
Carbon Electrode based Urea Sensor
Modification of Graphite and New Polymeric Carriers for Enzyme Immobilization
Julija Razumiene
1
, Ieva Sakinyte
1
, Tatjana Kochane
2
, Sandra Maciulyte
2
, Antanas Straksys
2
,
Saulute Budriene
2
and Jurgis Barkauskas
3
1
Institute of Biochemistry, Vilnius University, Mokslininku 12, 08662, Vilnius, Lithuania
2
Department of Polymer Chemistry, Vilnius University, Naugarduko 24, 03225, Vilnius, Lithuania
3
Department of General and Inorganic Chemistry, Vilnius University, Naugarduko 24, 03225, Vilnius, Lithuania
Keywords: Urea Biosensor, Modified Graphite, Polymeric Carrier, Enzyme, Amperometry.
Abstract: The amperometric biosensor for urea determination was designed based on the electrochemical oxidation of
urea decomposition products produced by urease. The enzyme electrode, made of a specially developed
modified graphite (MG) paste, was produced by covering the electrode surface with new polymeric carriers
poly(urethane-urea) (PUU) microparticles containing immobilized urease from Canavalia ensiformis (E.C.
3.5.1.5.).
1 INTRODUCTION
Urea is a final product of metabolism of aliphatic
nitrogen in organisms. Generally, abnormal urea
concentration indicates kidney disease. Rapid
determination of urea is important not only in
clinical analysis but urea detection is great problem
in fertilizes industry and in agriculture as well. This
small molecule yet is a very important part for milk
component. A high concentration of milk urea
shows dietary disbalance, potential milk losses and
risk of infertility (Miglior et al., 2007). Milk urea
nitrogen levels are known to vary with the amount of
protein in the diet, amount of urine excreted, amount
of water intake, dry matter intake, sampling
methods, breed, parity, and days in milk, season and
herd management (Renny et al., 2005). These levels
increase with adulteration and are detrimental to
human health. Therefore, it is essential to test urea
level in milk in all stages - from producing to
consuming.
The most enzymatic methods of urea
determination are based on enzymatic hydrolysis of
urea in presence of urease with following
determination of hydrolysis products. A number of
photometric methods for the determination of NH
4+
(
Patton and Crouch, 1977) and using a piezo-
electric sensor
(Miglior et al., 2007) were created.
However obviously, their application for express
analysis, and especially in turbid media, is rather
complicated. In a field of amperometric biosensors
the most critical issues impeding their large-scale
application is still the inefficient and power-
demanding signal mediation from the biological
sensing element to the transducer as well as poor
stability of the employed biocatalyst (
Muti et al.,
2011). Thus, development of new electrode
materials promising for effective electron transfer
and immobilization of enzymes still is in a growing
interest. Carbon nanomaterials and various
polymeric carriers (PC) have been extensively
studied and proved to be ideal materials for these
purposes (
Agui et al., 2008; Wang et al., 2004;
Fan et al., 2006; Qureshi et al., 2009)
.
The goal of this work was to design urea
biosensor based on specially developed MG
electrode and newly proposed method of urease
immobilization onto polymeric carriers and
implement such electrochemical system for
detection of urea in milk or other biological liquids.
2 EXPERIMENTAL
2.1 Preparation of Polymeric Carriers
and Immobilization of Urease
Poly(urethane-urea) (PUU) microparticles from
197
Razumiene J., Sakinyte I., Kochane T., Maciulyte S., Straksys A., Budriene S. and Barkauskas J..
Carbon Electrode based Urea Sensor - Modification of Graphite and New Polymeric Carriers for Enzyme Immobilization.
DOI: 10.5220/0004326901970201
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 197-201
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

poly(vinyl alcohol) (PVA) and hexamethylene
diisocyanate (HMDI) were synthesized by one-step
method in dimethyl sulfoxide/water (99/1 vol.%)
solution according to previously described protocol
(Budriene et al., 2007).
.
Initial concentration of PVA
was 0.1 M. Initial molar ratio of PVA and HMDI
was 1.0:5.0. SEM image of lyophilized PC
microparticles is shown in Fig. 1.
