
An Approach to the Electronic Textbook of Basic Chemistry Linking
Chemical Experiments
CG Teaching Materials based on Quantum Chemical Calculation
Akira Ikuo,
Yusuke Yoshinaga and Haruo Ogawa
Department of Chemistry, Tokyo Gakugei University, Tokyo 184-8501, Japan
Keywords: Chemical Experiment, Teaching Material, Tablet Computer, CG, Visualization, Quantum Chemical
Calculation.
Abstract: We tried to make CG teaching materials toward electronic textbook of basic chemistry linking chemical
experiment for university student. The CG teaching materials could demonstrate the nature of the reaction
such as structural change by ball-and-stick model or space filling model with electrostatic potential, and
potential energy change by the reaction profile. The materials included 1) formation of di-atomic molecule
such as hydrogen iodide, 2) hydroxylation of methyl chloride as a model of Walden’s inversion. These CG
teaching materials enabled to load with desktop, laptop, tablet computer, and smart phone. The CG teaching
material of hydroxylation of methyl chloride was tried to combine with chemical experiments to make
electronic textbook of basic chemistry.
1 INTRODUCTION
Chemistry is the subject that has been studied
through the experiment. Understanding the observed
phenomena, chemists use to imagine and explain
observations in terms of molecules. Observed
phenomena and molecular-level models are then
represented in terms of mathematics and chemical
equation. These three thinking levels of observable
level, symbolic level, and molecular level,
respectively was mentioned (Tasker and Dalton,
2010). Visualization is great help for students to
have images of phenomena, chemical concepts, and
molecular world. It is our aim to produce computer
graphics (CG) teaching material, which provides
realizable images of the nature of chemical reaction
(Ikuo et al., 2006).
The reaction of simple molecule such as
hydrogen halide and related compounds plays a
fundamental role in the development of chemical
kinetics and theoretical chemistry (Allison et al.,
1995; Eyring and Polanyi, 1913; Sullivan, 1962).
The reaction of equation (1) is often used for
explanation of reaction rate and
I + H
2
→ HI + H
(1)
chemical equilibrium in “Chemistry II” of Japanese
high school (Sanseido, 2004). Generally, reaction
profile is used to represent relationship between
potential energies (PE) and reaction coordinate. The
profile is often used in high school chemistry
textbooks (Daiichigakusyusya, 2004;
Jikkyosyuppan, 2004; Keirinkan, 2003; Sanseido,
2004; Tokyosyoseki, 2004). It is sometimes difficult
for student to realize the meaning of reaction
coordinate in the profile because of the
representation by a diagram of PE surface in two-
dimensions (PE-2D) except the rare case of rough
sketch of analogues in three-dimensions (PE-3D) in
physical chemistry textbook of university (Atkins
and Paula, 2002; Moor, 1982). Also images of
synchronization with successive changes of the
structure of objective molecules and distribution of
electrical character can provide clear images of the
reaction.
We developed CG teaching material based on
quantum chemical calculation of chemical reaction
for university student, which can be used to desktop,
laptop, and tablet computer, as well as smart phone.
This paper introduces our works of CG visualization
of fundamental chemical reactions for realizing
certain images of the reaction mechanism and an
approach to the electronic textbook of basic
chemistry linking chemical experiments, which
integrates the observable level experiment and the
molecular world.
688
Ikuo A., Yoshinaga Y. and Ogawa H..
An Approach to the Electronic Textbook of Basic Chemistry Linking Chemical Experiments - CG Teaching Materials based on Quantum Chemical
Calculation.
DOI: 10.5220/0004387406880691
In Proceedings of the 5th International Conference on Computer Supported Education (CSEDU-2013), pages 688-691
ISBN: 978-989-8565-53-2
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

2 PROCEDURE
2.1 Quantum Chemical Calculation
The semi-empirical molecular orbital calculation
software MOPAC (Stewart, 1989a, b, 1991) with
AM1, PM3, and PM5 Hamiltonians in CAChe Work
System for Windows (ver. 6.01, FUJITSU, Inc.) was
used in all of calculations (Ikuo et al., 2009) for
optimization of geometry, for search of potential
energies of various geometries of intermediates, for
search of transition state, and search of the reaction
path from the reactants to the products via the
transition state. The optimized structure of the
transition state was verified by the observation of a
single absorption peak in the imaginary number by
the use of the program Force in MOPAC (Stewart,
1989a, b, 1991) for vibration analysis. If the peak
was observed, Intrinsic Reaction Coordinate (IRC)
(Fukui, 1970) calculation was done and the reaction
path was confirmed.
