
Hospital Risk Management using Healthcare Failure Mode
and Effects Analysis
A Case Study on Ventilators Whithin an Intensive Care Unit
M. Alberto Ibarra-Sánchez
1
, Ana B. Pimentel-Aguilar
2
and Martha R. Ortiz-Posadas
1
1
Department of Electrical Engineering, Univerisdad Autónoma Metropolitana Iztapalapa,
Av. San Rafael Altixco #186, Col. Vicentina, C. P. 09340, México City, D. F. Mexico
2
Department of Biomedical Engineering, National Institute of Respiratory Diseases,
Calz. Tlalpan No. 4502, Col. Sección XVI, Deleg. Tlalpan, CP. 14080, México City, D. F. Mexico
Keywords: Hospital Risks Management, Healthcare Failure Mode and Effects Analysis, Mechanical Ventilators,
Intensive Care Unit.
Abstract: The objective of this work was to analyze the potential risks associated to the use of invasive mechanical
ventilators located in the intensive-care unit (ICU) of the Institute of Respiratory Diseases from Mexico.
The study was addressed by applying the Healthcare Failure Mode and Effects Analysis (HFMEA),
identifying possible/potential failure modes and its effects, and determining the severity and the probability
of occurrence for each of these failures. We determine the risk score, and if this score was 8 or higher, we
proposed a preventive action in order to develop an action plan. We identify six types of risks (electrical,
mechanical, due to medical gases, biological, catastrophic and those related to human factor) and 26
potential causes related with these risks. Base on the evidence acquired by the HFMEA, we proposed a
contingency plan for those potential causes.
1 INTRODUCTION
Risk is defined as the probability of harmful
consequences, or expected losses (deaths, injuries,
property, livelihood, economic activity disrupted or
environment damaged) resulting from interactions
between natural or human-induced hazards and
vulnerabilities (WHO, 2007). There are hazards
arise in the use of medical devices due to the
inherent risk of medical treatment, from device
failures (or malfunctions), and from device use.
Hazards resulting from medical devices impact
patients, family members, and professional
healthcare providers (Kaye and Crowley, 2000).
Risk management is defined as the systematic
process of identifying, evaluating and addressing
potential and actual risk. Risk management has
emerged as an integral element in the operational
activities of hospitals. The process is a mechanism
for self-protection in co-operative, self-insurance
arrangements and to secure premium adjustments.
Many trends have been recognized, that would
suggest a predisposition toward the proliferation of
risk management programs (Keddy et al., 1988).
Because of these, patient safety has become a
matter of interest to healthcare professionals,
governments and researchers worldwide. During the
last decade, many studies have been conducted to
assess the prevalence, severity and causes of a large
variety of different types of adverse events in
hospitals, as well as the effectiveness of various
approaches to enhance safety (Wolf et al., 2001;
Oliver et al., 2004; Marwick et al., 2009). The risks
present in the hospital are widespread and complex.
These risks are electrical, mechanical, biological,
environmental and radiological, among others.
The initial steps to develop a risk management
program include assessing current risk, control
activities and implementing structural elements. As
well, a program must address its relationship to
quality assurance activities in the hospital.
The objective of this work was to analyze the
potential risks of invasive mechanical ventilators
(invasive ventilation is defined as mechanical
ventilation via an artificial airway which can either
be via endotracheal tube or tracheostomy tube),
located in the intensive-care unit (ICU) of the
328
Alberto Ibarra-Sánchez M., B. Pimentel-Aguilar A. and R. Ortiz-Posadas M..
Hospital Risk Management using Healthcare Failure Mode and Effects Analysis - A Case Study on Ventilators Whithin an Intensive Care Unit.
DOI: 10.5220/0004536203280335
In Proceedings of the International Conference on Knowledge Discovery and Information Retrieval and the International Conference on Knowledge
Management and Information Sharing (KMIS-2013), pages 328-335
ISBN: 978-989-8565-75-4
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

National Institute of Respiratory Diseases (INER for
its Spanish acronym), which is a third level public
hospital in Mexico City. We address the study
applying the Healthcare Failure Mode and Effects
Analysis (HFMEA) (VA-NCPS, 2013) and propose
a contingency plan in order to manage the risks
associated with the use of this technology.
