
An Experimental Platform Aimed at Long Lasting
Electrophysiological Multichannel Recordings of Neuronal Cultures
G. Regalia
1
, E. Biffi
1
, A. Lucchini
2
, M. Capriata
1
, S. Achilli
1
, A. Menegon
3
, G. Ferrigno
1
, L.
Colombo
2
and A. Pedrocchi
1
1
Department of Electronics, Information and Bioengineering, Politecnico di Milano, via G. Colombo 40, Milan, Italy
2
Energy Department, Politecnico di Milano University, via Lambruschini 4, Milan, Italy
3
Advanced Light and Electron Microscopy Bio-Imaging Centre, San Raffaele Institute, via Olgettina 58, Milan, Italy
Keywords: Microelectrode arrays, Neuronal cultures, Long-term recordings
Abstract: The elucidation of physio-pathological mechanisms expressed by a neuronal network over extended time
scales (i.e., from hours to weeks) is the goal of many neurophysiological and neuropharmacological in vitro
studies. In this context, a challenging technological requirement is the establishment of an experimental
setup able to collect long-term neuronal signals. In this work we report the development of a compact
environmental chamber designed to perform prolonged recordings of the bioelectrical activity exhibited by
neuronal networks grown on MicroElectrode Arrays (MEAs). To reproduce an environment suitable for
cells growth (temperature, pH and humidity) the chamber was coupled with a temperature control system
and an air humidifying module. Validation tests demonstrated that the environment inside the portable
chamber is comparable to standard cell incubators environment. To collect neuronal extracellular signals,
custom multichannel pre-processing boards have been developed and integrated with the chamber. With this
equipment, we were able to reliably record spontaneous neuronal electrical activity from hippocampal
cultures grown inside the chamber for several hours, which is not possible with the standard MEA recording
setup due to environmental fluctuations. This system can collect multichannel data from neuronal cultures
over long periods, providing an effective solution for long-term studies of neural activity.
1 INTRODUCTION
A central goal of the modern neuroscience is to
understand the relationship between the functional
connectivity of neuronal circuits and their
physiological or pathological features. The
dissociated culture of primary central neurons
provides a convenient test system to reach this aim.
Indeed, in vitro cultures retain many characteristics
of their in vivo counterparts, but they are simpler
and more accessible for investigations and
manipulations (Eckmann et al., 2007). In this
context, neuronal cultures grown on MicroElectrode
Arrays (MEAs) represent a powerful tool thanks to
the non invasive and multisite approach (Johnstone
et al., 2010, Rossi et al., 2011). However, in vitro
neuronal ensembles are extremely sensitive to
changes in the surrounding environment
(temperature, pH, humidity) (Biffi et al., 2012).
Thus, the establishment of an experimental setup
able to maintain stable conditions is an absolute
requirement in order to design truly significant
experiments and collect reliable data with MEAs.
Nowadays, standard MEA-based experimental
platforms are well-established setups for several
neurobiological applications where short recordings
(i.e., from 10 minutes to a couple of hours) are
adequate to gather the information of interest.
Nevertheless, the possibility to perform longer
investigations of neuronal activity is a challenge to
throw light on physiological or pathological
mechanisms evolving over longer time windows
(e.g. degeneration of functional connectivity).
Some effort has been already directed at
improving standard setups in order to reach this aim
(Potter and DeMarse, 2001). Among the proposed
solutions, an effective solution is represented by
551
Regalia G., Biffi E., Lucchini A., Capriata M., Achilli S., Menegon A., Ferrigno G., Colombo L. and Pedrocchi A..
An Experimental Platform Aimed at Long Lasting Electrophysiological Multichannel Recordings of Neuronal Cultures.
DOI: 10.5220/0004659005510557
In Proceedings of the 5th International Joint Conference on Computational Intelligence (SSCN-2013), pages 551-557
ISBN: 978-989-8565-77-8
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

compact commercial top stage incubators for
microscopy analysis (e.g., Ibidi GmbH, Okolab
s.r.l.) which provide an effective environment
control but they are hardly modifiable to be coupled
to MEA technology. On the other hand, custom
setups built up for MEA-based experiments, do not
include the control of all environmental parameters
and they usually provide temporary solutions
(Novellino et al., 2011). Recently, it was devised a
novel system which merges an effective
environmental control and multisite recordings
capability. This system is a pilot environmental
chamber for a single neuronal culture grown on a
MEA coupled to external commercial electronics
(Biffi et al., 2012). However, more effort has to be
done to improve the osmolarity control, to advance
the quality of recorded neuronal signals and to
increase the throughput of the system.
