role in acid-base balance:
H
2
O+CO
2
H
2
CO
3
In turn, dissociation of this weak acid yields
hydrogen ion and the conjugate base, bicarbonate
ion:
H
2
CO
3
H
+
+ HCO
3
-
Therefore carbonic acid can be viewed as a
traditional stage between the hydration of dissolved
carbon dioxide on one side, and the dissociation into
hydrogen and bicarbonate ions on the other side:
H
2
O+CO
2
H
2
CO
3
H
+
+ HCO
3
-
Increasing one of the chemical species in a system
pushes the equilibrium toward the opposite side.
Thus a rise in carbon dioxide levels in the blood
causes an increase in the hydrogen ion
concentration. Blood acid-base (pH) varies inversely
with hydrogen ion concentration according to the
relation below:
pH = log
1
H
(1)
Table 1: pH values with H
+
ion concentration.
pH Ion Concentration (gram
equivalent per liter)
Type of Solution
0 1.0
Acid Solution
- Hydrogen ions -
H
+
1 0.1
2 0.01
3 0.001
4 0.0001
5 0.00001
6 0.000001
7 0.0000001 Neutral Solution
8 0.000001
Basic (alkaline) Solution
- Hydroxide ions -
OH
-
9 0.00001
10 0.0001
11 0.001
12 0.01
13 0.1
14
1.0
The pure water have pH of 7 which considered
neutral, acid solutions have pH less than 7 like the
orange juice (3-4) while the basic solutions have pH
more than 7 like soapy water (12). Table 1 showed
the pH values with the corresponding hydrogen ion
concentration and the type of solution.
2 METHODS
Because of the direct relation between the carbon
dioxide in the blood and the pH, determining the
level of carbon dioxide in the blood is very
important. The air we inhale is roughly 78% by
volume nitrogen, 21% oxygen, 0.96% argon and the
rest 0.04% contain carbon dioxide, helium, water,
and other gases. The permanent gases in the breath
we exhale are roughly 4% to 5% more carbon
dioxide and 4% to 5% less oxygen than was inhaled.
There are different methods to determine the
CO
2
level in the blood, one of the important one
which is invasive is the Arterial Blood Gas Analysis
method which gives the partial pressure of the O2
and CO2, but what was accomplished in this
research is non-invasive method of determining
pCO
2
and pO
2
in the arterial blood. Measurements
using the Vista-MX device from (Vacu•Med) were
taken from 60 person (45 male, 15 female) in the
rest and for 5 Athletes during exercise. These
measurements give many parameters of the exhaled
breath, we use some of these parameters which are
VO
2
, VCO
2
, O
2
% and CO
2
%. The meaning of each
of these parameters is:
VO
2
: Oxygen consumption in liter per minute.
VCO
2
: CO
2
output in liter per minute.
O
2
% and CO
2
%: the concentrations of oxygen and
carbon dioxide in the exhaled breath.
According to the ideal gas law VO
2
and VCO
2
were
converted to pressure which is then multiplied by the
concentration of the related gas to give the partial
pressure of the oxygen and carbon dioxide in the
exhaled breath (pO
2
and pCO
2
).
P=
n. T.
K
V
(2)
n: number of moles, T: temperature, K: Boltzmann
constant and V: volume. Table 2 shows some gases
constants used to calculate the number of moles for
the above equation.
Table 2: O
2
and CO
2
constants.