2 METHODOLOGY
Healthcare Failure Mode and Effects Analysis
(HFMEA) is a prospective methodology that
identifies and improves steps in a process thereby
reasonably ensuring a safe and clinically desirable
outcome. HFMEA has been designed by the
National Center for Patient Safety (NCPS) of the
Department of Veterans Affairs (VA) specifically
for healthcare (VA-NCPS, 2013), and streamlines
the hazard analysis steps found in the traditional
failure mode and effect analysis process (IMCA,
2002). The purpose of the hazard analysis is to
develop a list of hazards that are of such significance
that they are reasonably likely to cause injury or
illness if not effectively controlled. The steps of the
HFMEA are described as follows.
2.1 Healthcare FMEA Steps
Step 1: Define the topic of the HFMEA along with a
clear definition of the process to be studied.
Step 2: Assemble a Multidisciplinary Team
including the subject matter expert(s) and an
advisor.
Step 3: Graphically describe the Process.
Step 4: Conduct a hazard Analysis:
a. List all possible/potential failure modes for the
process. Failure modes include anything that
could go wrong that would prevent the process
from being carried out. Consecutively number
these failure modes.
b. List all possible/potential effects of the failure
mode. Effects include anything that could
happen if the failure actually occurs.
c. Determine the severity (S) of each effect by
using the severity rating (Table 1).
d. Determine the potential causes of each failure
mode. Each failure mode may have multiple
failure mode causes. Document the causes.
e. Determine the probability of occurrence (O) for
each of the potential causes by using the
probability rating (PR) as follows:
Frequent (PR=4). Likely to occur immediately or
within a short period (may happen several times
in one year).
Table 1: Severity rating.
Event Severity rating
Catastrophic (4)
Patient Outcome: Death or major permanent
loss of function (sensory, motor, physiologic,
or intellectual).
Visitor Outcome: Death; or hospitalization of
three or more visitors.
Staff Outcome: A death or hospitalization of
three or more staff.
Equipment or Facility: Damage equal to or
more than $250,000.
Fire: Any fire that grows larger than
incipient/beginning stage cannot be controlled
with portable fire extinguisher or small hose.
Major (3)
Patient Outcome: Permanent lessening of
bodily function (sensory, motor, physiologic,
or intellectual), increased length of stay or
increased level of care, for three or more
patients.
Visitor Outcome: Hospitalization of two or
more visitors.
Staff Outcome: Hospitalization of one or two
staff or three or more staff experiencing lost
time or restricted duty injuries or illnesses.
Equipment or Facility: Damage equal to or
more than $100,000.
Moderate (2)
Patient Outcome: Increased length of stay or
increased level of care for one or two patients.
Visitor Outcome: Evaluation and treatment
for one or two visitors (less than
hospitalization).
Staff Outcome: Medical expenses lost time or
restricted duty injuries or illness for one or
two staff.
Equipment or Facility: Damage between
$10,000 -$100,000.
Fire: Incipient/beginning stage or smaller can
be controlled with portable fire extinguisher or
small hose.
Minor (1)
Visitor Outcome: Evaluation and no
treatment required or refused treatment.
Staff Outcome: First aid treatment only with
no lost time, nor restricted duty injuries or
illnesses.
Equipment or Facility: Damage less than
$10,000 or loss of any utility without adverse
patient outcome.
Occasional (PR=3). Probably will occur (may
happen several times in 1 to 2 years).
Uncommon (PR=2). Possible to occur (may
happen sometime in 1 or 2 years).
Remote (PR=1). Unlikely to occur (may happen
sometime in 5 to 30 year years).
f. Determine the risk score (RS) by multiplying the
probability score by the severity score.
HospitalRiskManagementusingHealthcareFailureModeandEffectsAnalysis-ACaseStudyonVentilatorsWhithinan
IntensiveCareUnit
329

g. Use the hazard decision matrix (Table 2) to
determine if the failure mode warrants further
action. If the score is 8 or higher, strong
consideration should be given to developing an
action plan.
Step 5: Actions and outcome Measures:
a. Identify an action for each failure mode that will
be corrected. Place the corrective actions in the
process at the earliest feasible point. Multiple
actions can be placed in the process to control a
single hazard. An action can be used more than
one time in the process.
b. Identify outcome measures that will be used to
analyze and test the redesigned process.
c. Identify a single, responsible individual by title
to complete the recommended action.
d. Indicate whether top management has concurred
with the recommended action.
e. Record the recommended action, responsibility
and target date.