To tackle the challenge of environmental
stability during in vitro electrophysiological
experiments and to fulfil the requirements of
environmental stability, good quality recordings and
multi-MEA format, we designed and validated a
stand-alone platform for multi site experiments with
neuronal networks. In particular, we present here: (i)
the design of the environmental chamber and a
preliminary quantitative characterization of the
environmental control (ii) the design and validation
of a custom modular multichannel front-end and (iii)
preliminary results regarding prolonged recordings
with this experimental platform.
2 MATERIALS AND METHODS
2.1 Multi-MEA Environmental
Chamber Realization
Figure 1 reports a schematic representation of the
environmental chamber. The chamber has been
realized by assembling two PMMA boxes: an
external one (220x220x45 mm) and an internal one
(180x180x30 mm), the latter being surrounded by a
water jacket. Both boxes are sealed with an airtight
top plate, through which we drilled openings for air
inlet and outlet, for the insertion of a temperature
probe and for medium exchange from outside. In the
internal box, we placed a reference well in the centre
and four 50x50 mm housings for 60 channel MEA
chips in the corners. The recording electronics
(paragraph 2.2) contacts the 60 pads of each MEA
chip by means of vertical gold spring probes. Signals
are carried outside by means of a 68-pin connector
inserted and sealed through the top plate. Openings
Figure 1: Schematic representation of the environmental
chamber (top) and top view of MEAs housings (bottom).
for microscope objective insertion were drilled
below the four MEA housings.
2.1.1 Temperature Control
The chamber heating is obtained by means of a
circulating bath (E306, Ecoline, Lauda GmbH) and a
feedback Proportional-Integral control, as previously
described (Biffi et al., 2012). Preliminary
simulations of the temperature distribution in the
MEA housings were performed with a FEM code
(Comsol). During experiments, the temperature of
culturing medium contained in Petri dishes located
in the four MEA housings was monitored by
thermo-couples to verify its maintenance in a
physiological range (37 ± 0.5 °C).
2.1.2 PH Stabilization
To maintain the pH of the medium in a physiological
range (7.2-7.4), an air flow enriched with CO
2
is
injected in the chamber from a gas cylinder.
Experimental tests have been conducted to link the
air flow rate to the CO
2
content inside the chamber.
20% O
2
, 75% N
2
, and 5% CO
2
air flow rates
spanning from 70 to 500 ml/min were set by means
of a flow meter (NG series, Platon SaS). For each
flow rate, the gaseous CO
2
percentage in the
chamber was measured by a CO
2
tester (Heraeus,
Thermo Scientific).
2.1.2 Humidity Regulation
To slow medium evaporation, the flow is warmed
and humidified by an independent bubbling module
placed onto the lab bench. This unit is formed by a
glass bottle containing a glass micro-filter candle to
improve the creation of bubbles, and thus the air
IJCCI 2013 - International Joint Conference on Computational Intelligence
552
humidification. A Nickel-Chrome heating wire (10
W), insulated by a silicon sheath, is placed into the
bottle to worm up the gas flow. This element is
powered on when a miniaturized humidity sensor
(SHT15, Sensirion AG), integrated into the chamber,
measures values of relative humidity (RH) lower
than 85% and it is switched off when RH reaches
95%. In its first version, the control is implemented
by software (USB6009 and Labview, National
Instruments). An heating resistive wire is bound to
the tube which connects the bubbling module to the
chamber, to prevent air cooling. To have a reference
for the miniaturized sensor, RH measurements after
the bubbling column and inside the environmental
chamber have been performed with a commercial
probe (HMP233, Vaisala Inc.).
A high RH level could induce condensation in
the chamber, thus raising the probability of water
droplets to drain on the cells and decreasing
visibility from outside. Condensation of the water
vapour over the inner face of the top plate would
arise if its temperature lowers under the dew point.
To avoid this drawback, heating elements could be
placed on the top plate. To suitably design the
heaters, a FEM model of the steady-state heat
transfer through the whole environmental chamber
has been implemented (Comsol).