O
2
CO
2
molecular weight 31.9989 44.01
Density(kg/m
3
) at 25
o
C 1.308 1.799
After obtaining the partial pressure of oxygen
NONINVASIVE MEASUREMENT OF BLOOD ACID-BASE (pH) USING CONCENTRATIONS OF EXHALED
GASES
265
and carbon dioxide (pO2, pCO2)
in the exhaled
breath, paO2 and paCO2 have to be calculated in the
arterial, this can be done by using the ventilatory
exchange ratio (R), which can be calculated from the
ratio between the carbon dioxide output and the
oxygen uptake:
R
=
VCO2
VO2
(3)
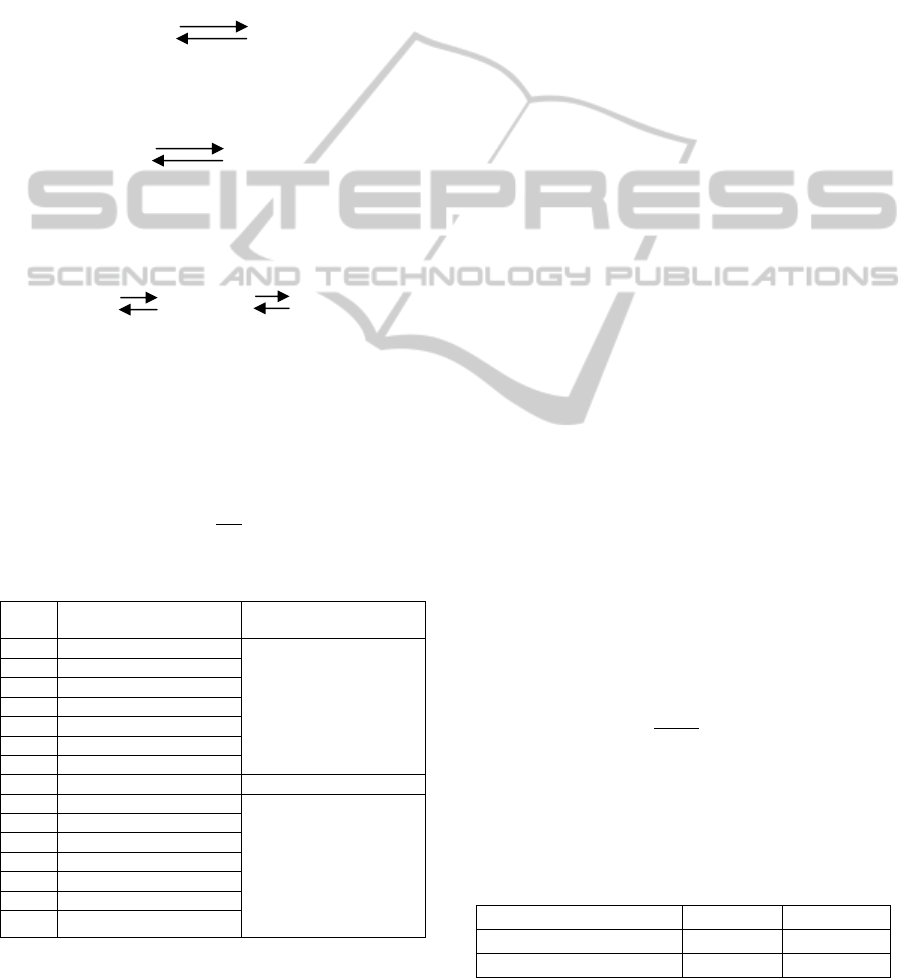
Figure 1 shows ventilatory exchange ratio (R=0.8)
plotted in the pO
2
- pCO
2
plane. The figure shows
four important points for the values of pO2 and
pCO2 (E: Exhaled, A:Alveoli i:ideal and a:arterial).
These four points play the main idea in the
calculations of the partial pressure of oxygen and
carbon dioxide in the arterial blood.
A method is developed in this research to
estimate the actual values of pAO2 and pACO2 in
the Alveoli according to the ventilatory exchange
ratio (R). The ideal values for the pO2 and pCO2 in
the arterial blood are 100 and 40 respectively. But
the actual values may be different from these values.
Most of the references state that the partial pressure
of the carbon dioxide is the same in the alveoli and
the alveoli arteries. The arterial partial pressure of
oxygen was calculated from alveoli pAO2 using the
equation below which gives the normal gradient
between the partial pressure of oxygen in the alveoli
and the arterial:
Normal A-a gradient = (Age+10) / 4 (4)
After getting the arterial partial pressure of the
oxygen and carbon dioxide, a neural network was
built to determine the level of blood acid-base (pH).
The neural network based on the arterial pO2 and
pCO2 which are directly related to the pH of the
blood.
Figure 1: Ventilatory exchange ratio (R).
3 RESULTS
The theoretical chemical reaction states that increase
the carbon dioxide in the blood causes increasing
hydrogen ion in the blood which results in lower pH
(the logarithmic relation), that means the blood
becomes acidic. Also vice versa is correct, when the
carbon dioxide decreases in the blood causes
decreasing the hydrogen ion which results in higher
pH and the blood becomes alkaline.