Step 6: Follow-up on Actions Taken
a. After the target date for the recommended
actions, follow-up to make sure the actions were
implemented and on what date.
b. Now that the recommended actions have been
implemented, the hazard score should be lower.
So, revisit the probability of that failure mode
cause using the probability rating table (Table 2)
and document the new rating.
c. Obtain the new hazard score by multiplying the
severity times the probability and document the
result. The new hazard score should now be <8.
If not, revisit the recommended actions.
Table 2: Risk decision matrix.
Probability Severity of Effect
Catastro
phic (4)
Major
(3)
Moderate
(2)
Minor
(1)
Frequent (4) 16 12 8 4
Occasional
(3)
12 9 6 3
Uncommon
(2)
8 6 4 2
Remote (1) 4 3 2 1
3 RESULTS
This work was developed by a multidisciplinary
team of biomedical engineers, respiratory therapists
and nurses. The knowledge acquisition (the process
of extracting, structuring and organizing knowledge
from one source, usually human experts), was made
through interviews to technology users and by
studying the procedures of handling and use of the
ventilators, and management of medical technology.
Six risk-types associated with mechanical
ventilators in the ICU were identified: electrical,
mechanical, due to medical gases, biological,
catastrophic and those related to human factor. Its
failure modes and effects, and potential causes were
analyzed for every case. The results shown in this
work are only those with a risk score greater or
equal to 8, because according to the hazard decision
matrix (Table 2) these need further corrective
actions. Therefore some operative actions were
proposed and related with the hospital service
responsible for its implementation.
3.1 Electrical Risk
Electrical risk is defined as a dangerous condition
such that contact or equipment failure can result in
electric shock, arc-flash burn, thermal burn, or blast.
(NFPA, 2004).
For this risk one failure mode and effect was
identified and associated to four potential causes,
that got RS=8 (Table 3). This failure means that the
ventilator has discharged battery. For all cases the
potential causes have catastrophic severity (S=4),
because if the ventilator stops working the patient’s
life is threatened, although the probability is
uncommon (P=2).
3.2 Mechanical Risk
Mechanical devices are necessary for many
treatments in the modern hospital. These devices
include mobility aids, transfer devices, prosthetic
devices, mechanical-assist devices, and patient-
support equipment. Each of these devices embodies
numerous life and limb threats to patients as well to
hospital staff. These devices must be subject to
careful design review, failure indication, and the
establishment of complete specifications for safe use
(Freeman, 1979).
For this risk two failure modes and effects were
identified and associated to three potential causes
(Table 3). Note that the potential cause 5 got a
RS=12, because it is related to the localization of the
electrical outlets, hence an infrastructure issue. The
other two potential causes got a minor RS (RS=9),
because these problems are related to the distribution
of the ventilators into the ICU cubicle.
KMIS2013-InternationalConferenceonKnowledgeManagementandInformationSharing
330

Table 3: HFMEA for the risks associated to the ventilators in the Intensive Care Unit.
Risk
Failure mode Failure effect Potential cause S O RS
Electrical
Discharged batteries
The ventilator does not
work
1. The ventilator is unplugged to the
electrical system.
4 2
8
2. The ventilator is plugged to an
electrical outlet that doesn’t work.
4 2
8
3. The ventilator is stored for a long
time without being plugged to the
electrical system.
4 2
8
4. There is not an area for plugging
the ventilators in order to charge
the batteries.
4 2
8
Mechanical
No free access to the
electrical outlets.
By plugging the
ventilator, other device
may be unplugged
(e.g., infusion pumps).
5. The electrical outlets may be in a
high position and the staff may
require a bench to plug the
ventilator.
3 4
12
The ventilator blocks the
free staff’s circulation.
The staff cannot access
to the patient for
emergency procedures.
6. Lack of space in the patient cubicle. 3 3
9
7. Crossed hoses and wires block the
access of the staff to the patient.
3 3
9
Medical Gases
Insufficient medical gases
supply pressure.
The ventilator does not
work.
8. Insufficient gas compressor power. 4 3
12
9. Leaking hoses. 4 2
8
10. Drop of the medical gases supply
pressure.
4 3
12
11. Leaking medical gases outlets or
ventilator connectors.
