
LOW-COST ENZYME-BASED BIOSENSOR FOR LACTIC
ACID AMPEROMETRIC DETECTION
Electrical Modeling and Validation for Clinical and Food Processing Applications
M. Scaramuzza
1
, A. Ferrario
1
, E. Pasqualotto
1
, G. Rosati
1
, A. De Toni
1
, M. Quarta
2
,
A. Paccagnella
1
and C. Reggiani
2
1
Department of Information Engineering, University of Padova, via Gradenigo 6/B, Padova, Italy
2
Department of Human Anatomy and Physiology, University of Padova, via Marzolo 3, Padova, Italy
Keywords: Lactate oxidase, Amperometric detection, Low-cost, Clinical applications, Food processing applications.
Abstract: In this work we present the preliminary resulting measurements of an enzyme-based biosensor for the
amperometric detection of lactic acid (LA). The sensor is based on low-cost gold electrodes on polymeric
substrate. The redox catalytic enzyme used for analyte amperometric detection is lactate oxidase (LOx)
from Pediococcus sp. This enzyme has been immobilized over electrodes surfaces by direct adsorption
methodologies. Analysis of the enzyme-modified electrodes have been carried out by means of
Electrochemical Impedance Spectroscopy (EIS) and with the development of an equivalent electrical model,
in order to improve the adsorption process. Biosensors performance have been evaluated with Cyclic
Voltammetry (CVM) measurements in different lactic acid solutions with concentrations from 1 μM up to
300 mM. The lactate sensitivity of this disposable biosensor results in about 6.24 µA mM
-1
cm
-2
.
1 INTRODUCTION
Lactic acid detection with low-cost devices
represents a growing need both in medical and food
processing applications, where large-scale
screenings are required (Castillo and Gáspár, 2004).
In food processing applications, LA
measurements are an easy and effective way to
determine food quality because lactate is correlated
with degradation processes in milk products
(Palmisano, Quinto et al., 2001). Lactic acid is
noticeable in biotechnology, health care, and sports
medicine applications because its levels are tightly
correlated with mortality of patients in shock status
or during hypoxia. Normal physiological blood
lactate concentrations are below 2 mM, and lactate
levels exceeding 7–8 mM are usually associated
with multiple organ failure (Sung, Bae et al., 2006).
The most widely used technique for LA
detection is a colorimetric and chromatographic
analysis, which are expensive and time-consuming
due to sample pre-treatment. Conversely, both
amperometric (Boujtita and Chapleau, 1996) and
potentiometric (Lee, Wu et al., 2008)
electrochemical analysis allow high sensitivity and
fast response. Two principal electrochemical
enzyme-modified sensors refer to L-lactate oxidase
(LOx) and L-lactate dehydrogenase. This work
investigate LOx-modified gold electrodes for LA
detection. The related catalytic reaction pathway is
(Gamero and Pariente, 2010):
L-lactate + (LOx)
OX
→ pyruvate + (LOx)
RED
(LOx)
RED
+ (Med)
OX
→ (LOx)
OX
+ (Med)
RED
(Med)
RED
→ (Med)
OX
+ e
−
where ferricianyde/ferrocianyde has been used as
redox mediator (Med). EIS measurements and an
equivalent electrical model have been used to
analyze enzyme adsorption on gold surfaces and its
role on sensor efficiency.
2 DEVICE AND
MEASUREMENTS SET-UP
EIS measurements have been performed with a
Solartron SI1260 impedance analyzer in a frequency
range between 1 Hz and 1 MHz. An Ag/AgCl in
KCl 1 M (CHI111, CH Instruments Inc., US) has
380
Scaramuzza M., Ferrario A., Pasqualotto E., Rosati G., De Toni A., Quarta M., Paccagnella A. and Reggiani C..
LOW-COST ENZYME-BASED BIOSENSOR FOR LACTIC ACID AMPEROMETRIC DETECTION - Electrical Modeling and Validation for Clinical and
Food Processing Applications.
DOI: 10.5220/0003867603800383
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 380-383
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

been used as reference electrode. A custom-built
Labview program has been developed to drive the
instrumentation and acquire measurements data.
CVM measurements have been performed with a
CHI 440A potentiostat (CH Instruments Inc., US)
with the electrochemical cell composed by the
previous Ag/AgCl electrode and a platinum counter
electrode (CHI129, CH Instruments Inc., US). The
potential range investigate was -0.20 V to +0.70 V
for Fe(CN)
6
4-/3-
with a scan rate of 50 mV/s. All data
have been analyzed with MATLAB 7.9, while fitting
results have been obtained with ZVIEW
2.80
(complex fitting, data-modulus weighting) software.
