
POLYSSACHARIDE-BASED MAGNETIC HYDROGELS AS
POTENTICAL VECTORS FOR EXTERNAL-CONTROLLED
SOLUTE RELEASE
Alexandre T. Paulino
1,2
, Laurence A. Belfiore
2
, Matt J. Kipper
2
and Elias B. Tambourgi
1
1
School of Chemical Engineering, Department of Chemical System Engineering, Separation Process Laboratory
State University of Campinas, Av. Albert Einstein, 500, Bloco A, Cidade Universitária, Campinas, SP 13083-852, Brazil
2
Department of Chemical & Biological Engineering, Polymer Physics & Engineering Laboratory
Colorado State University, Fort Collins, CO 80523, U.S.A.
Keywords: Magnetic hydrogel, Polysaccharide, External-controlled solute release.
Abstract: This work describes the synthesis of polysaccharide-based magnetic hydrogels with the introduction of
magnetite nanoparticles in the polymer network. The magnetic hydrogels were characterized by Fourier-
transform infrared spectroscopy (FTIR) and magnetization curves. FTIR analysis confirmed the efficiency
of the polysaccharide-modifying process. The amounts of diffused water into or out-of a hydrogel network
were measured. The degree of swelling of the polysaccharide-based magnetic hydrogels was less than that
found for the regular polysaccharide-based hydrogels and there was no variation in the water diffusion
mechanism. The absence of hysteresis loops and coercivity observed through magnetization curves
indicated that magnetic hydrogels can be applied in external-controlled solute release.
1 INTRODUCTION
In recent years, there has been substantial interest in
the potential applications of functionalized
hydrogels. These types of hydrogels have many
important properties and offer advantages over non-
functionalized hydrogels (Chaterji et al., 2007);
(Bajpai et al., 2008). The advantages of such
hydrogels have been observed through some studies
on controlled drug release systems, cell
proliferation, water treatment, soil conditioning and
so forth. (Oh et al., 2008); (Arizaga et al., 2010);
(Deligkaris et al., 2010).
Functionalized hydrogels based on
polysaccharides, proteins and amino acids have been
extensively studied for medical, pharmaceutical and
biological application due to their properties of
biocompatibility with living organisms,
biodegradability, accessibility and renewability
(Morelli and Chiellini, 2010). In this contribution,
external magnetic field-sensitive functionalized
hydrogels based on gum arabic, chitosan and
maltodextrin were synthesized with the introduction
of magnetite nanoparticles in the polymer network.
The potentiality of these hydrogels as magnetic
vectors for external-controlled solute release was
investigated by FTIR, magnetization curves and
water absorption kinetic.
2 EXPERIMENTAL
PROCEDURES
2.1 Materials
Chitosan (Aldrich), acetic acid (Merck), acrylic acid
(Merck), glycidyl methacrylate (Across Organics),
methylenebisacrylamide (Merck), hydrochloric acid
(Merck), ammonium persulfate (Aldrich), gum
arabic (Sudan), sodium hydroxide (Nuclear),
acrylamide (Aldrich), potassium acrylate (Aldrich),
maltodextrin (Aldrich), ethanol (TEDIA), magnetite
nanoparticles (Fe
3
O
4
) purchased from Fisher
Scientific and characterized elsewhere (Paulino et
al., 2009). All experiments were performed using
Milli-Q
®
water.
2.2 Hydrogel Synthesis
1% chitosan solution was prepared by diluting the
263
T. Paulino A., A. Belfiore L., J. Kipper M. and B. Tambourgi E..
POLYSSACHARIDE-BASED MAGNETIC HYDROGELS AS POTENTICAL VECTORS FOR EXTERNAL-CONTROLLED SOLUTE RELEASE.
DOI: 10.5220/0003870802630268
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 263-268
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

appropriate amounts of the polysaccharide with
acetic acid in a 50-mL beaker. Gaseous nitrogen
was purged into the system for 30 min in order to
remove the dissolved oxygen. Known amounts of
ammonium persulfate were then introduced to
initiate chitosan for the generation of radicals. The
complete polymerization reaction took place at 70
ºC for three hours after adding 15 mL of Milli-Q
®
water, acrylic acid, methylene-bis-acrylamide and
magnetite nanoparticles. The hydrogel was purified
and dried in an oven at 50 ºC for 12 to 24 hours.