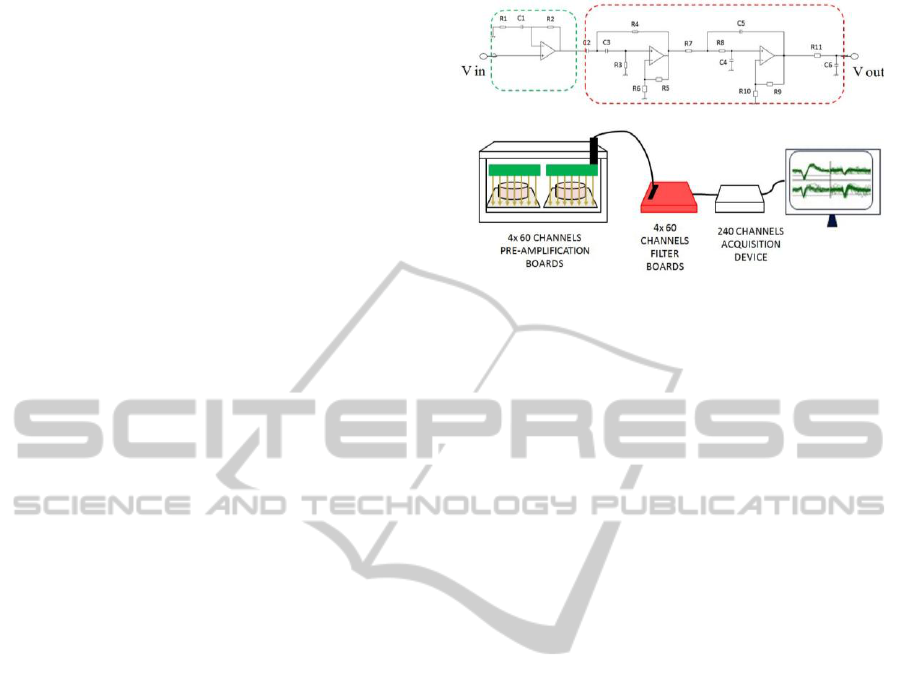
2.2 MEA Recording Boards Design
To perform electrical recordings from cultures
grown on four 60-channel MEA chips, custom pre-
processing boards have been designed and coupled
to a multichannel commercial data acquisition
system (USB-ME240, 50 kHz, 16 bit, Multi Channel
Systems, MCS, GmbH).
The whole processing chain was designed to
fulfil the main following requirements: (1) high gain
(~ 60 dB), suitable for the amplification of neuronal
signals in vitro (20-400 μV peak to-peak ) (2)
neuronal spike bandwidth (300Hz - 5 kHz), (3) low
noise (< 20 μV peak-to-peak, i.e., typical thermal
and background biological noise at the
microelectrodes). The defined circuitry (Figure 2,
top) consists of a pre-amplification stage (gain 100,
high pass cut frequency at 300 Hz), a Butterworth
high-pass filter (gain 3, cut frequency at 300 Hz) and
a Butterworth low pass filter followed by a RC low
pass (gain 3, cut frequency at 5 kHz). The
performance of the circuit was simulated with Spice
software (LTSpice, Linear Technology).
Figure 2: Schematic of the custom front-end circuitry (top)
and assembly of the boards (bottom).
For each 60 channel MEA, the pre-amplification
stage was designed to be implemented on two 30-
channel boards (65x65 mm) placed inside the culture
chamber (Figure 2, in green), in order to avoid the
degradation of the signal-to-noise ratio (SNR) of
recordings. A squared hole has been drilled in the
centre of each board in order to allow visibility of
the cultures and to insert tubes from medium
exchange from the top. The following filter stage
was implemented on two 100x100 mm 30-channel
external boards (Figure 2, in red). Surface mounted
components and low noise, precision opamps were
chosen. Four copies of pre-amplifiers and filter
modules were realized in order to record
simultaneously from four MEAs (i.e., 240 channels).
The assessment of the real frequency response
gain of each board was obtained by providing sine
waves (peak-to-peak amplitude equal to 100 μV for
the pre-amplification stage and 10 mV for the filter
stage) by means of a sinusoidal wave generator with
frequency varying between 1 Hz and 10 kHz, for
each channel. The input-referred noise (300 Hz - 5
kHz) of each board was tested measuring the
channel output and dividing it by the overall
nominal gain, with inputs connected to ground. The
channel crosstalk was measured by sending a 1 kHz
controlled sine wave to one channel, and recording
from directly adjacent ones (with inputs grounded).
After testing each board independently, pre-
amplification and filter boards were connected and
tested together.