4 2
8
12. Bad medical gases supply
connection.
4 2
8
The ventilator cannot be
connected to the medical
gases outlets.
The patient could not
receive ventilatory
support.
13. Incompatibility between the
medical gases outlets and the
ventilator’s connectors.
4 3
12
Biological
Contaminated ventilators
not identified.
Use of contaminated
ventilators.
14. Clean and contaminated ventilators
are stored in the same place
(transfer).
3 3
9
15. No label for contaminated
ventilators.
3 3
9
3.3 Risks by Medical Gases
Medical gases are widely used around the hospital
and are supplied in cylinders or piped into wards and
clinical areas. They are safe if handled correctly,
however, misuse or mishandling can have
catastrophic consequences (NHS, 2012).
For this risk two failure modes and effects were
identified and associated to six potential causes
(Table 3). Those related with the gas supply pressure
got the mayor risk score (RS=12), because the
correct operation of the ventilators depends on this;
and the last three, related with the ventilator’s
connection to the gas outlet, got RS=8.
3.4 Biological Risk
Biological health risks are linked to the exposure to
bacteria, viruses, fungi, other micro-organisms and
associated toxins. These micro-organisms are
widespread in nature and represent a potential
danger for public health (EC, 2013).
The main biological risk arises when the staff
cannot identify the contaminated ventilators, as it
may cause a nosocomial infection if one of these
devices is used in another patient. For this case two
potential causes with a RS=9 related to the lack of a
label to identify between contaminated ventilators
that need cleanup and those clean ready for usage
were determined.
Once the risks were analyzed, with the RS
obtained a plot was made to see how the potential
causes cluster and to define the priority in order to
develop its prevention actions (Figure 1). Note that
the risk by medical gases has the set of potential
causes with mayor RS. It means that the first actions
HospitalRiskManagementusingHealthcareFailureModeandEffectsAnalysis-ACaseStudyonVentilatorsWhithinan
IntensiveCareUnit
331
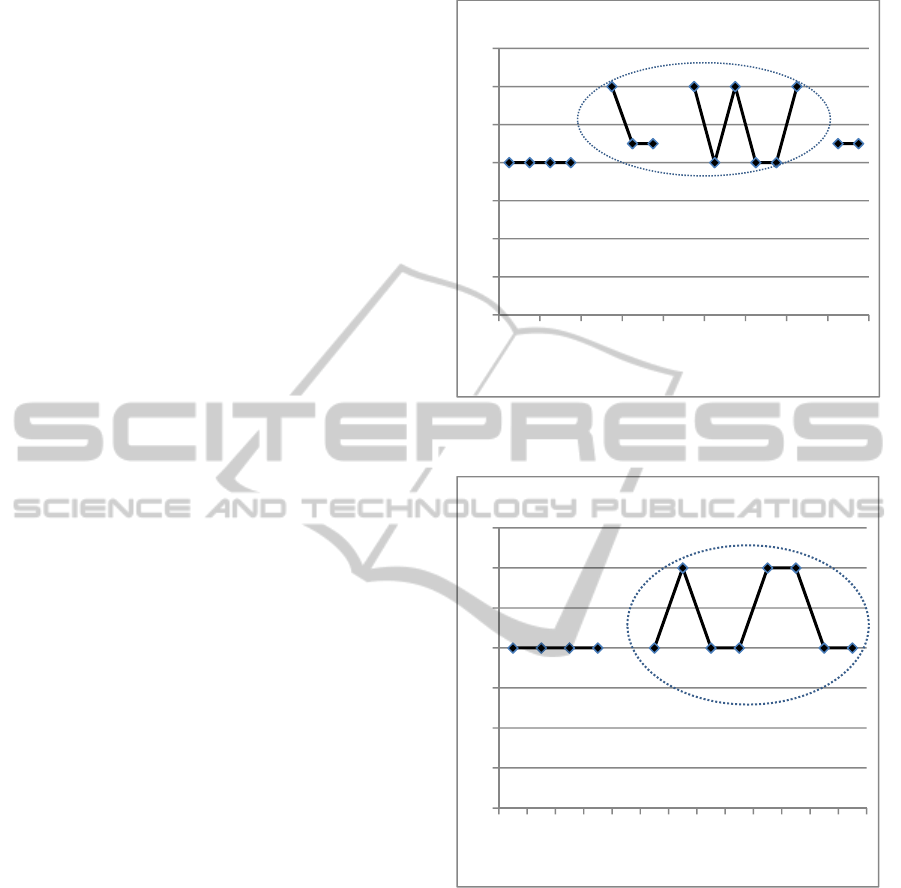
to develop in the contingency plan will be for the
potential causes of this risk. Then those for
mechanical, biological and electrical risk would
follow.