Figure 1: Optical microscopy images of PC.
Immobilization of urease onto PUU
microparticles was carried out in 0.1 M phosphate
buffer solution, pH 7.2. The mixture of the enzyme,
buffer and PUU carrier (in different ratios) was
stirred at 25 °C for 30 min (immediately after
synthesis) and then left at 4 °C overnight. It was
prepared and investigated folowing ratios: using
1540 U of urease for 0.5 g of PC (1 PC); using of
770 U for 0.5 g of PC (2 PC); using of 389 U for 0.5
g of PC (3 PC) and using of 112 U for 0.5 g of PC (4
PC). Next day the immobilized enzyme was
thoroughly washed with buffer.
2.2 Preparation of MG and
Amperometric Biosensor
Modified graphite particles were synthesized from
pristine graphite (Merck KGaA) by oxidizing it with
potassium ferricyanide K
3
[Fe(CN)
6
] in alkaline
media. The obtained batches of MG were examined
by titration and AFM analysis methods (Fig. 2).
Titration analysis revealed the presence of small
amount (0.14 – 0.17 mmol/g) of basic surface
functional groups. AFM images show that sonication
procedure causes the formation of finely dispersed
MG particles (Fig. 2 A and B).
It was determined that the MG sample suitable
for biosensor design contains a fine fraction of 63 %
with an average diameter of the graphite particles of
20 nm.
MG powder was mixed with the pasting liquid
consisting of 10 % polyvinyl dichloride in acetone
and used for design of the electrodes.
Aiming to design working electrodes MG mixed
with pasting liquid was extruded by forming tablet
(Voitechovic et al., 2010). The tablet was sealed in a
Teflon tube. Electrodes were washed with bidistilled
water, and dried before use. Working urease-MG
electrode (biosensor) was designed by mechanically
attaching the polymeric carriers containing
immobilized enzyme urease to the surface of MG.
Further the constructed biosensor was protected by
using semipermeable terylene film.
Figure 2: AFM images of MG. (A) batches prepared
without sonication, and (B) bathes prepared including a
sonication procedure.
2.3 Electrochemical Measurements
Electrochemical measurements were performed
using an electrochemical system “PARSTAT 2273”
(Princeton Applied Reasearch, USA) with a
conventional three-electrode system comprised of a
platinum plate electrode as auxiliary electrode, a
saturated Ag/AgCl electrode as reference and
urease-MG (2 mm diameter) as working electrode.
The response of the prepared enzyme electrode
to the addition of substrate was investigated under
potentiostatic conditions at 0.4 V (vs. Ag/AgCl) in a
stirred buffer solution. As a substrate was used
phosphate buffer solution, pH 7.2, containing 1 M of
urea. The program Origin Pro 8.0 (free trial version
from http://www.originlab.com, OriginLab
Corporation, US) was used for data analysis.
2.3.1 Measurements in Milk
Commercial milk was analysed using the developed
biosensor. Taking into account that the concentration
of urea in dairy products is outside the working
range of the biosensor, a dilution of the samples
were necessary prior to analysis to adjust the sample
concentration to the linear range of the biosensor.
For this purpose, 1 M of urea solution was prepared
in milk. For each measurement 2, 3, 5, 7 and 10 µl
of the dairy product were added into electrochemical
cell containing of 1 ml of buffer solution. Thus, the
final dilution factor was from 50 to 500. Analogous
experiments were carried out by adding in the
AB
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
198
electrochemical cell of 1 M urea prepared in buffer
solution.