Prior to synthesizing the hydrogels based on
either gum arabic or maltodextrin, the specific
polysaccharide was modified through
functionalization using glycidyl methacrylate
(Dorkoosh et al., 2002
a
, 2002
b
). Each synthesis was
carried out through the solubilization of the specific
modified polysaccharide in an aqueous solution
containing acrylamide, potassium acrylate,
ammonium persulfate and magnetite nanoparticles.
The solution was heated to 65 °C for some minutes
under constant stirring. The hydrogel obtained was
purified and dried in an oven at 50 ºC for 12 to 24
hours.
2.3 Water Uptake Mechanism
Pieces of previously dried hydrogel with masses
ranging from 50 to 100 mg were placed in contact
with 100 mL of water for different contact times,
with pH controlled at around 6.5. The degree of
swelling (DS) was calculated using Eq. 1:
DS=
m
−m
m
(1)
in which m
s
and m
d
are the masses of swollen and
dried hydrogel, respectively.
The absorption mechanism of water in a three-
dimensional hydrogel structure has been described
based on the diffusion phenomena and
macromolecular relaxation of the three-dimensional
structure. This approach is related to Fickian
diffusion processes, in which the coefficient (n) is a
parameter that describes the adsorption mechanism
(Hallinan et al., 2010), the values (1/min) of which
are determined from the fraction curves of diffused
water (M
t
/M
eq
) in function of time, as presented
mathematically in Eq. 2:
M
M
=kt
(2)
in which M
t
and M
eq
are the masses (g) of water
absorbed by a hydrogel at a specific time and in
equilibrium, respectively, and k (dimensionless) is
the proportionality constant of the polymer network
of a particular hydrogel.
The Fickian diffusion model sets the absorption
of water in a three-dimensional structure until 70 %
of initial absorption. Above 70 %, there is no
linearity in the graph curve ln(M
t
/M
eq
) versus time
(t). The linear regression of Eq. 2 is seen in Eq. 3:
log
=log+ log
t
(3)
The n values for different released solutes have been
commonly characterized by Fickian diffusion, non-
Fickian diffusion (anomalous) and Super Case II
models. For n values equal to 0.45, the solute
transport is characterized by the Fickian diffusion
model. In this case, it is considered that water
molecules may simply diffuse through the polymer
network by diffusion processes. For n values
between 0.45 and 0.89, the solute diffusion is
characterized by the non-Fickian diffusion model. In
this case, the diffusion mechanism is characterized
by two processes occurring simultaneously –
diffusion through the pores and macromolecular
structure relaxation. When the phenomenon of
macromolecular relaxation is involved, there is a
direct relationship with the flexibility of the polymer
network. Finally, when the n value is higher than
0.89, the diffusion mechanism of solutes in three-
dimensional polymer structures is governed
exclusively by macromolecular structure relaxation,
i.e., Super Case II model (Halligan et al., 2010;
Paulino et al., 2011).
2.4 FTIR Analysis
The samples of the polysaccharide-based hydrogels
were characterized in potassium bromide pellets
using FTIR spectra (FT-GO
max
Bomem Easy
MB−100, Nickelson). To achieve the best resolution
(4.0 cm
−1
), 21 scans min
−1
were run for each
spectrum.
2.5 Magnetization Analysis
Magnetization curves for the polysaccharide-based
magnetic hydrogel were measured using a vibrating-
sample magnetometer (physical properties
measurement system (PPMS)−9, Quantum Design,
SQID magnetometer), with a maximal magnetic
field of 7 T and sensibility of 10
-6
emu at a
temperature of 573 K.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
264

3 RESULTS AND DISCUSSIONS
3.1 Degree of Swelling
The degree of swelling decreased from 8.78 to 6.48
g of water per gram of chitosan-based dried
hydrogel concurrently with the increase in magnetite
concentration from 0.0 to 5.5 wt.-%, respectively.
The degree of swelling of the chitosan-based
magnetic hydrogels was less than that found for the
chitosan-based hydrogel without magnetic behavior
(0 wt.-% of magnetite). The same behavior was
observed with hydrogels based on modified
maltodextrin and modified gum arabic, as a lesser
degree of swelling occurred in magnetic hydrogels.