2.3 Electrophysiological Recordings
From Neuronal Cultures
An Experimental Platform Aimed at Long Lasting Electrophysiological Multichannel Recordings of Neuronal Cultures
553
Neuronal cultures on MEAs were obtained with a
standard protocol, described in (Ghezzi et al., 2008,
Biffi et al., 2012 b). Short recordings (< 30 min)
from hippocampal neurons (CD1 mice, E17.5,
200.000 cells/MEA) grown on standard MEA chips
(60 electrodes, MCS GmbH) were performed during
the 2
nd
and 3
rd
week of maturation, with the aim of
evaluating signal quality and spikes morphology.
Moreover, to evaluate the feasibility of prolonged
experiments with the system, we performed
recordings lasting several hours (>2 h). Spikes were
detected by a comparison with a threshold based on
noise level (as described in Biffi et al., 2010). Then,
time stamps were analyzed by means of standard
signal processing (Matlab®) (Biffi et al., 2011). The
SNR for a single channel has been defined averaging
the spikes amplitudes over the recording window
and dividing by the noise over the first 500 ms. The
mean firing rate was chosen as a global descriptor of
network activity (Biffi et al., 2011), computed as the
ratio between the total number of spikes and the
number of active channels, reported in Hz.
3 RESULTS
3.1 Multi-MEA Environmental
Chamber
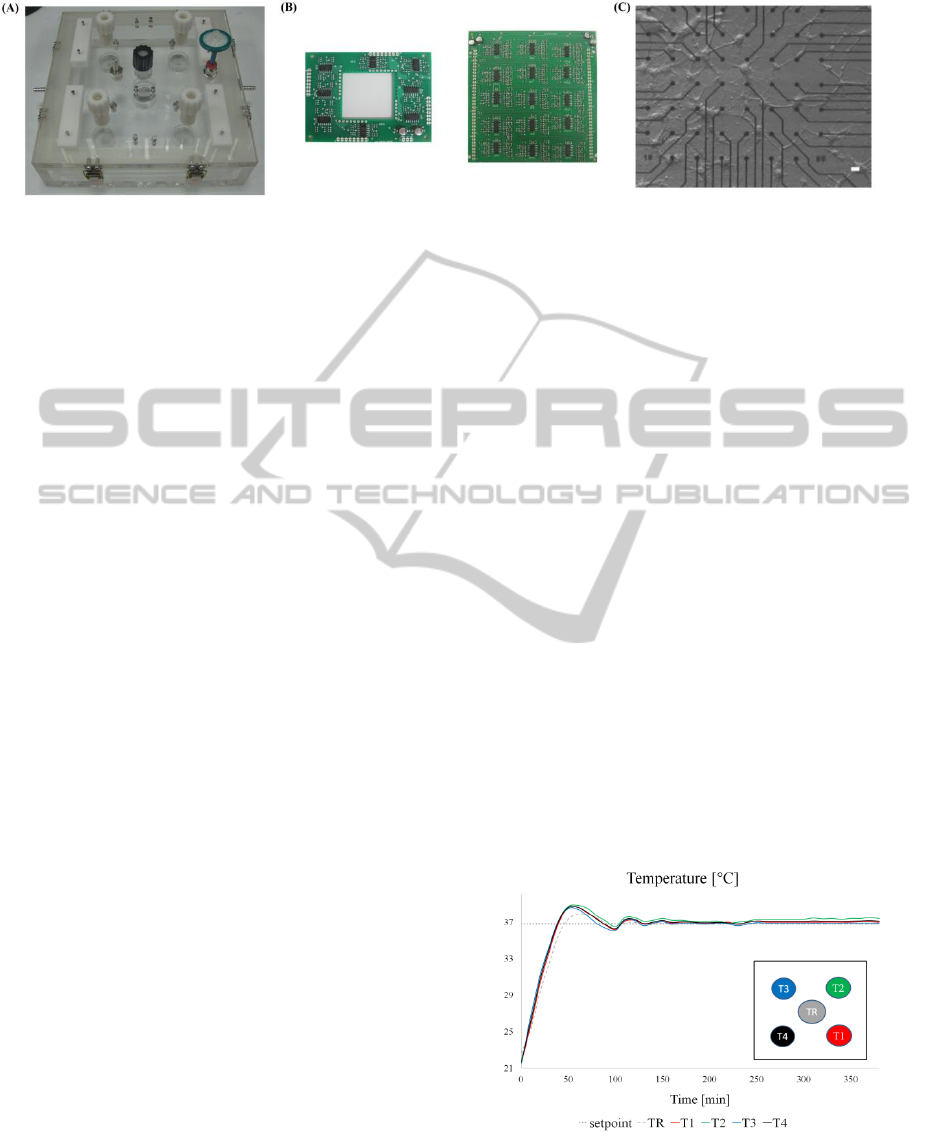
Figure 3A reports the chamber assembled. Figure 3B
shows the custom pre-amplifier board (left) and
filter board (right). Figure 3C shows a neuronal
network on microelectrodes of a MEA chip. The
image was taken by using an inverted microscope
(Axiovert 135 TV, Zeiss). The 5x differential
interference contrast objective was inserted beneath
the MEA housing through the opening in the bottom
wall. The quality of the picture attests the possibility
of duly monitoring cells inside the chamber during
network maturation.