3 RESULTS
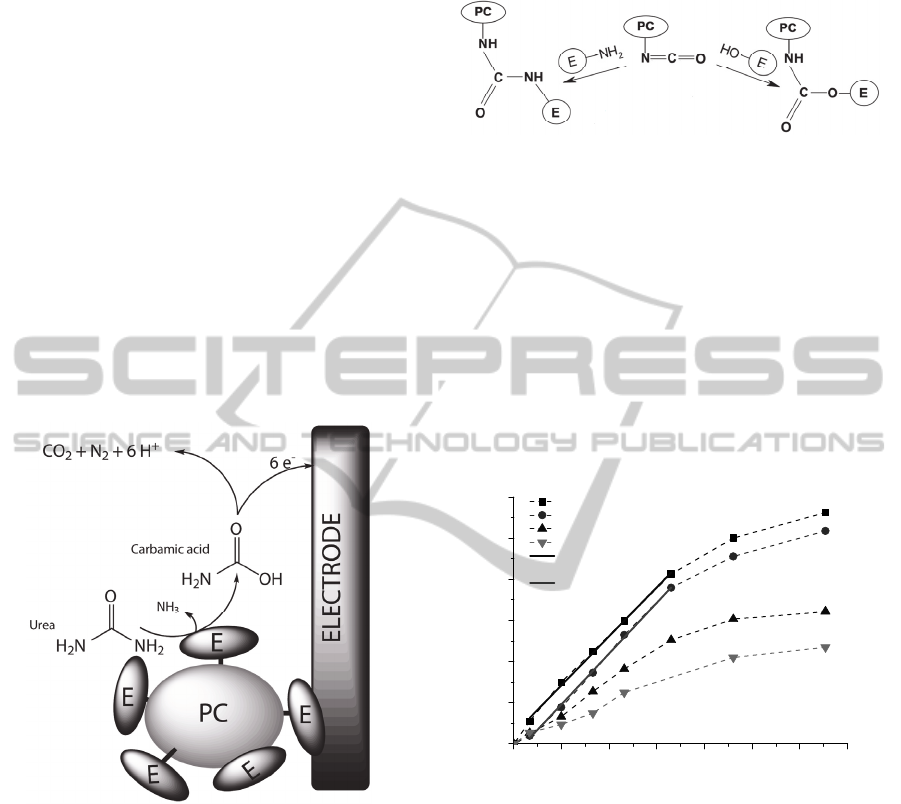
3.1 Principle of Urea Detection
The urea biosensor and the amperometric detection
principle based on the urease-catalyzed hydrolysis of
urea are shown in Fig. 3. Carbamic acid and
ammonia are the initial enzymatic reaction products
of urea, and the carbamic acid is further hydrolyzed
to ammonia and carbon dioxide. The final products
namely, ammonia and carbon dioxide are
electroinactive, thus, oxidation current observed
during the enzymatic reaction must be attributed to
the intermediate product. It can be assumed that the
carbamic acid undergoes electrooxidation by
forming nitrogen and carbon dioxide.
Figure 3: The biosensor and the amperometric detection
principle based on the urease-catalyzed hydrolysis of urea.
Aiming to obtain easy reproducible, sensitive
and stable biosensing system the enzyme was
immobilized onto polymeric carriers. Enzymes,
which have amino and hydroxyl groups, may be
covalently immobilized by attachment to PUU
microparticles, which have unreacted NCO groups
and urea or urethane linkages are formed (
Fig. 4).
NCO groups of PUU at low temperature react faster
with amino than with primary alcohol groups or
water (Randall and Lee, 2002).
Whereas
immobilization procedure followed in aqueous
media remained free NCO groups react with water
by formation of CO
2
and they do not have any
inactivation effect on enzyme latter (Budriene et al.,
2007).
Figure 4: Immobilization scheme of the enzyme onto PC.
3.2 Characterization of Urea Biosensor
Biosensor based on the urease-MG electrode after
addition of urea in to electrochemical cell shows
substrate-dependent anodic response. The biosensor
shows fast response (90 % of steady state current
achieved in 1 min) and this feature is desirable for
analytical instruments. The urea calibration curves
obtained using biosensors based on different amount
of enzyme immobilized onto polymeric carriers at
applied electrode potential 0.4 V are shown in Fig.
5.
Figure 5: The urea calibration curves and linear range
obtained using different amount of enzyme immobilized
onto polymeric carriers. Applied electrode potential 0.4 V,
phosphate buffer solution, pH 7.2.