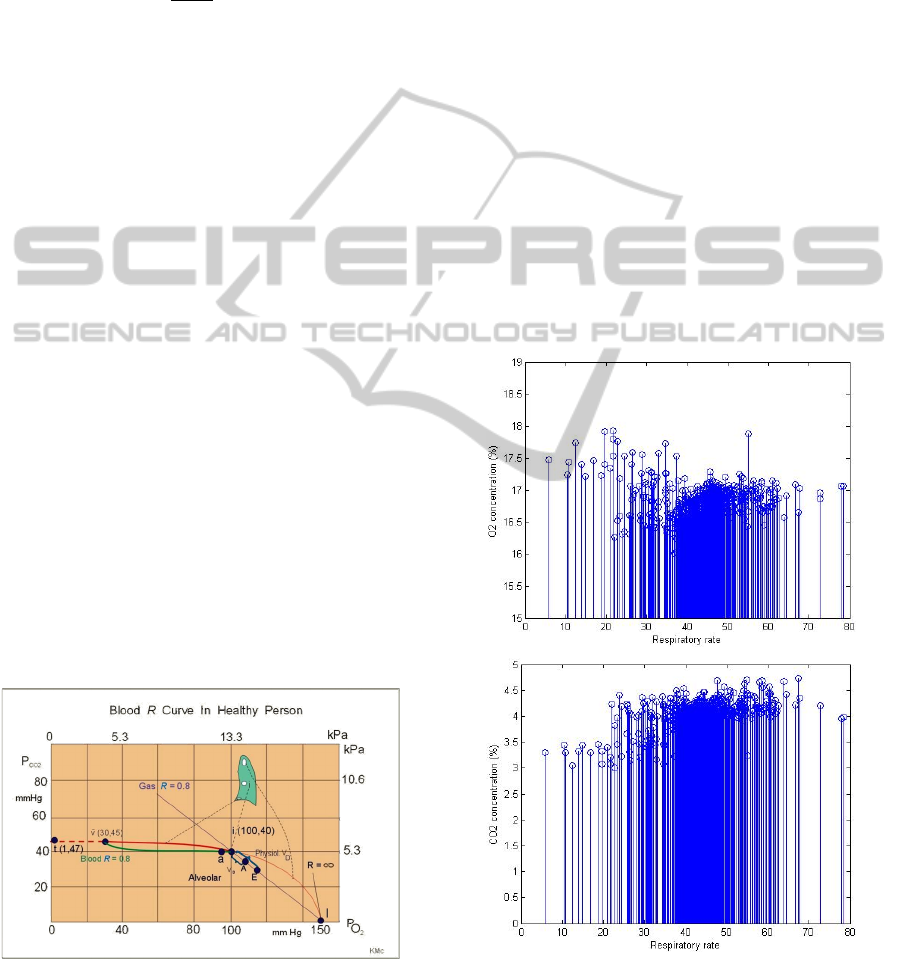
At the beginning the developed algorithm was
applied to the data obtained from the athletes. As
stated in the introduction section about the
consumed oxygen which is increased during the
exercise that results the concentration of the exhaled
oxygen to be decreased. Figure 2 (Up) clarifies the
decrease in oxygen concentrations with the
respiratory rate in the exhaled breath during
exercise. While the opposite happened with the
carbon dioxide concentration which is increased
with increasing the respiratory rate as shown in
figure 2 (Down).
Figure 2: Up: change of the O2 concentration with the
respiratory rate. Down: change of the CO2 concentration
with the respiratory rate.
When the muscles are working harder they
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
266

release more carbon dioxide, this will causes the
partial pressure of carbon dioxide in the blood to be
raised, and as a result the concentration of the CO2
in the exhaled breath will be increased. The body
respond to this change in gases concentrations by
raises the respiratory rate to throw out more CO2
from the body. Figure 3 shows the change of the
CO2 concentration in the exhaled breath with the
partial pressure of CO2 in the Blood. Figure 4 shows
the increase of the partial pressure of the carbon
dioxide with the respiratory rate.
Figure 3: Change of the pCO2 in the arterial with the CO2
concentration in the exhaled breath.
Figure 4: Change of the pCO2 in the arterial with the
respiratory rate.