3.1.1 Temperature Control
The FEM simulations confirm that in all the possible
operative conditions, the temperature difference
among the MEA allocations is negligible. To
maintain the desired temperature in the MEA
housings the set-point has been set to 36.8 °C. After
the initial heating phase (almost 60 minutes),
temperature measurements compare well among the
4 housings and show small oscillations around 37°C
(i.e., <0.5°C peak-to-peak) (Figure 4).
3.1.2 PH Stability
As a result of the air flow rate characterization, flow
rates ranging from 140 ml/min to 500 ml/min allow
to balance CO
2
losses occurring along the tubing
system and in the chamber and to reach 5% CO
2
,
i.e., the value needed to maintain cell culture pH at
7.4 (Biffi et al., 2012). After these experiments we
chose a flow rate equal to 200 ml/min for further
experiments as a trade-off between a physiological
pH value, a good humidification (better for higher
flow rates) and the gas cylinder consumption.
Figure 4: Temperature time course measured at the
reference well (dotted line) and at the four MEAs
allocations (T1-T4). The insert is a top view of the
temperature measurement points.
Figure 3: (A) Picture of the realized environmental chamber. (B) Assembled electronic boards for the internal pre-amplifier
stage (left) and external filter stage (right). (C) Inverted microscope image of a neuronal culture grown on a MEA taken
from the chamber (bar scale 10 μm).
IJCCI 2013 - International Joint Conference on Computational Intelligence
554
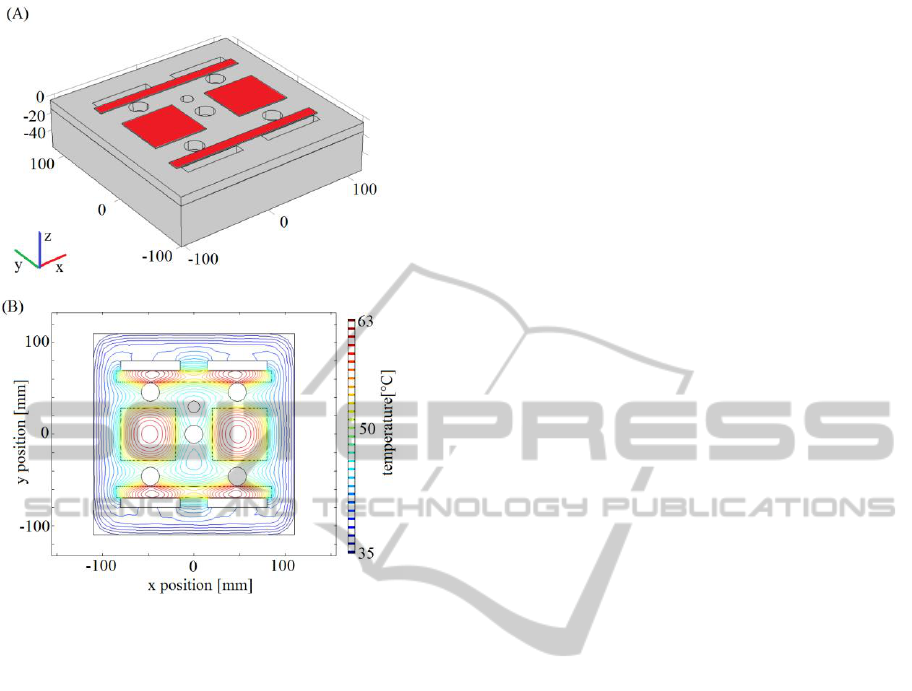
Figure 5: (A) 3D chamber geometry with heating elements
on the top plate (red). (B) Temperature contour in the
lower side of the top plate.