The efficiency of electron transfer expressed by
sensitivity of the biosensor depends on amount of
immobilized enzyme. The best results were obtained
by using 1540 U of urease for 0.5 g of PC (1 PC in
Fig. 5). This enzyme and PC ratio was taken as
optimal and used for measurements in milk. Linear
response of this type of urea biosensor lies in the
range of 1 – 10 mM (Fig. 5). The obtained results
show that the method of immobilization of enzyme
plays crucial role in biosensor design. Doubtless, the
proper immobilization avoids the enzyme from fast
inactivation and affords an effective electron transfer
0 3 6 9 12 15 18 21
0
40
80
120
160
200
240
I, nA
C
(
Urea
),
mM
1 PC
2 PC
3 PC
4 PC
Linear fit of 1 PC,
R = 0.9985
Linear fit of 2 PC,
R = 0.9988
CarbonElectrodebasedUreaSensor-ModificationofGraphiteandNewPolymericCarriersforEnzymeImmobilization
199
from the active centre of the enzyme via
intermediate products toward the electrode surface.
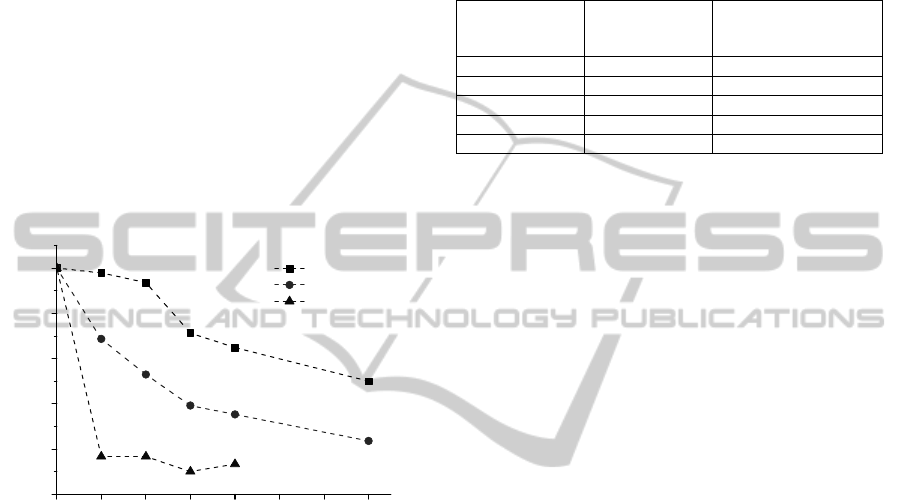
3.3 Urea Biosensor Stability
Stability of the biosensor designed using MG and
immobilized onto polymeric carriers urease was
investigated during one week (Fig. 6). The responses
to the standard urea solution (5 mM) were
periodically recorded at 20 °C. The residual
response of the best biosensor operated at potential
of 0.4 V was not less than about 50 % of initial
magnitude over the period of one week. Highest rate
of inactivation of the 4 PC biosensor activity
indicates that in this case the stability of the
biosensor was determined not by the inactivation
process of urease but just desorption of not cross-
linked to the PC enzyme (4 PC in Fig. 6).
Figure 6: Stability of the biosensors designed using MG
and different amounts of urease immobilized onto
polymeric carriers. Applied electrode potential 0.4 V,
phosphate buffer solution, pH 7.2.
3.4 Urea Determination in Milk
Amperometric type of sensors beside other well
known advantages such as comparable instrumental
sensitivity and amenability to miniaturization also
has one of very important feature – acceptability for
functioning in turbid media. Thus, in this report, we
present simple approach of the biosensor for
determination of urea in diluted milk. The
measurements have been carried out in conventional
electrochemical cell by adding urea spiked both
buffer solution and milk. The anodic current was
registered and the urea concentration was calculated
using urea calibration curve. The data are presented
in Table 1.
It was observed good correlation between data
obtained in buffer solution and in media containing
different concentrations of urea as well as amount of
milk (Table 1). The results encouraged us to carry
on further experiments concerning analysis of other
biological liquids.
Table 1: Comparison of urea concentration obtained in
buffer solution and in diluted milk using proposed
biosensor.