LOx, PBS (Phosphate Buffer Saline), redox
mediators and other chemicals have all been
provided by Sigma Aldrich. LOx (50 units/mg) has
been obtained as lyophilized powder. All solution
has been prepared with Millipore water.
The biosensor consists of circular 50 nm thick
gold electrodes with 1 mm diameter and of a gold
contact pad both on polymeric substrate. Electrodes
have been contacted through micro-positioned
probes (Wentworth Laboratories).
Figure 1: CVM measurements on 1 mm diameter
electrodes for different redox mediator concentrations with
scan rate of 50 mV/s.
3 REDOX MEDIATOR
CONCENTRATION
During gold electrodes surfaces preliminary
electrical characterization, EIS and CVM
measurements have been performed in “fresh”
condition, i.e. gold electrodes without any biological
coverage. The aim of these measurements was to
determine the best trade-off between SNR (Signal-
to-Noise Ratio) of interfacial charge transfer and
redox mediator concentration (see Figure 1). From
these tests the measurement solution has been
chosen as ferri/ferrocyanide 1 mM in PBS 100 mM.
4 COVERAGE STUDY AND
FITTING TECHNIQUE
An equivalent electrical model has been developed
to assess electrodes enzymatic coverage (see Figure
2). The main feature of this model is the a-weighted
contribution of two different electrical impedances
(Huang, Nguyen et al., 2004).
Figure 2: Equivalent electrical model for electrode
coverage studies. Parameter a is the numerical impedance
multiplier. Free electrode interface equivalent element
CPE
fresh
is multiplied for (1-a), while full enzyme-covered
electrode impedance for a.
Enzyme-free electrodes have been measured
with EIS in PBS and results have been fitted with an
equivalent electrical model composed of a series
between a Constant Phase Element CPE (Onaral,
Sun et al., 1984), CPE
fresh
, and a parallel between
R
Au
and C
Au
: the former is related to electrochemical
interfacial processes between electrodes and
electrolyte, while the other takes into account the
electrodes material. Impedance frequency
dependence is modeled with CPE
fresh
=
1/C
fresh
(j2πf)
nfresh
, where f is the frequency and n
fresh
is
a number between 0 and 1. Values for R
Au
and C
Au
(see Table I) have been obtained using COMSOL
Multiphysics 3.4 (AC/DC In-plane electric currents
module) to simulate the sensor electrical impedance
(2 cm length, 50 nm thick) with 1 mm diameter
active circular area and 100 μm probe contact area.
Gold electrical parameters have been set to
conductivity σ = 4.5
10
7
S/m and relative dielectric
permittivity ε
r
= 6.9. Electrolyte spreading resistance
R
e
value has been obtained by fitting EIS
measurements of PBS. To obtain second branch
model parameters values, electrodes surfaces have
been full covered with 25 mg/mL LOx in PBS 100
mM solution. The immobilization protocol consisted
in the deposition of 1 μL of enzyme solution directly
onto the electrodes for 1 hour and then rinsed with
0 0.1 0.2 0.3 0.4
-1
-0.5
0
0.5
1
x 10
-5
Voltage [V]
Current [A]
0.5 mM
1 mM
2 mM
5 mM
LOW-COST ENZYME-BASED BIOSENSOR FOR LACTIC ACID AMPEROMETRIC DETECTION - Electrical
Modeling and Validation for Clinical and Food Processing Applications
381

de-ionized water (Parra and Casero, 2006).
Functionalization data have been fitted with similar
equivalent circuit with CPE
LOx
. In the complete
electrical model the weighting parameter a is the
only fitting variable. The behavior of a as a function
of immobilized LOx concentration C
LOx
has been
obtained from EIS measurements: each curve
depicted in Figure 3 has been fitted using the
coverage model, obtaining a values described in
Figure 4.
Figure 3: EIS measurements on round gold electrodes (1
mm diameter) with different concentrations of
immobilized LOx. Box: EIS response |Z| at 1 kHz.
Figure 4: Variation of coverage parameter a as a function
of enzyme concentration C
LOx
. Fitted parameters are: m =
1.086, n = -0.7414, p = -0.8473. With the described power
function the goodness of fit is R
2
= 0.9979.
As can be seen from Figure 5, parameter a trend
reaches a saturation level for LOx concentrations
above about 10 mg/mL, which corresponds to a =
0.94, therefore in these conditions an adequate
electrode coverage degree is obtained. Moreover,
sensors electrical response as a function of C
LOx
has
been evaluated with CVM measurements in order to
assess the electric transduction efficiency. Figure 5
shows CVM reduction currents peaks with 1 mM
and 100 mM LA in measurement solution: the trade-
off between SNR and electrodes coverage can be
obtained for C
LOx
= 10 mg/mL.