2.2 CG Teaching Material
A movie of the reaction path was produced by the
software DIRECTOR (ver. 8.5.1J, Macromedia,
Inc.) or Flash CS4 software (Adobe, Inc.) following
the display of the bond order of the structure of the
reactants in each reaction stage, which was drawn by
the CAChe. It was confirmed that the Cast members
were arranged on the stage and the molecular
models of reactants moves smoothly. The ball was
arranged on the reaction profile and the movement
of the ball and the reactants was confirmed. The
movie file was converted to the Quick Time movie
by the Quick Time PRO (ver. 7.66, Apple, Inc.) and
was saved to iPad (Apple, Inc.) by using the iTunes
(ver. 10.7, Apple, Inc.).
2.3 Practice of Teaching Material
Teaching material was practiced to the first year
students of teacher training course for elementary
school and the second year students of natural
environmental science course, of “Chemistry
laboratory” at Tokyo Gakugei University. Teaching
material used for the trial was the CG movie shown
by the tablet computer.
3 RESULTS AND DISCUSSION
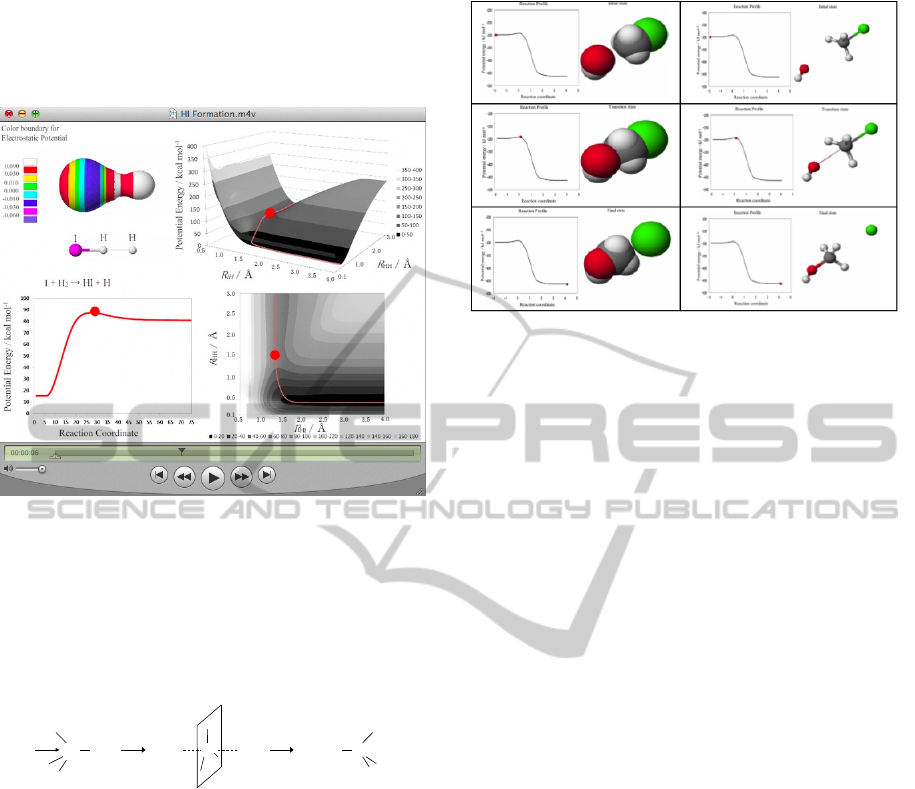
3.1 I + H
2
→ Hi + H
The CG teaching material of rearrangement by
collision of diatomic molecule and one atom as
shown in equation (1) was developed. PE of 2-D and
3-D is shown in figure 1. The figure clearly shows
these changes of PEs with display on PE surface in
3-D, which offers a bird-eye view of the reaction
profile. Two Valleys of lower energies and hilltop
on the transition state at the saddle point can be
recognized boldly. Possible pathways of the reaction
from the reactants of I and H
2
to the products of HI
and H via the transition state at saddle point can be
readily traced. The CG teaching material is able to
provide information about change of the PE and
structure of reactants in a certain state
simultaneously.