3.5 Catastrophic Risk (Seismic)
Catastrophic risks are those that can result in
substantial loss of life or livelihood, call an
organization’s existence into question or cause
significant environmental damage. These risks
include a diverse range of events such as floods,
pandemic infections, nuclear accidents, wars,
seismic, economic collapse, etc. (World Economic
Forum, 2012). In this sense, seismic activity occurs
in many areas of Mexico, and Mexico City is
particularly at risk due to unique geological
characteristics coupled with an extraordinarily high
concentration of exposure (USGS, 2012). This is the
reason why we consider the analysis of seismic risk
in this study.
For this risk one failure mode, two effects and
three potential causes related with the infrastructure
and movement of the ventilator in the ICU were
identified (Table 4) and got a RS=8 for the three
cases.
3.6 Risks by Human Factors
Hazards associated with device use are a common
and serious problem. Evidence suggests that the
frequency and consequence of hazards resulting
from medical device use might far exceed those
arising from device failures. Therefore, it is essential
to ensure safe and effective device use if all hazards
are to be controlled effectively (Kaye and Crowley,
2000).
Here we addressed hazards resulting from
interactions between users and the mechanical
ventilators in the ICU. We identify three failure
modes and effects, associated to eight potential
causes (Table 4). The ones with the mayor risk score
(RS=12) were those related with the out-of-order
ventilators and with the lack of staff capacitation in
the correct use and handle of the equipment.
For these two last risks (biological and
catastrophic) a plot with the gotten RS also was
made (Figure 2), in which it’s clearly seen that the
first prevention actions to be developed are those for
the risk related to human factor.
Figure 1: Graphic of the risk scores of the electrical,
mechanical, by medical gases and biological risks.
Figure 2: Graphic of the risk scores for the seismic and
human factors risks.
3.7 Contingency Plan
A contingency plan is a process that prepares an
organization to respond coherently to an unplanned
event. The contingency plan can be also used as an
alternative for action if expected results fail to
materialize. The HFMEA study goes on to make
recommendations on how to address the failure
modes, ranging from better education, better visual
displays, "time outs", bar-codes, etc.
For developing a contingency plan for the risks
associated to the use of the mechanical ventilator in
0
2
4
6
8
10
12
14
1 3 5 7 9 11 13 15 17
Electrical
Risks
Biological
Risks
Risks byMedicalGases
Mechanical
Risks
Riskscore
Potential causenumber
0
2
4
6
8
10
12
14
135791113
Riskscore
Seismic
Risk
RisksbyHumanFactor
Potencialcausenumber
KMIS2013-InternationalConferenceonKnowledgeManagementandInformationSharing
332

the ICU, we used the risk score and the risk matrix
(Table 2) to identify which critical failure modes
need correction, but then is still need to make those
corrections and take more action.
In this case, the Hospital would need to address
26 different potential causes. That doesn't
necessarily mean that 26 separate remedial and
corrective actions need to take place. A single
corrective action might be able to address multiple
failure modes, so a few key changes might address
many failure modes at once.
Following we discuss the corrective actions for
each type of risk, in order to develop the
contingency plan as well as the responsible
department for their implementation.
3.7.1 Electrical Risk
The proposed actions to diminish the electrical risk,
in general, are of surveillance.
Inspect that the ventilator is effectively plugged
to the electrical system, at least once per shift, to
guarantee charged batteries. Respiratory Therapy is
the area in charge for this action.
Supervise that all the electrical outlets in the ICU
have electrical supply, are connected to the
emergency power system and its voltage is
periodically checked. Hospital maintenance is the
area in charge for these actions.
To allocate an exclusive area for ventilators
storage and to have a control strategy for the
batteries charge process. Respiratory Therapy is the
area in charge for this action.
3.7.2 Mechanical Risk
Some infrastructure modifications are proposed in
order to diminish this risk, like to change the place
of the electrical outlets to guarantee the staff free
access to them. Hospital maintenance is the area in
charge for this action.