3.1.3 Humidity Maintenance
With the selected flow rate of 200 ml/min, the
control system maintains almost 95% RH in the
bubbling column (water temperature 65 °C, air
temperature 27 °C), which corresponds to almost
85% RH in the chamber (air temperature 35°C). The
simplest solution to overcome condensation on the
inner side of the top plate turns out to be the use of
electrical strip heaters on the outer surface of the
cover. A suitable heater configuration consists of
two couples of heaters (170×12 mm, 0.75 W and
60×57 mm, 1 W, respectively), shown in Figure 5A.
They keep the top plate inner surface temperature
above the dew point (i.e., 35 °C, 85% RH) inducing
a maximum temperature of almost 60°C, which is
lower than PMMA melting point (Figure 5B).
Moreover, their allocation preserve visibility from
the outside of the chamber. To limit the power
consumption and to avoid accidental contact with
hot surfaces, an insulating, removable layer of
rubber foam, 10 mm thick, has been simulated above
the heaters. Preliminary experiments with real
heaters seem to confirm the simulations (maximum
temperature of almost 58 °C) but further tests have
to be performed to verify that the heaters do not
interfere with cells viability and quality of the
recordings.
3.2 Boards Performances
The defined front-end circuit is suitable for the
analog processing of in vitro neuronal signals. The
measured whole gain of pre-amplifiers and filter
boards is in agreement with calculations and Spice
simulations (absolute error equal to ~1.6 dB in the
bandwidth, Figure 6). Also, noise performances of
the whole chain in terms of input-noise (4 μV RMS,
mean of the 60 channels) and cross-talk gain (-36
dB, mean of the 60 channels) are comparable to
noise as measured from the commercial equipment
and other custom setups (Bottino et al., 2009,
Rolston et al., 2009).
3.3 Long Lasting Recordings of
Neuronal Networks
The environmental chamber and the custom
electronic described above, were used to perform
electrophysiological multichannel recordings from
hippocampal neuronal cultures. We observed a mean
SNR equal to 5.4 dB, which is comparable to
recordings performed with standard equipment in
our lab. Furthermore, the recorded biphasic spike
waves compare well to those described in the
literature (Rolston et al., 2009) (Figure 7).
Moreover, preliminary results show that neuronal
cells inside the controlled environment do not
undergo the activity decline, that typically occurs
with the standard setup when the recording time
windows is longer than 2 hours. Several cultures
were recorded for time windows lasting from 3 to 12
hours. As an example, Figure 8 reports the spike rate
of a neuronal culture briefly recorded in the standard
setup (with only temperature control) and
immediately after in the environmental and
recording chamber for almost 4 hours (temperature,
RH% and gaseous CO
2
control). Apart from the
initial adaptation due to the repositioning, neuronal
activity inside the chamber is stable over the time
window. Furthermore, it is characterized by a mean
value (2.6 Hz) and fluctuations (± 0.78 Hz)
comparable to the activity recorded by the standard
setup over a shorter time window (2.3 Hz ± 0.68
Hz), which demonstrates the reliability of long-term
data (i.e., the culture kept staying in a physiological
state throughout the 4 hours).
An Experimental Platform Aimed at Long Lasting Electrophysiological Multichannel Recordings of Neuronal Cultures
555

Figure 6: Comparison between the simulated gain and the
measured one (pre-amplifiers + filter boards).
Figure 7: Overlapped neuronal spikes recorded from a
MEA channel inside the environmental chamber.
Figure 8: Spike rate of a neuronal culture recorded in the
standard setup and in the environmental recording
chamber. Mean spike rates over 1minute bins are reported.
4 DISCUSSION
To tackle the challenge of environmental stability
during in vitro electrophysiological experiments, we
designed and validated a stand-alone platform aimed
at maintaining a controlled environment while
growing and recording from neuronal networks on
MEAs.
To realize a controlled environment, we
connected the chamber to a temperature controller
and a system to inject air enriched with CO
2
and
water vapour. We demonstrated that the chamber
maintains a stable physiological temperature in each
of the four MEA housings. Moreover this
preliminary measurements suggest that a flow rate of
200 ml/min is optimal to obtain (i) a CO
2
percentage
almost equal to the quantity contained in the air
delivered by the gas source (ii) a quite high level of
RH in the chamber, i.e. 85% RH, which is
comparable to other commercial top stage incubators
(Ibidi GmbH).