The electrostatic potential on electron density
(EPED) model and ball-and-stick model of the
intermediate, I-H-H, and the reaction profile were
combined in the left side of figure 1 for easier
recognition of those three. The electrostatic potential
(Kahn et al., 1986) was calculated based on the
coordinates of atoms from the IRC calculation
(Fukui, 1970) and superimposed on to the iso-
surface of the electron density at the value of 0.01 e
Å
-3
as shown in the upper left part of the CG. The
values of electrostatic potentials were represented in
different colour on the model of intermediate. The
model by EPED provides information about
electrostatic distribution of the intermediate with
realistic shape on the way of the reaction. In the
middle of the CG, skeletal structure in the ball-and-
stick model in which diameter of the stick reflects
calculated bond order is shown. The lower left part
of the CG shows the reaction profile, which
demonstrates the degree of the reaction progress by
the ball indicating the PE versus the reaction
coordinate. Student could correlate this reaction
profile with the reaction path in the right side of CG.
The left side of the CG is able to provide
information about characteristics of intermediate of
molecule in a certain state on the progress of
reaction.
From the posteriori survey, number of correct
answers in question about “Energy” increased 28%
compared with the preliminary survey (n=49).
Students described their comments in the free
description section of the questionnaire, such
as,“With image, it was easier for me to understand
the way of reaction and changes of energy.” and “I
could see that reaction mechanism and energy
AnApproachtotheElectronicTextbookofBasicChemistryLinkingChemicalExperiments-CGTeachingMaterials
basedonQuantumChemicalCalculation
689
change is closely related.” These comments suggest
that many students were able to obtain the concept
of energy change in chemical reaction from the CG
teaching material.
Figure 1: CG teaching material of I + H
2
→ HI + H.
3.2 OH
-
+ CH
3
Cl → CH
3
OH + Cl
-
Structural change of reactants in the reaction shown
in equation (2) was studied as a model of Walden’s
inversion, which is also shown in scheme 1.
OH
-
+ CH
3
Cl → CH
3
OH + Cl
-
(2)
Scheme 1: Images of Walden’s inversion.
Reaction of hydroxide and chloromethane is a
typical example of the Nucleophilic Substitution in
the 2nd order reaction. Carbon atom at the centre to
which halogen attaches is attacked by the
nucleophile, hydroxide, from a position 180 degrees
from chlorine and then methyl alcohol forms.
Therefore, the transition state was searched from the
reactants where the bond angle of O-C-Cl was
adjusted to 180°.
The inter-atomic distances of C-Cl in CH
3
Cl was
calculated as 1.87 Å (1.87 Å) (Weast, 1982), and C-
O in CH
3
OH was 1.41 Å (1.43 Å) (Shida, 1981).
These values were in good agreement with the
literature values in the parentheses. Energy between
the initial state of reactants and the final state of
products was 165.01 kJmol
-1
. The value was in fairly
good agreement with literature (Shida, 1981) value
of 162.90 kJ mol
-1
.
Figure 2: Selected picture of CG movies: from the CG
teaching material; Reaction profile and image of reactants;
in space filling and ball-and-stick model.
Selected picture of CG movies are shown in the
figure 2. The CG shows the reaction profile, which
demonstrates the degree of the reaction progress by
the ball indicating the potential energy versus the
reaction coordinate. Movies were made by using not
only the space filling model which shows realistic
shape but also the ball-and-stick model which shows
change in molecular configuration easily. A student
is expected to obtain the image of an umbrella
reverse like motion in Walden’s inversion. In the
space filling, the existence probability of the
electron is 90 %. In the ball-and-stick, the thickness
of stick changes by bond order.
When the CG is touched by student, the Quick
Time control bar appears and the red ball can move
by student’s choice. This manual control feature
provides “Hands-on” feeling to student. This CG
teaching material could provide not only images of
energy change during reaction but also images of
dynamical structure change during chemical
reaction.
The CG teaching material could demonstrate the
structural change of reactants with both space filling
and ball-and-stick models along with the reaction
profile, which can provide image of energy change
during the reaction.
From the result of the questionnaires (n=103),
the answer judged to be able to acquire the image of
Walden’s inversion (the image to which an umbrella
reverses) was follows; the image obtained from the
reaction formula was 24% and from the CG teaching
material was 51%. The number of CG teaching
material was better than that of the reaction formula.
Students were abele to obtain the image of drastic
change of the structure in Walden’s inversion from
the CG teaching material.
C Cl
H
H
H
OH
‐
C
Cl
H
H
H
OH
-
C
Cl
-
H
H
H
OH
+
CSEDU2013-5thInternationalConferenceonComputerSupportedEducation
690

The CG teaching material can be loaded with note
PC, tablet PC, and smart phone.