On the other hand, it is necessary that a correct
distribution of the equipment in the patient cubicles
of ICU be done. The ventilators must be placed near
to the medical gases and electrical outlets, and so
vital signs monitors and infusion pumps must be
correct placed. ICU is the area in charge of this
action.
3.7.3 Risks by Medical Gases
It is important to be aware of pressures at which
gases are stored and used. Therefore the medical
gases supply pressure and each outlet in every
cubicle must be verified, at least once a day, and so
the connectors. Hospital maintenance is the area in
charge of this action.
Table 4: HFMEA for the risks associated to the use of invasive ventilators in the Intensive Care Unit.
Risk Failure mode Failure effect Potential causes S O
RS
Catastrophic
Seismic
The ventilator does not
work.
16. The medical gases supply is
interrupted due to damage to the
hospital infrastructure.
4 2
8
17. The ventilator gets disconnected
from the medical gases supply,
electrical system or breathing
circuit.
4 2
8
The ventilator may hinder
the evacuation of patient and
staff.
18. The ventilator moves and blocks
the staff’s evacuation of the
cubicle.
4 2
8
Human Factors
Lack of available
ventilators.
Patient does not receive
ventilatory support.
19. Contaminated ventilators. 4 2
8
20. Out-of-order ventilators. 4 3
12
21. Not enough accessories (breathing
circuits, hoses, etc.).
4 2
8
22. Cleaning verification not passed. 4 2
8
Not enabled staff in
the use of
ventilators.
Patient does not receive
ventilatory support.
23. Invasive ventilator used for
patient transfer.
4 3
12
24. Lack of capacitation to the user. 4 3
12
The ventilator
stops working
during patient
transfer.
Patient does not receive
ventilatory support.
25. The ventilator sustains a
breakdown during the patient
transfer.
4 2
8
26. Drop of the medical gas tank
pressure.
4 2
8
HospitalRiskManagementusingHealthcareFailureModeandEffectsAnalysis-ACaseStudyonVentilatorsWhithinan
IntensiveCareUnit
333

To check the hose and breathing circuit state at
least once per shift and to have a replacement
strategy in accordance to the manufacturer’s
specifications, also the compatibility between
medical gases outlets and ventilator connectors must
be assured, and those that do not meet this
requirement must be replaced. Respiratory Therapy
is the area in charge of this action.
3.7.4 Biological Risks
In this case developing an infection control plan is
fundamental; it will allow identifying contaminated
ventilators in order to start effective
decontamination procedures. The Respiratory
Therapy is the area in charge of these actions.
3.7.5 Catastrophic Risk (Seismic)
In case of an earthquake, having the sufficient
equipment and accessories to maintain the maximum
technology capacity is necessary.
Also, portable oxygen tanks to keep the
ventilators working until the regular gas supply is
reestablished, enough transfer ventilators and
compatible invasive transfer ventilators circuits to
avoid patients’ re-intubation are required. The
Respiratory Therapy is the area in charge of these
actions.
Furthermore, it is very important to verify the
wheel brakes of both the ventilator and the bed to
avoid displacements during an earthquake. ICU is
the area in charge of this action.
3.7.6 Risks by Human Factors
For these risks the following actions are proposed:
Supervise the effective cleaning of the
ventilators. Acquire enough equipment, accessories
and consumables according to the demand of
ventilators. Use transfer ventilators for patient
mobilization. Respiratory Therapy is the area in
charge of this action.
On the other hand, it is necessary to schedule
daily equipment review routines to guarantee the
availability of verified ventilators. Improve the
ventilators’ preventive and corrective maintenance
response. To develop a continuous training program
for the staff and a continuous ventilators’
functionality test program. The Biomedical
Engineering Department is in charge of these
actions.
Distribute the workload of the ICU according to
the staff available and to promote the recruitment of
more staff.
4 CONCLUSIONS
The HFMEA application showed evidence that
allowed to analyze the potential causes associated to
six identified risks (electrical, mechanical, due to
medical gases, biological, catastrophic and those
related to human factors), in the use of mechanical
ventilators in the ICU.
With the RS obtained for each one of the 26
potential causes, its priority was determined and
preventive actions were proposed, aiming for a risk
management contingency plan development.