An electrostatic repulsion generated between the
ionized groups of both the magnetic hydrogels and
the hydrogels without magnetic properties may
expand the polymer network (Jiang et al., 2010).
This phenomenon helps destabilize the structures of
the material, thereby allowing the diffusion of water
and solutes in and out-of the polymer matrix
(Paulino et al., 2009; 2010). Thus, a greater amount
of magnetite used in the hydrogel synthesis leads to
a greater degree of crosslinking due to the formation
of covalent bonds between the iron and hydroxyl or
amine groups. With this logic, a greater density of
crosslinking decreases the swelling rate.
3.2 Water Diffusion Mechanism
Table 1: Water diffusion exponent values (n) at 25
o
C into
hydrogels based on chitosan, modified maltodextrin and
modified gum arabic (pure hydrogel) and with known
amounts of magnetite (magnetic hydrogel).
Chitosan-based hydrogel (Pure hydrogel) and magnetic
hydrogels
(n) (R
2
)
Pure hydrogel
1.9 wt.-% magnetite
0.5478
0.5474
0.9918
0.9907
2.9 wt.-% magnetite 0.5910 0.9841
5.5 wt.-% magnetite 0.6046 0.9885
Modified-maltodextrin-based hydrogel (Pure hydrogel)
and magnetic hydrogels
(n) (R
2
)
Pure hydrogel 0.5430 0.9950
1.9 wt.-% magnetite 0.5545 0.9993
2.9 wt.-% magnetite 0.5479 0.9973
5.5 wt.-% magnetite 0.5492 0.9982
Modified-gum-arabic-based hydrogel (Pure hydrogel) and
magnetic hydrogels
(n) (R
2
)
Pure hydrogel 0.6201 0.9982
1.9 wt.-% magnetite 0.6307 0.9927
2.9 wt.-% magnetite 0.6385 0.9857
5.5 wt.-% magnetite 0.6496 0.9921
Table 1 displays the water diffusion exponent (n) for
hydrogels based on chitosan, modified maltodextrin
and modified gum arabic, containing magnetite
concentrations ranging from 0.0 to 5.5 wt.-%. The n
values ranged from 0.5430 to 0.6496, indicating the
non-Fickian diffusion model, with a tendency
toward macromolecular structure relaxation.
Moreover, these results indicate that there is no
variation in the water diffusion mechanism between
regular and magnetic hydrogels, confirming
previous studies (Paulino et al., 2009; 2010; 2011).
3.3 FTIR Analysis
Fig. 1 displays the FTIR spectra of the chitosan-
based hydrogel and chitosan-based magnetic
hydrogel. All hydrogels exhibited a broad absorption
band between 1715 and 1724 cm
-1
, corresponding to
the carbonyl group stretch in protonated carbonyl
acid, which means that these hydrogels are
polycations. This carbonyl comes from the
acetylated chitosan with a 10% degree of
acetylation. In the chitosan-based magnetic hydrogel
spectrum, the peak shifted to lower values due to
hydrogen bonds and interactions with magnetite
nanoparticles. The band appearing at 1574 cm
-1
corresponds to the amine group vibrations (amide II)
in the chitosan molecule, which interacted with
magnetite nanoparticles, appearing at 1552 cm
-1
in
the magnetic hydrogel spectrum. The band at 1451
cm
-1
may be attributed to the methyl groups. The
peak at 1401 cm
-1
may be associated to CH
2
scissoring of the six carbons of the glucosamine
residues. The sharp bands around 1322 cm
-1
reveal
the presence of carboxylic groups and appeared at
1249 cm
-1
in the magnetic hydrogel spectrum due
the interaction with the magnetite nanoparticles. The
remaining broad absorption bands between 1110 and
1155 cm
-1
may be associated to vibration modes of
the saccharide rings and C-N stretching. The C-O-C
stretching vibration is also expected to take place
around 1000 cm
-1
and appears as a small shoulder on
the broad absorption band. There were important
differences between the chitosan-based hydrogel and
chitosan-based magnetic hydrogel spectra, which
were used to characterize the modification achieved
with the embedding of magnetite nanoparticles. The
main differences were related to the shifting to lower
values of the absorption bands at 1724, 1574 and
1322 cm
-1
due the formation of covalent bonds with
magnetite molecules.