We tried to produce electric textbook for
chemical laboratory (Figure 3), which integrates the
observable level experiment and the molecular
world.
Figure 3: Prototype electronic textbook.
4 CONCLUSIONS
We produced CG teaching materials included 1)
formation of di-atomic molecule such as hydrogen
iodide, 2) hydroxylation of methyl chloride as a
model of Walden’s inversion. These teaching
materials could demonstrate the nature of the
reaction such as structural change by ball-and-stick
model or space filling model with electrostatic
potential, and potential energy change by the
reaction profile. The CG teaching materials enabled
to load with note PC, tablet PC, and smart phone.
The CG teaching material of hydroxylation of
methyl chloride was tried to combine with chemical
experiments to make electronic textbook of basic
chemistry.
ACKNOWLEDGEMENTS
This work was supported by JSPS Grant-in-Aid for
Scientific Research (C) (22500803).
REFERENCES
Allison, T. C., Mielke, S. L., Schwenke, D. W., Lynch, G.
C., Gordon, M. S., and Truhlar, D. G., (1995).
Dynamics of Cl + H
2
<=> HCl + H on a new
potential energysurface: the photosynthesis of
hydrogen chloride revisited 100 years after
MaxBodenstein, Chem. Phys. 61, 111-124.
Atkins P and Paula, Y., (2002). ATKINS Physical
Chemistry 7th. Ed., 966-969, Oxford University Press.
Daiichigakusyusya, (2004). Chemistry II ( in Japanese).
Eyring, H., Polanyi, M., Z., (1913). Phys. Chem., B12,
279.
Fukui, K. (1970). A Formulation of the Reaction
Coordinate, J. Phys. Chem., 74, 4161-4163.
Ikuo, A., Ikarashi, Y., Shishido, T., and Ogawa, H. (2006).
User-friendly CG Visualization with Animation of
Chemical Reaction: Esterification of Acetic Acid and
Ethyl Alcohol and Survey of Textbooks of High School
Chemistry, Journal of Science Education in Japan, 30
(4), 210-215.
Ikuo A., Nagashima H., Yoshinaga Y., and Ogawa H.
(2009). Calculation of potential energy in the reaction
of
“
I + H
2
→
HI + H
”
, and its visualization, The Che-
mical Education Journal (CEJ), Registration #13-2.
Jikkyosyuppan, (2004). Chemistry II ( in Japanese).
Keirinkan, (2003). Chemistry II (in Japanese).
Kahn, S. D., Pau, C. F., Overman, L. E. and Hehre, W. J.
(1986). Modeling chemical reactivity. 1.
Regioselectivity of Diels-Alder cycloadditions of
electron-rich dienes with electron-deficient
dienophiles, J. Am. Chem. Soc., 108, 7381-7396.
Moor, W. J., (1982). Physical Chemistry, 4th. Ed., pp.
382-387, Tokyo Kagakudojin (in Japanese).
Sanseido, (2004). Chemistry II ( in Japanese).
Shida, S. (1981). Kagakujiten, Morikitasyuppan, p.1251.
Stewart, J. J. P. (1989a). Optimization of parameters for
semiempirical methods I. Method, J Comp. Chem., 10
(2), 209–220.
Stewart, J. J. P. (1989b). Optimization of parameters for
semiempirical methods II. Applications, J. Comp.
Chem., 10 (2), 221– 264.
Stewart, J. J. P. (1991). Optimization of parameters for
semiempirical methods. III Extension of PM3 to Be,
Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb,
and Bi, J. Comp. Chem., 12 (3), 320–341.
Sullivan, J. H., (1962). Rates of Reaction of Hydrogen
with Iodine. II, J. Chem. Phys., 38(7), 1925-1932.
Tasker, R. and Dalton, R. (2010). Visualizing the
Molecular World-Design, Evaluation, and Use of
Animation, In Gilbert, J. K., Reiner, M., and Nakhleh,
M (Eds.), Visualization: Theory and Practice in
Science Education, Springer, 105.
Tokyosyoseki, (2004). Chemistry II ( in Japanese).
Weast, R. C. (1982). CRC Handbook of Chemistry and
Physics (63rd ed.), CRC Press, Inc., F180-181.
AnApproachtotheElectronicTextbookofBasicChemistryLinkingChemicalExperiments-CGTeachingMaterials
basedonQuantumChemicalCalculation
691