Figure 5: CVM peak currents with different concentrations
of immobilized LOx and 1 mM (blue) and 100 mM (red)
LA. For C
LOx
below 10 mg/mL the solutions cannot be
properly detected.
Table 1: Electrical parameters for Figure 2 model.
Parameter Value Error [%]
R
Au
0.6078 Ω -
C
Au
2.62 10
-18
F -
CPE
fresh
C
fresh
4.4 10
-7
F 1.82
n
fresh
0.89784 0.35
R
e
821 Ω 0.94
CPE
LOx
C
LOx
2.3 10
-7
F 1.71
n
LOx
0.88817 0.31
5 LACTIC ACID DETECTION
The enzymatic biosensor has been tested with LA
racemic mixture at different concentrations. First 2
mm diameter gold electrodes have been used to
detect LA concentration range between 70 μM up to
1.2 M in order to verify transduction saturation
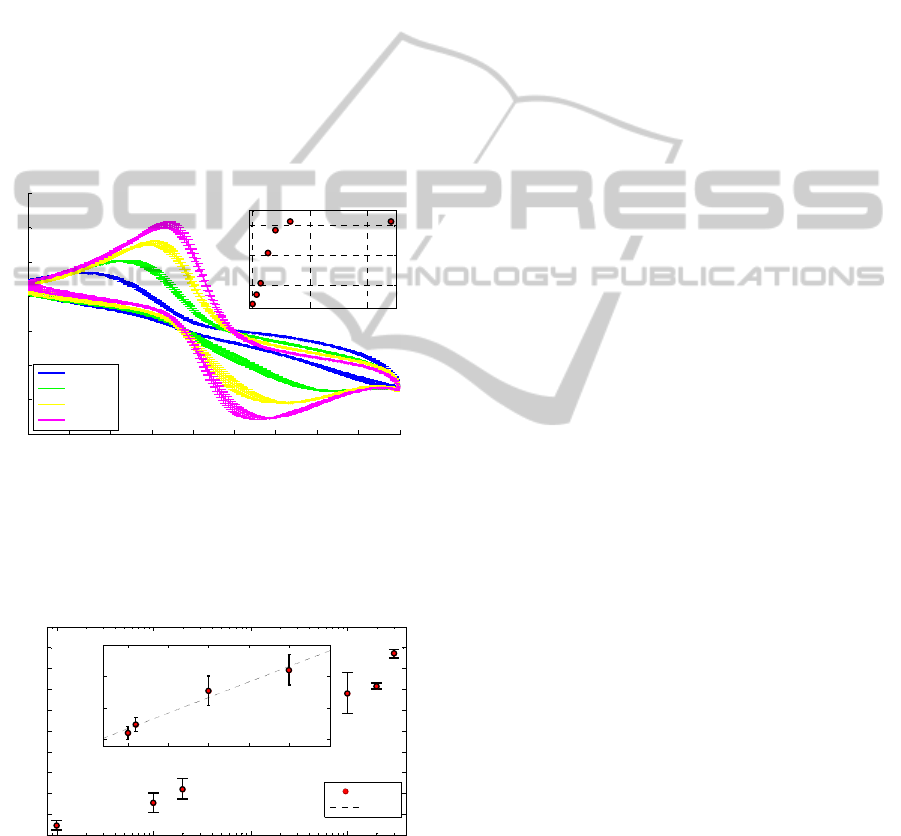
levels. Results of CVM measurements are depicted
in Figure 6. In order to study sensor lower detection
limits and sensitivity, CVM measurements have
been carried out in a lower LA concentration range,
i.e. down to 1 μM, with 1 mm diameter electrodes.
Figure 7 depicts reduction peaks currents as a
function of LA concentration and sensor linear
response range comparable with other works in
literature (Gamero and Pariente, 2010). The
corresponding LA sensitivity is about 6.24 µA mM
-1
cm
-2
(Jena and Raj, 2006).
6 CONCLUSIONS
Low-cost gold electrodes-based sensors have been
functionalized with immobilized lactate oxidase
enzymes using direct adsorption protocols.
Electrodes surface coverage has been investigated
10
2
10
3
10
4
10
5
10
6
10
3
10
4
Frequency [Hz]
|Z| [
Ω
]
PBS
1 mg/mL
2 mg/mL
5 mg/mL
7 mg/mL
10 mg/mL
25 mg/mL
0 5 10 15 20 25
1500
2000
2500
C
LOx
[mg/mL]
|Z| [
Ω
]
1 2 5 10 25
0
0.5
1
1.5
x 10
-6
C
LOx
[mg/mL]
i
p,red
[A]
LA 1 mM
LA 100 mM
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
382
with EIS measurements in concentration range
between 1 mg/mL up to 25 mg/mL. An equivalent
electrical model based on a weighted contribution
approach has been developed in order to obtain a
numeric parameter that is related to the electrode
coverage. Both this methodology and CVM
measurements have identified in 10 mg/mL the
enzyme concentration that leads to adequate
electrodes coverage. The sensors lactic acid
detection performance have been evaluated using
CVM measurements: the upper saturation levels is
reached at about 300 mM, and the sensitivity is
about 6.24 µA mM
-1
cm
-2
.