After the practical proof of the direct relation
between the concentration of the exhaled gases with
the partial pressure of these gases in the blood, a
neural network has been designed to determine the
blood acid-base level depending on the partial
pressure of the gases. A neural network was built,
two types of the neural networks achieved the goals,
the characteristics of the two neural networks chosen
are listed in the table 3.
The network was trained by data sets (from rats)
have been taken from Bioinformatics Program of
Human & Molecular Genetics Center-Medical
College of Wisconsin, USA. Two data groups were
used the first one contain 673 data sets and the
second contain 860 data sets. During the network
training many of learning functions were used and
many performances of the network were obtained.
One of the best performances of the neural network
is shown in the figure 5.
Table 3: Neural network characteristics.
Network type Layer
Recurrent
Elman bachprop
Training function Traingdm Traingdm
Adaption learning
function
Learngdm Learngdm
No. Of Layers 2 2
No. Of neurons 10 10
Transfer function Purelin Purelin, Tansig
Figure 5: Neural network performance.
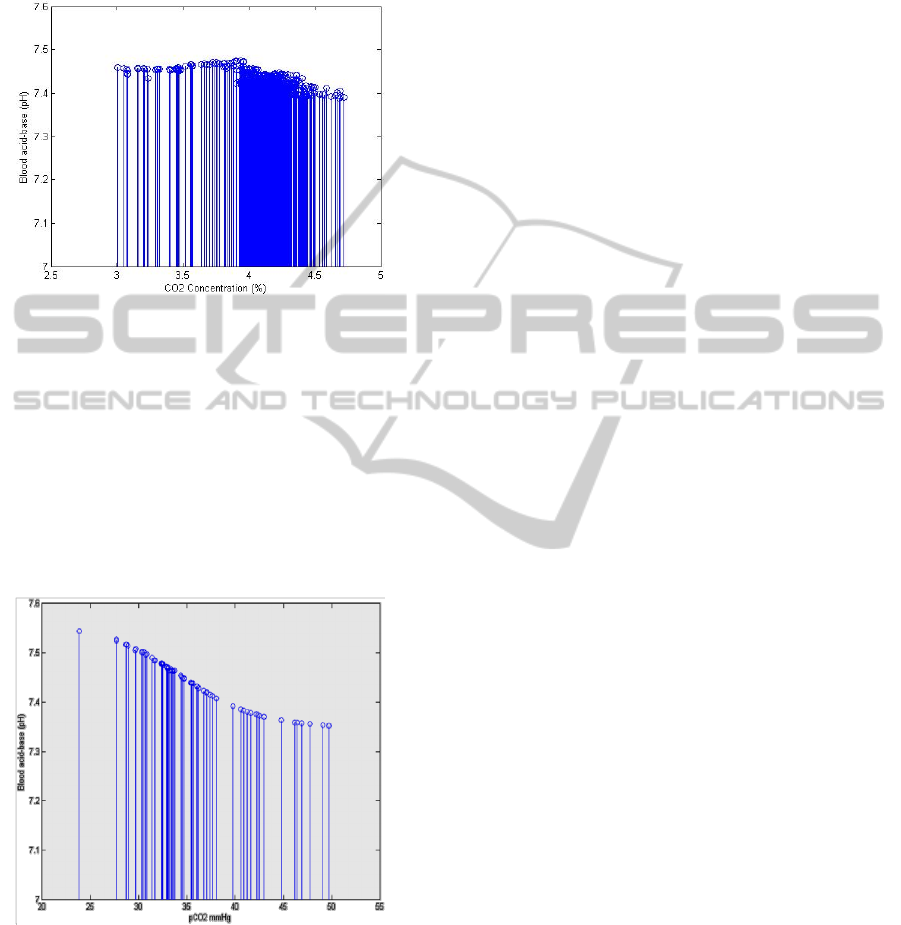
The results given by the neural network are clear.
They give that the change of the blood acid-base
level is directly related to the change of the partial
pressure of the CO2 in the blood. Figure 6 shows the
clear inversely change of the pH with the partial
pressure of the carbon dioxide in the blood.
Figure 6: Change of the pH with the pCO2.
NONINVASIVE MEASUREMENT OF BLOOD ACID-BASE (pH) USING CONCENTRATIONS OF EXHALED
GASES
267
Then it will be obvious that the pH will be
changed with the concentration of the CO2 in the
exhaled breath because of the change of pCO2 with
CO2 concentration, figure 7 shows this change.