Once the contingency plan in the ICU is
established, the tracing and feedback actions that
allow to recalculate the RS and to evaluate the
effectiveness of the preventive measures
implemented will be carried out, and, if so, keep or
modify them. As we know, a common outcome of
risk analysis is to re-emphasize the training and
procedure-following by staff members.
By the other hand, an equipment control program
must be implanted in the ICU, in order to enforce the
contingency plan. The control program provides a
structure for the clinical utilization of equipment in
the hospital, and directs the effort by the entire
institution to apply technical competence,
management techniques, and organizational skills to
the control and application of technology (Furst,
1979).
In this work is shown the usefulness of the
HFMEA for the evaluation and management of risks
associated with mechanical ventilators use in the
ICU. However it is a tool that can be used for
analyzing and evaluating risks of any medical
technology in any clinical service.
As further work, an evaluation of the risk
management framework is going to be conducted by
a pilot program (a preliminary study) to see how part
of the ICU, using the proposed contingency plan
performs better than part of the ICU not using it, in
order to evaluate feasibility, time, cost, adverse
events, and effect size.
REFERENCES
WHO - World Health Organization, 2007. Risk reduction
and emergency preparedness. WHO six-year strategy
for the health sector and community. WHO Document
Production Services, Geneva, Switzerland.
Kaye, R. and Crowley J., 2000. Medical Device Use-
Safety: Incorporating Human Factors Engineering into
Risk Management. U. S. Department of Health and
Human Services. FDA.
KMIS2013-InternationalConferenceonKnowledgeManagementandInformationSharing
334

Keddy, W. R., Johnson M. W. and McKerrow W., 1988.
Hospital risk management: the second decade.
Healthcare Manage Forum, 1 (1):12-7
Wolff, A. M., Bourke, J., Campbell, J. A. and
Leembruggen, D. W., 2001. Detecting and reducing
hospital adverse events: outcomes of the Wimmera
clinical risk management program. Medical Journal of
Australia, 174 (12): 621-625.
Oliver D., Daly F., Martin F. C. and McMurdo M. T.,
2004. Risk factors and risk assessment tools for falls
in hospital in-patients: a systematic review. Age and
Ageing, 33: 122–130.
Marwick, C. and Davey, P., 2009. Care bundles: the holy
grail of infectious risk management in hospital?
Current Opinion in Infectious Diseases, 22:364–369.
VA-NCPS - National Center for Patient Safety. The
Basics of Healthcare Failure Mode and Effect
Analysis (HFMEA). Available at http://
www.patientsafety.gov/SafetyTopics/HFMEA/HFME
AIntro.pdf consulted on March 2013.
IMCA - International Marine Contractors Association,
2002. Guidance on Failure Modes & Effects Analyses.
IMCA-M-166.
NFPA - National Fire Protection Association, 70E
Standard for Electrical Safety Requirements for
Employee Workplaces, 2004 Edition. Available at
http://www.nfpa.org/assets/files/PDF/CodesStandards/
TIAErrataFI/TIA70E-04-1.pdf consulted on March
2013.
Freeman, J., 1979. Safety program. In: Clinical
engineering. Principles and practices, Webster J (Ed.).
Prentice Hall, NJ.
NHS - Salford Royal National Health Service Foundation
Trust, 2012. Design Services, Basic Medical Gas
Safety. Available at: http://elearning.hope-
academic.org.uk/srht_elearn_dept/medical_gases_wor
kbook.pdf consulted on March 2013
EC - European Commission, Biological Risks, Health-EU,
available at http://ec.europa.eu/health-eu/my_
environment/biological_risks/index_en.htm, consulted
on March 2013.
World Economic Forum. Global Agenda Council on
Catastrophic Risks 2012. Available at:
http://www.weforum.org/content/ global-agenda-
council-catastrophic-risks-2012 consulted on March
2013.
USGS - US Geological Survey. Mexico Earthquake
Information. Available at :http://earthquake.usgs.gov/
earthquakes/world/index.php?regionID=3 consulted
on March 2013.
Furst, E., 1979. The equipment control program. In:
Clinical engineering. Principles and practices, Webster
J (Ed.). Prentice Hall, NJ.
HospitalRiskManagementusingHealthcareFailureModeandEffectsAnalysis-ACaseStudyonVentilatorsWhithinan
IntensiveCareUnit
335