Added urea
concentration, mM
Detected urea
concentration in
milk, mM
Detected urea
concentration in buffer
solution, mM
1.99 2.10 2.08
2.99 3.04 3.00
4.98 4.94 4.89
6.95 6.77 6.70
9.90 9.52 9.50
4 CONCLUSIONS
For this research especially devoted MG and
polymeric carriers for enzymes have been fabricated
and tested as the electrode materials for the
amperometric urea biosensor.
It was revealed that the proposed biosensor can
be used for rapid and simple detection of urea in
diluted milk.
The biosensors exhibited fast response and
sensitivity dependent on amount of immobilized
enzyme. Although, synthesis of PC and enzyme
immobilization methodology was not optimized
properly yet, new polymeric carriers seems are very
promising for biosensors design. Thus, our future
investigations will be focused on improving of
synthesis of PC by using different initial molar ratios
of PVA and HMDI and immobilization of other
important biocatalysts.
ACKNOWLEDGEMENTS
This work was funded by the European Social Fund
under National Integrated Programme
Biotechnology and Biopharmacy, grant VP1-3.1-
SMM- 08-K01-005.
REFERENCES
Agui, L., Yanes-Sedeno, P., Pingarron, J.M., (2008). Role
of carbon nanotubes in electroanalytical chemistry: a
review. Anal. Chim. Acta 622, 11-47.
Budriene, S., Romaskevic, T., Pielichowski, K.,
Pielichowski, J., (2007). Synthesis and
characterization of polyurethane microspheres and
01234567
0
20
40
60
80
100
Response to 5 mM of urea
Time, days
2 PC
3 PC
4 PC
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
200

their application for immobilization of maltogenase.
Polym. Adv. Technol., 18, 67-71.
Fan, J., Yudasaka, M., Miyawaki, J., Ajima, K., Murata,
K., Lijima, S., (2006). Control of hole opening in
single-wall carbon nanotubes and single-wall carbon
nanohorns using oxygen. J. Phys. Chem. B, 110, 1587-
1591.
Miglior, F., Sewalem, A., Jamrozik, J., Bohmanova J.,
Lefebre, D. M., Motore, R. K., (2007). Genetic
Analysis of Milk Urea Nitrogen and Lactose and Their
Relationships with Other Production Traits in
Canadian Holstein Cattle. J. Dairy Sc. 90, 2468-2479.
Muti, M., Sharma, S. A. Erdem, Papakonstantinou, P.,
(2011). Electrochemical Monitoring of Nucleic Acid
Hybridization by Single-Use Graphene Oxide-Based
Sensor. Electroanalysis, 23 (1), 272-279.
Patton, C. J., Crouch, S. R., (1977). Spectrophotometric
and kinetics investigation of the Berthelot reaction for
the determination of ammonia. Anal. Chem. 49, 464-
469.
Randall, D. and Lee, S., (2002). The Polyurethane Book,
John Wiley & Sons, New York, USA.
Renny, E. F., Daniel, D. K., Krastanov, A. I., Zachariah,
C. A., Elizabeth, R., (2005). Enzyme based sensor for
detection of urea in milk. Biotechnol. & Biotechnol.
Equip. 19 (2), 198-201.
Voitechovic, E., Razumiene, E., Sakinyte, E., Barkauskas,
J., (2010). Investigation of bioelectrocatalytic systems
with PQQ-dependent GDH and carbonaceous
materials. Biologija. 56. (1-4), 83-87.
Wang, J., Kawde, A. N., Jan, M. R., (2004). Carbon-
nanotube-modified electrodes for amplified enzyme-
based electrical detection of DNA hybridization[J].
Biosens. Bioelectron. 20, 995-1000.
Qureshi, A., Kang, W. P., Davidson, J. L., Gurbuz, Y.,
(2009). Review on carbon-derived, solid-state, micro
and nano sensors for electrochemical sensing
applications. Diamond & Related Materials, 18, 1401-
1420.
CarbonElectrodebasedUreaSensor-ModificationofGraphiteandNewPolymericCarriersforEnzymeImmobilization
201