Regarding the custom front-end, it is suitable to
be coupled with the chamber, both in terms of sizes,
environmental compatibility and recording
performances. Moreover, the realized boards are
cheaper and more easily replicable than commercial
recording front-end devices or custom CMOS-based
systems (Rolston et al., 2009). Preliminary results
assessed the feasibility of performing experiments
with MEAs longer than standard ones (i.e., 2 hours)
thanks to the stable, physiological environment.
Regarding the throughput of the system, the actual
prototype houses four 60-channel MEA chips, which
means up to 24 cultures if 6-well MEAs (9
electrodes per well) are used (MCS GmbH).
Future work will include an improvement of the
humidification system, the integration of gas sensors
in the chamber and repeated experimental tests to
assess the reproducibility of the system.
5 CONCLUSIONS
In this work we presented the design and
preliminary validation of a challenging stand-alone
platform for parallel prolonged experiments from
neuronal cells grown on MEAs. Our final aim is to
provide a compact technological tool for an
electrophysiological laboratory, independent from
both bulky incubators and expensive front-end
equipments, and easy to handle for experimenters.
This system provides new perspectives for in vitro
long-term, high-throughput electrophysiological
studies on neuronal cultures on MEAs.
ACKNOWLEDGEMENTS
Authors would like to thank people from the
Alembic facility for their support and Dr. De Ceglia
for the dissection of hippocampi.
REFERENCES
Biffi, E., Ghezzi, D., Pedrocchi, A. et al., 2010.
Development and validation of a spike detection and
classification algorithm aimed to be implemented on
hardware devices. Comput Intell and Neurosci. DOI
10.1155/2010/659050.
Biffi, E., Menegon, A., Regalia, G. et al., 2011. A new
cross-correlation algorithm for the analysis of "in
vitro" neuronal network activity aimed at
IJCCI 2013 - International Joint Conference on Computational Intelligence
556

pharmacological studies. J Neurosci Methods.
199:321-327.
Biffi, E., Piraino, F., Pedrocchi, A. et al., 2012b. A
microfluidic platform for controlled biochemical
stimulation of twin neuronal networks. Biomicroflu.
6:24106-2410610.
Biffi, E., Regalia, G., Ghezzi, D. et al., 2012a. A novel
environmental chamber for neuronal network multisite
recordings. Biotechnol Bioeng. 109:2553-2566.
Bottino, E., Massobrio, P., Martinoia, S. et al., 2009. Low-
noise low-power CMOS preamplifier for multi-site
extracellular neuronal recordings. Microelec J.
40:1779- 1787.
Eckmann, J., Feinerman, O., Gruendlinger, L., Moses, E.
et al., 2007. The physics of living neural networks.
Physics Reports 449: 54-76.
Ghezzi, D., Menegon, A., Pedrocchi, A. et al., 2008. A
Micro-Electrode Array device coupled to a laser-based
system for the local stimulation of neurons by optical
release of glutamate. J Neurosci Methods. 175:70-78.
Johnstone, A.F., Gross, G.W., Weiss, D.G. et al., 2010.
Microelectrode arrays: a physiologically based
neurotoxicity testing platform for the 21st century.
Neurotoxicology. 31:331-350.
Novellino, A., Scelfo, B., Palosaari, T. et al., 2011.
Development of micro-electrode array based tests for
neurotoxicity: assessment of interlaboratory
reproducibility with neuroactive chemicals. Front
Neuroeng. DOI 10.3389/fneng.2011.00004.
Potter, .SM., DeMarse, T.B., 2001. A new approach to
neural cell culture for long-term studies. J Neurosci
Methods. 110:17-24.
Rolston, J.D., Gross, R.E., Potter, S.M., 2009. A low-cost
multielectrode system for data acquisition enabling
real-time closed-loop processing with rapid recovery
from stimulation artefact. Front Neuroeng. DOI
10.3389/neuro.16.012.2009.
Rossi, S., Muzio, L., De Chiara et al., 2011. Impaired
striatal GABA transmission in experimental
autoimmune encephalomyelitis. Brain Behav Immun.
25:947-56.
An Experimental Platform Aimed at Long Lasting Electrophysiological Multichannel Recordings of Neuronal Cultures
557