Fig. 2 displays the FTIR spectra of the purified
maltodextrin, glycidyl methacrylate, modified
maltodextrin and modified maltodextrin-based
POLYSSACHARIDE-BASED MAGNETIC HYDROGELS AS POTENTICAL VECTORS FOR
EXTERNAL-CONTROLLED SOLUTE RELEASE
265
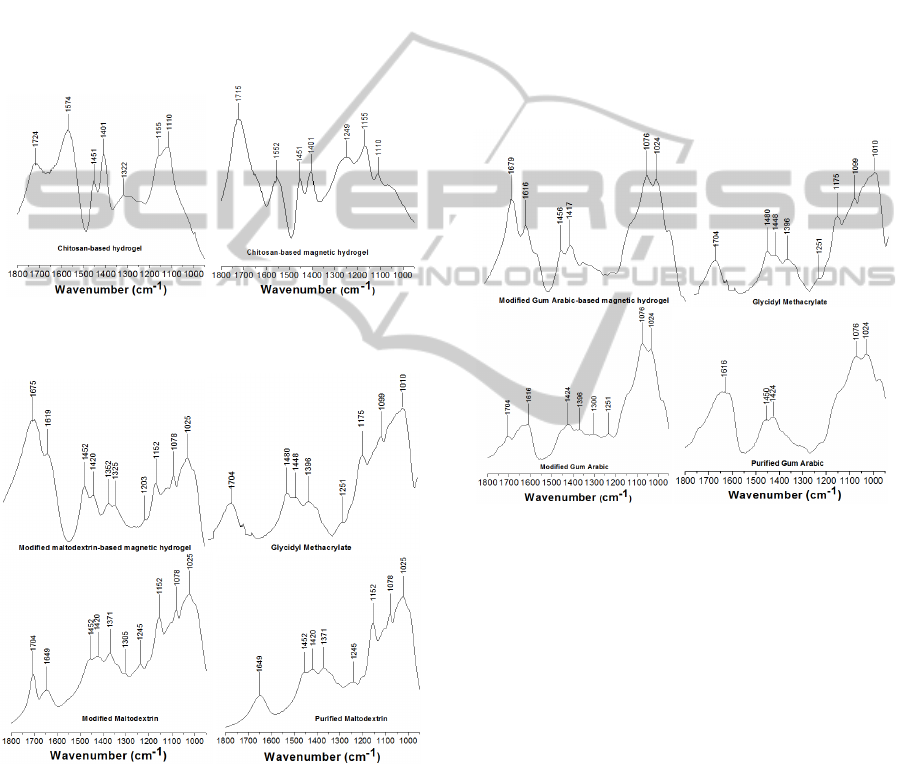
magnetic hydrogels. The band appearing at 1649 cm
-
1
in the purified maltodextrin spectrum corresponds
to the C-OH group stretching of polysaccharides.
The absorption band at 1452 cm
-1
was attributed to
the methyl groups and the band at 1420 cm
-1
was
attributed to CH
2
scissoring. The peak at 1371 cm
-1
may be attributed to -OH in plane bending vibration.
These peaks were also noted in the modified
maltodextrin spectrum. Moreover, purified
maltodextrin, modified maltodextrin and modified
maltodextrin-based magnetic hydrogels exhibited
similar peaks between 1025 and 1245 cm
-1
. The C-O
group stretching may appear at ~1245 cm
-1
.
Figure 1: Transmission FTIR spectra for chitosan-based
hydrogel and chitosan-based magnetic hydrogel.
Figure 2: Transmission FTIR spectra for modification of
purified maltodextrin with glycidyl methacrylate and
modified maltodextrin-based magnetic hydrogels.