Figure 7: Change of the pH with the concentration of CO2
in the exhaled breath.
As stated in the beginning of the result section all
of the above results were for the athletes during
exercise. Also the same algorithms were applied to
individuals at the rest. The obtained results show the
inverse changes of the pH level with the
increase/decrease of CO2 in the blood. Figure 8
shows the results of inversely change of pH with the
partial pressure of CO2 in the arterial blood for
individuals.
Figure 8: Change of the pH with the pCO2 for persons at
the rest.
4 CONCLUSIONS
Blood pH is tightly regulated by a system of buffers
that continuously maintain it in a normal range of
7.35 to 7.45 (slightly alkaline). Carbon dioxide is
one of the central roles in this blood pH abnormality.
Resting metabolism produces more than 15,000
mmol of CO2, or 15,000 mmol/L of carbonic acid,
and this acid leaves the body through the lungs.
More CO2 in the blood causes more hydrogen ion
which is the major factor specifies the blood acid-
base level pH. This means that the respiratory
system is playing an important role in the regulation
of blood acid-base level pH. This fact lead us to
develop a noninvasive method for finding pH
depending on exhaled gases.
A direct method to noninvasively determine the
blood acid-base level pH was developed. The
developed method depends on the concentration of
the carbon dioxide that the person exhaled. The CO2
concentration is associated with the level of the
partial pressure of CO2 in the blood which we used
to determine the pH level. The increase of CO2
causes the blood to be acidic while decrease CO2
makes the blood more alkaline. Increasing the CO2
in the blood causes increasing the respiratory rate to
exhaled more CO2 which results the pH to be
returned to its normal level.
ACKNOWLEDGEMENTS
We thank the staff and the students in the
Department of Physical Education-College of
Basic Education-Mosul University in Iraq, who
help us to get the data of more than 80 people.
REFERENCES
T. Tiger, J. K. Kirk, R. J. Solomon, 1999. Mathematical
Concepts in Clinical Science, Prentice Hall.
Instruction Manual, Manual No. X17001-5, TurboFit_
Software for Windows Version 5.11, Last Update 17
February 2010.
Poul-Erik Paulev, M. D., D.Sci, 1999 – 2000. Medical
Physiology And Pathophysiology Essentials and
clinical problems, Copenhagen Medical Publishers.
J. S. Gravenstein, Michael B. Jaffe, David A. Paulus,
2004. Capnography: clinical aspects, Cambridge
University Press, United Kingdom.
Rob Law, H. Bukwirwa, 1999. The Physiology of Oxygen
Delivery.
http://www.shapesense.com/fitnessexercise/articles/vo2-an
d-vo2max.aspx#whatareVO2andVO2max
Terry Des Jardins, MEd, RRT, 2002. Cardiopulmonary
Anatomy & Physiology Essentials for Respiratory
Care, Delmar, a division of Thomson Learning, Inc.
Thomson Learning Fourth Edition.
Michael Krause, Andrea Doescher, Beate Zimmermann,
BIOSIGNALS 2012 - International Conference on Bio-inspired Systems and Signal Processing
268

and Thomas H. Müller, 2010, Noninvasive pH
measurement to monitor changes during suboptimal
storage of platelet concentrates, Transfusion
2010;50:2185-2192.
Bhavani-Shankar K, Moseley H, Kumar A Y et al. 1992
Capnometry and Anaesthesia. Can J Anaesth; 39:
617–32.
Lawrence Martin, M. D., 1999, All You Really Need to
Know to Interpret Arterial Blood Gases , 2
nd
edition.
Babs R. Soller, PhD, Ronald H. Micheels, PhD, John
Coen, B. S., Bhairavi Parikh, M. S., Ling Chu, PhD,
and Charles Hsi, MiD, 1996, Feasibility of Non-
invasive Measurement of Tissue Ph using Near-
infrared Reflectance Spectroscopy, Journal of Clinical
Monitoring 12: 387-395.
Arthur C. Guyton, M. D., 2006, John E. Hall, Ph.D.,
Textbook of Medical Physiology, Elsevier Inc.
NONINVASIVE MEASUREMENT OF BLOOD ACID-BASE (pH) USING CONCENTRATIONS OF EXHALED
GASES
269