This work is part of a University of Padova
research project for the development of an
innovative cell-based Lab-On-Chip for clinical and
food-processing massive screening applications.
Figure 6: CVM response of 2 mm diameter gold sensors
with different LA concentration in measurement solution.
Box: CVM reduction peaks currents as a function of LA
concentrations. The response saturates for LA
concentrations above 300 mM.
Figure 7: CVM reduction peaks currents as a function of
LA concentrations for round gold electrodes (1 mm
diameter). The tested LA concentrations vary from 1 μm
up to 300 mM. Box: magnification in milli-molar range.
Regression line: i
p,red
= 4.9 10
-2
C
LA
+ 1.1 10
-6
.
ACKNOWLEDGEMENTS
This project is partially supported by the University
of Padova project “Design and validation of a
biosensor to monitor myogenic cell growth in vitro”
(Department of Human Anatomy and Physiology,
Department of Information Engineering). This
project is partially supported by University of
Padova project MISCHA (Microfluidic laboratory
for Scientific and teCHnological Applications).
REFERENCES
Boujtita, M.; Chapleau, M. and Mum, N. E. (1996)
Enzymatic Electrode for the Determination of L-
Lactate, Electroanalysis, 8 (5), 485-488.
Castillo, J. and Gáspár, S. (2004). Biosensors for life
quality: Design, development and applications.
Sensors and Actuators B, 102, 179-194.
Gamero, M. and Pariente, F. (2010). Nanostructured rough
gold electrodes for the development of lactate oxidase
– based biosensor. Biosensors and Bioelectronics, 25,
2038-2044.
Huang, X.; Nguyen D.; Greve, D. W. and Domach M. M.
(2004). Simulation of Microelectrode Impedance
changes Due to Cell Growth, IEEE Sensors Journal, 5
(4), 576-583.
Jena, B. K. and Raj, C. R. (2006) Electrochemical
Biosensor Based on Integrated Assembly of
Dehydrogenase Enzymes and Gold Nanoparticles,
Anal. Chem., 78, 6332-6339.
Lee, H. C.; Wu, W. Y.; Lin, J. L.; Chin, Y. L.; Lee, K. Y.
and Sun, T. P. (2008) Evolution of the TiO2
membrane on ITO PET substrate applied to a lactate
biosensor using a potentiometric differential readout,
circuit Sensors, 2008 IEEE, 898-901.
Onaral, B.; Sun H. H. and Schwan, H. P. (1984). Electrical
Properties of Bioelectrodes, IEEE Transactions on
Biomedical Engineering, 12 (BME-31), 827-832.
Palmisano, F.; Quinto M; Rizzi R. and Zambonin (2001)
Flow injection analysis of L-lactate in milk and
yoghurt by on-line microdialysis and amperometric
detection at a disposable biosensor, Analyst, 126, 866-
870.
Parra, A. and Casero, E. (2006). Design and characteri-
zation of a lactate biosensor based on immobilized
lactate oxidase onto gold surfaces. Analytica Chimica
Acta, 555, 308-315.
Sung, W. J. and Bae, Y. H. (2006) Glucose oxidase,
lactate oxidase, and galactose oxidase enzyme
electrode based on polypyrrole with polyanion/PEG/
enzyme conjugate dopant, Sensors and Actuators B,
114, 164-169.
-0.2 -0.1 0 0.1 0.2 0.3 0.4 0.5 0.6 0.
7
-6
-4
-2
0
2
4
6
8
x 10
-6
Voltage [V]
Current [A]
w/o LA
0.07 mM
0.13 mM
1.2 mM
0 0.5 1
4
5
6
x 10
-6
C
LA
[M]
i
p,red
[A]
0 0.5 1 1.5 2 2.5
-2
0
2
x 10
-7
residuals
10
-4
10
-3
10
-2
10
-1
1.15
1.2
1.25
1.3
1.35
1.4
1.45
1.5
1.55
x 10
-6
C
LA
[M]
i
p,red
[A]
0 0.5 1 1.5 2 2.5
1.1
1.15
1.2
1.25
x 10
-6
C
LA
[mM]
i
p,red
[A]
data
linear
LOW-COST ENZYME-BASED BIOSENSOR FOR LACTIC ACID AMPEROMETRIC DETECTION - Electrical
Modeling and Validation for Clinical and Food Processing Applications
383