Absorption bands between 1000 and 1152 cm
-1
may be attributed to ether bonds. These bands were
also observed in the modified maltodextrin-based
magnetic hydrogel. After the modification of
purified maltodextrin using glycidyl methacrylate, a
new absorption band appeared at 1704 cm
-1
, which
was attributed to carbonyl stretching of the
conjugated ester groups derived from a glycidyl
methacrylate molecule. The appearance of this band
was associated to the efficiency of the
polysaccharide-modifying process (Chatterjee et al.,
2003). The absorption bands at 1704 and 1649 cm
-1
shifted to lower values (1675 and 1619 cm
-1
,
respectively) after the modified maltodextrin-based
magnetic hydrogel synthesis. This was associated to
interactions between these specific groups and the
magnetite molecules, as previously described in the
FTIR spectra for chitosan-based magnetic hydrogels.
Fig. 3 displays the FTIR spectra of the purified
gum arabic, glycidyl methacrylate, modified gum
arabic and modified gum arabic-based magnetic
hydrogels.
Figure 3: Transmission FTIR spectra for the modification
of gum arabic with glycidyl methacrylate and modified
gum arabic-based magnetic hydrogels.
Considering the purified gum arabic spectrum,
an absorption band at 1616 cm
-1
was observed,
corresponding to the C-OH groups from purified
polysaccharides. The absorption bands at 1450 and
1424 cm
-1
correspond to the methyl groups and CH
2
scissoring, respectively. The bands at 1024 and 1076
cm
-1
correspond to the ether bond vibrations. The
new absorption band at 1704 cm
-1
appearing in the
modified gum arabic spectrum was attributed to the
carbonyl stretching frequency of the conjugated
ester groups derived from a glycidyl methacrylate
molecule. As observed for the modified
maltodextrin-based magnetic hydrogel, the
absorption bands at 1704 and 1616 cm
-1
shifted to
lower values (1679 and 1616 cm
-1
, respectively) due
the presence of hydrogen bonds and covalent bonds
between hydrogel groups and magnetite molecules.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
266

3.4 Magnetization Curves
Fig. 4 displays the magnetization loops
[magnetization versus applied magnetic field (B−H)]
of the chitosan-, modified maltodextrin- and
modified gum arabic-based magnetic hydrogels,
containing 1.9 wt.-% magnetite nanoparticles.
Figure 4: Magnetization vs. applied magnetic field for
magnetic hydrogels based on chitosan, modified
maltodextrin and modified gum arabic; magnetic
hydrogels containing 1.9 wt.-% magnetite.
The saturation magnetization values ranged from
150 to 160 emu g
-1
. Neither remanence nor
coercivity was observed for the magnetic hydrogels
due to the absence of hysteresis loops. This is a
characteristic of nanoparticulate materials embedded
in hydrogels (Chatterjee et al., 2003); (Mahkam,
2010). Therefore, it may be assumed that the
polysaccharide-based magnetic hydrogels support
nanoparticulate structures. It may also be stated that
the magnetite nanoparticles embedded in the
magnetic hydrogels through covalent bonding are
magnetic/superparamagnetic (Chatterjee et al.,
2003); (Mahkam, 2010). The superparamagnetic
properties supported by the magnetic hydrogels may
be directly related to the smaller size of magnetite
nanoparticles and their satisfactory dispersion
throughout the hydrogel network (Paulino et al.,
2010). Otherwise, a lack of either magnetization or
superparamagnetic properties would be observed. A
ferromagnetic material with either low or no
coercivity is said to be soft and may be used in some
kind of electronic devices. In many applications,
small hysteresis loops are driven around points in
the B-H plane. Loops near the origin have greater
magnetic permeability (Chatterjee et al., 2003);
(Mahkam, 2010).
4 CONCLUSIONS
A hydrogel with and without magnetic properties
may be synthesized by conventional methods and
characterized through methods such as FTIR,
magnetometry and water absorption kinetic studies.
Detained FTIR results were particularly useful in
demonstrating that the magnetic hydrogels were
formed by a cross-linking reaction of the natural
polymers in the presence of magnetite nanoparticles.
The superparamagnetic properties obtained through
magnetization may be directly related to the smaller
size of magnetite nanoparticles and their satisfactory
dispersion throughout the hydrogel network;
otherwise, a lack of either magnetization or
superparamagnetic properties would have been
observed. A ferromagnetic material with either low
or no coercivity is said to be soft and may be used in
many kinds of electronic devices. The water uptake
analysis revealed that a greater amount of magnetite
in the magnetic hydrogel network led to lesser water
uptake. On the other hand, a greater amount of
magnetite made the hydrogel more sensitive to a
externally applied magnetic field. If the main goal of
these materials is the application in remote-
controlled drug release, it could be supposed that
after applying an external magnetic field to a loaded
magnetic hydrogel with a specific drug encapsulated
in its structure, magnetic spins of magnetite would
be aligned in the same direction as the applied
magnetic field and, consequently, an enhanced
attractive force between north and south poles would
narrow the hydrogel network and deliver both water
and drug toward-out from the polymer network.
Accordingly, remote-controlled drug release can be
monitored and controlled by an external applied
magnetic field through a non-invasive procedure.
Furthermore, the magnetic hydrogels synthesized
here could effectively be applied as a magnetic
biosorbent, a magnetic biosensor, a soil conditioner
or even in cancer cell treatment.
ACKNOWLEDGEMENTS
A. T. Paulino and E. B. Tambourgi thank the State
of São Paulo Research Foundation (FAPESP,
Brazil) for the post-doctorate fellowship (Process N
0
2008/00285-7). A. T. Paulino, L. A. Belfiore and M.
J. Kipper thank the Coordination of Improvement of
Higher Education Personnel (CAPES, Brazil) for the
post-doctorate fellowship abroad (Process N
0
5267/09-9).
POLYSSACHARIDE-BASED MAGNETIC HYDROGELS AS POTENTICAL VECTORS FOR
EXTERNAL-CONTROLLED SOLUTE RELEASE
267

REFERENCES
Arizaga, A., Ibarz, G., Piñol, R., 2010. J. Colloid Interf.
Sci., 348, 668–672.
Bajpai, A. K., Shukla, S. K., Bhanu, S., Kankane, S.,
2008. Prog. Polym. Sci., 33, 1088–1118.
Chaterji, S., Kwon, I. K., Park, K., 2007. Prog. Polym.
Sci., 32, 1083–1122.
Chatterjee, J., Haik, Y., Jen Chen, C., 2003. Colloid
Polym. Sci., 281, 892–896.
Deligkaris, K., Tadele, T. S., Olthuis, W., van den Berg,
A., 2010. Sensors Act. B, 147, 765–774.
Dorkoosh, F. A., Coos Verhoef, J., Ambagts, M. H. C.,
Rafiee-Tehrani, M., Borchard, G., Junginger, H. E.,
2002
b
. Eur. J. Pharmaceut. Sci., 15, 433–439.
Dorkoosh, F.A., Verhoefm, J.C., Borchard, G., Rafiee-
Tehrani, M., Verheijden, J.H.M., Junginger, H.E.,
2002
a
. Int. J. Pharm., 247, 47-55.
Hallinan, D. T., De Angelis, M. G., Baschetti, M. G.,
Sarti, G. C., Elabd, Y. A., 2010. Macromolecules, 43,
4667–4678.
Jiang, G. Q., Liu, C., Liu, X. L., Zhang, G. H., Yang, M.,
Chen, Q. R., Liu, F. Q., 2010. J. Macromol. Sci. Pure.
Appl. Chem., 47, 663-670.
Mahkam, M., 2010. J. Bioact. Compat. Polym., 25, 406-
418.
Morelli, A., Chiellini, F., 2010. Macromol. Chem. Phys.,
211, 821-832.
Oh, J. K., Drumright, R., Siegwart, D. J., Matyjaszewski,
K., 2008. Prog. Polym. Sci., 33, 448–477.
Paulino, A. T., Fajardo, A. R., Junior, A. P., Muniz, E. C.,
Tambourgi, E. B., 2011. Polym. Int., 60, 1324–1333.
Paulino, A. T., Guilherme, M. R., Almeida, E. A. M. S.,
Pereira, A. G. B., Muniz, E. C., Tambourgi, E. B.,
2009. J. Magn. Magn. Mater., 321, 2636 – 2642.
Paulino, A. T., Guilherme, M. R., Mattoso, L. H. C.,
Tambourgi, E. B., 2010. Macromol. Chem. Phys., 211,
1196-1205.
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
268
